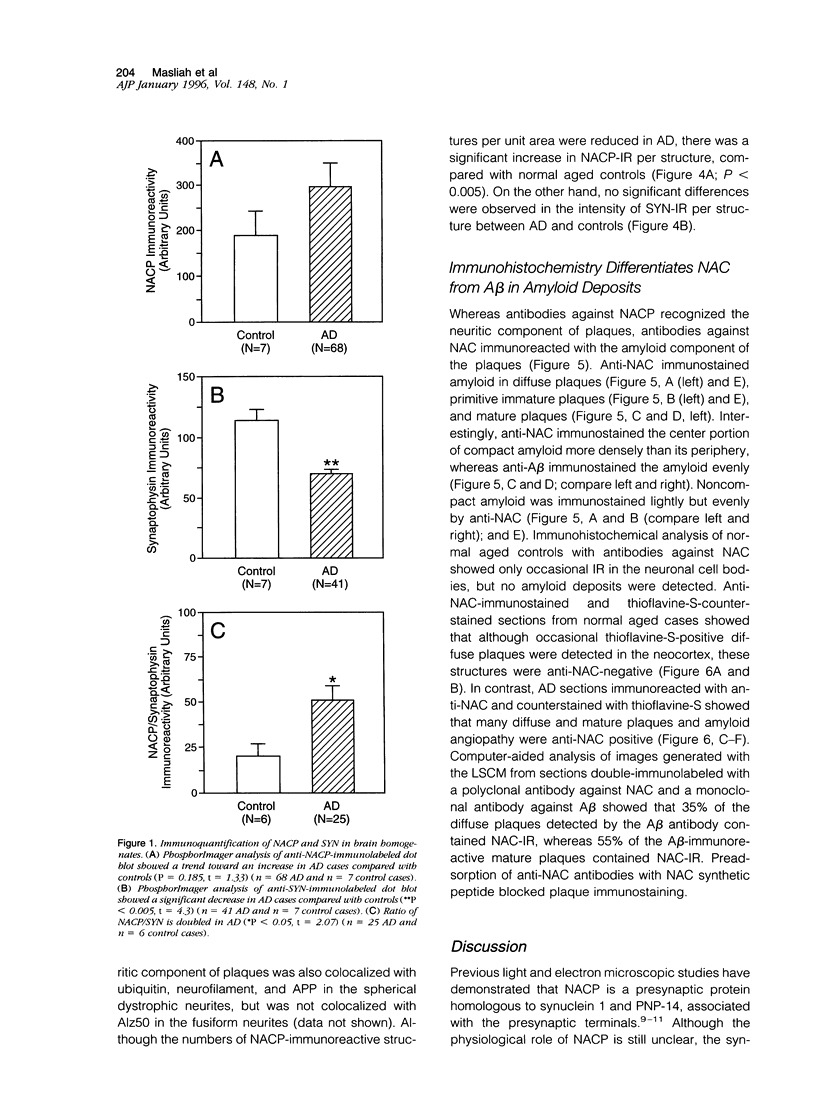
Abstract
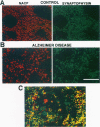
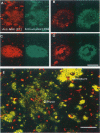
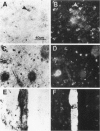
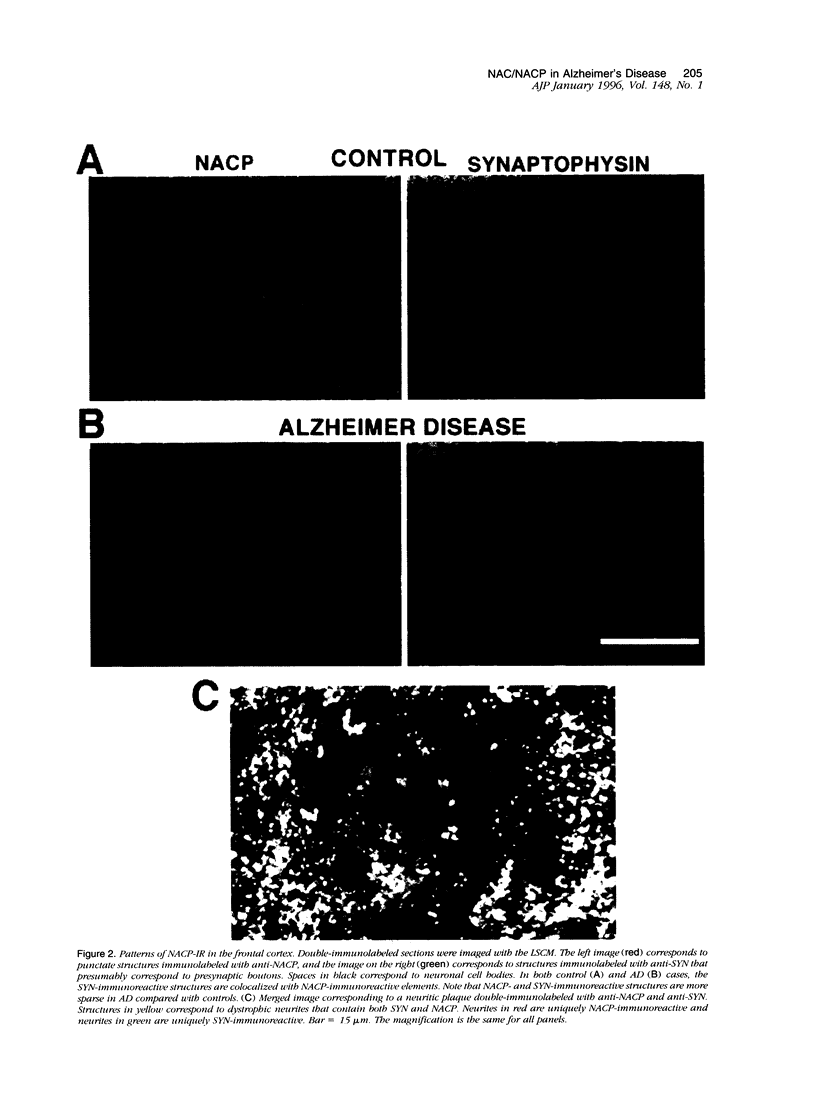
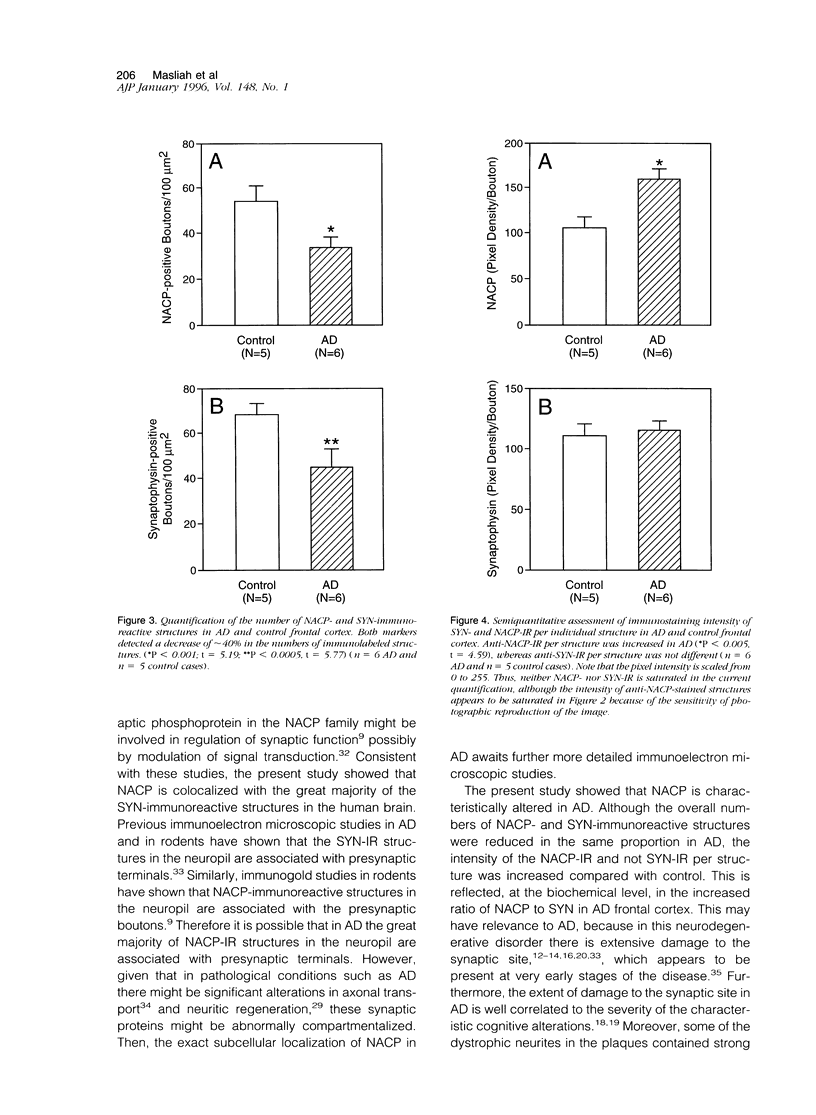
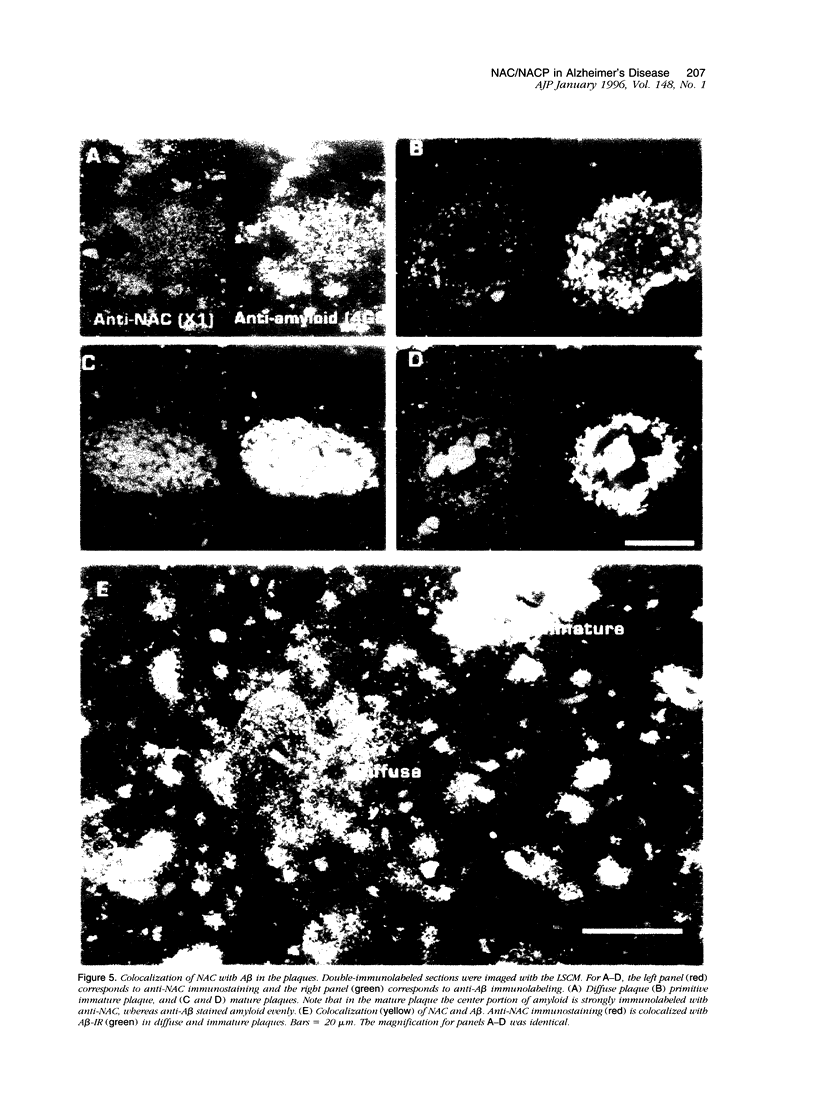
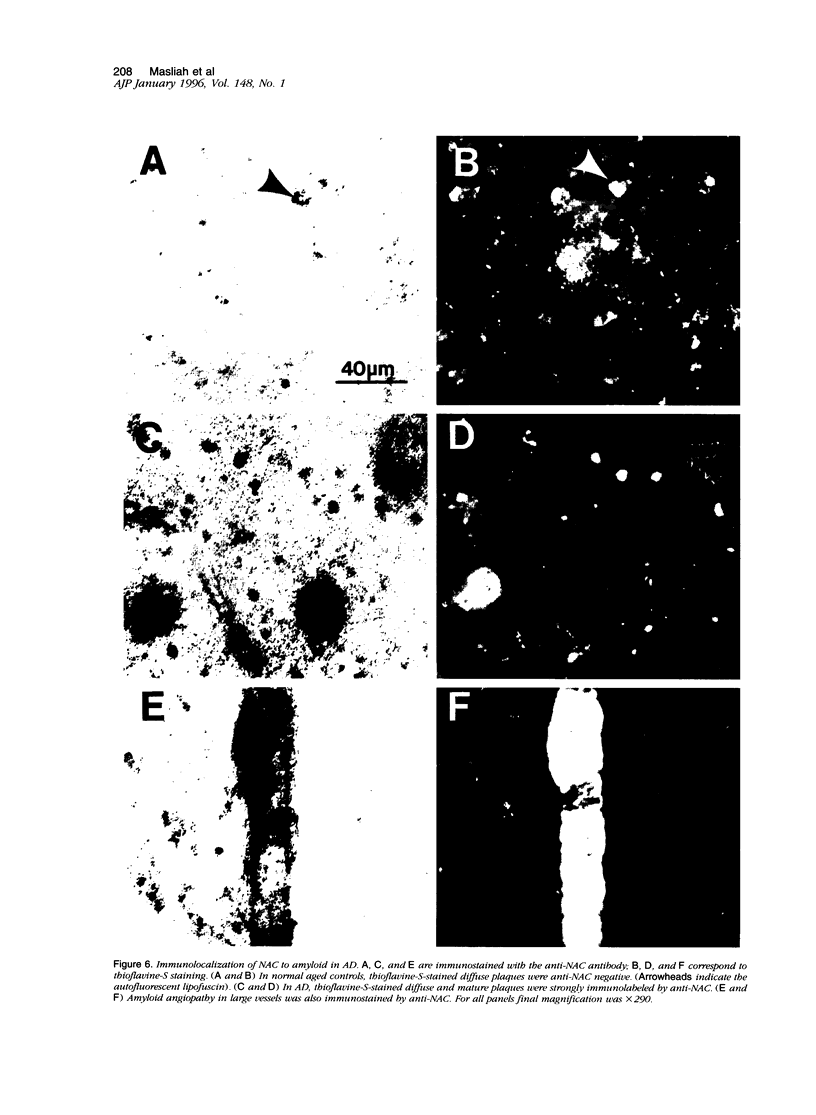
We have recently identified, in the brain tissue of patients afflicted with Alzheimer's disease (AD), the non-A beta component of AD amyloid (NAC) as a new constituent of amyloid. NAC is derived from a larger precursor, NACP, a presynaptic protein. To better understand the role of NACP/NAC in the pathogenesis of AD, we used semiquantitative immunoblotting and combined double-immunocytochemistry/laser scanning confocal microscopy to study the concentration and distribution of NACP/NAC in human brain, and compared them to the concentration and distribution of the presynaptic marker synaptophysin and the amyloid marker A beta. The semiquantitative immunoblotting demonstrated that the NACP concentration is slightly increased in the AD frontal cortex without statistical significance, whereas synaptophysin was reduced in its levels in AD. Consequently the proportion of NACP/synaptophysin was more than double in the AD frontal cortex as compared with controls. In the AD neocortex, NACP was colocalized with approximately 80% of the synaptophysin-immunoreactive structures (presumably the presynaptic terminals) and with the dystrophic neuritic component of the plaques. Computer-aided analysis showed that numbers of NACP-immunoreactive structures along synaptophysin-immunoreactive structures were significantly diminished (30 to 40%) in AD. Although the overall numbers of NACP-positive structures were decreased, there was a significant increase in the intensity of NACP-immunoreactivity per structure in AD. This increased intensity of NACP immunoreactivity per structure in AD was not observed with anti-synaptophysin, consistent with immunoblotting-based quantification. Antibodies against NAC immunoreacted with amyloid in 35% of the diffuse plaques and 55% of the mature plaques. Normal aged control brains containing small groups of diffuse plaques were negative with anti-NAC. Double-immunolabeling studies with A beta antibodies showed that NAC immunoreactivity is more abundant in the center portion of amyloid rather than in the periphery. These studies suggest that there is a connection between metabolism of presynaptic proteins and amyloid formation, and that NAC might follow diffuse A beta accumulation resulting in the formation of compact amyloid and mature plaques.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham C. R., Selkoe D. J., Potter H. Immunochemical identification of the serine protease inhibitor alpha 1-antichymotrypsin in the brain amyloid deposits of Alzheimer's disease. Cell. 1988 Feb 26;52(4):487–501. doi: 10.1016/0092-8674(88)90462-x. [DOI] [PubMed] [Google Scholar]
- Cataldo A. M., Thayer C. Y., Bird E. D., Wheelock T. R., Nixon R. A. Lysosomal proteinase antigens are prominently localized within senile plaques of Alzheimer's disease: evidence for a neuronal origin. Brain Res. 1990 Apr 16;513(2):181–192. doi: 10.1016/0006-8993(90)90456-l. [DOI] [PubMed] [Google Scholar]
- Davies C. A., Mann D. M., Sumpter P. Q., Yates P. O. A quantitative morphometric analysis of the neuronal and synaptic content of the frontal and temporal cortex in patients with Alzheimer's disease. J Neurol Sci. 1987 Apr;78(2):151–164. doi: 10.1016/0022-510x(87)90057-8. [DOI] [PubMed] [Google Scholar]
- DeKosky S. T., Scheff S. W. Synapse loss in frontal cortex biopsies in Alzheimer's disease: correlation with cognitive severity. Ann Neurol. 1990 May;27(5):457–464. doi: 10.1002/ana.410270502. [DOI] [PubMed] [Google Scholar]
- Glenner G. G., Wong C. W. Alzheimer's disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun. 1984 May 16;120(3):885–890. doi: 10.1016/s0006-291x(84)80190-4. [DOI] [PubMed] [Google Scholar]
- Han H., Weinreb P. H., Lansbury P. T., Jr The core Alzheimer's peptide NAC forms amyloid fibrils which seed and are seeded by beta-amyloid: is NAC a common trigger or target in neurodegenerative disease? Chem Biol. 1995 Mar;2(3):163–169. doi: 10.1016/1074-5521(95)90071-3. [DOI] [PubMed] [Google Scholar]
- Ishii T., Haga S. Complements, microglial cells and amyloid fibril formation. Res Immunol. 1992 Jul-Aug;143(6):614–616. doi: 10.1016/0923-2494(92)80043-k. [DOI] [PubMed] [Google Scholar]
- Iwai A., Masliah E., Yoshimoto M., Ge N., Flanagan L., de Silva H. A., Kittel A., Saitoh T. The precursor protein of non-A beta component of Alzheimer's disease amyloid is a presynaptic protein of the central nervous system. Neuron. 1995 Feb;14(2):467–475. doi: 10.1016/0896-6273(95)90302-x. [DOI] [PubMed] [Google Scholar]
- Iwai A., Yoshimoto M., Masliah E., Saitoh T. Non-A beta component of Alzheimer's disease amyloid (NAC) is amyloidogenic. Biochemistry. 1995 Aug 15;34(32):10139–10145. doi: 10.1021/bi00032a006. [DOI] [PubMed] [Google Scholar]
- Koo E. H., Sisodia S. S., Archer D. R., Martin L. J., Weidemann A., Beyreuther K., Fischer P., Masters C. L., Price D. L. Precursor of amyloid protein in Alzheimer disease undergoes fast anterograde axonal transport. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1561–1565. doi: 10.1073/pnas.87.4.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassmann H., Weiler R., Fischer P., Bancher C., Jellinger K., Floor E., Danielczyk W., Seitelberger F., Winkler H. Synaptic pathology in Alzheimer's disease: immunological data for markers of synaptic and large dense-core vesicles. Neuroscience. 1992;46(1):1–8. doi: 10.1016/0306-4522(92)90003-k. [DOI] [PubMed] [Google Scholar]
- Maroteaux L., Scheller R. H. The rat brain synucleins; family of proteins transiently associated with neuronal membrane. Brain Res Mol Brain Res. 1991 Oct;11(3-4):335–343. doi: 10.1016/0169-328x(91)90043-w. [DOI] [PubMed] [Google Scholar]
- Masliah E., Cole G. M., Hansen L. A., Mallory M., Albright T., Terry R. D., Saitoh T. Protein kinase C alteration is an early biochemical marker in Alzheimer's disease. J Neurosci. 1991 Sep;11(9):2759–2767. doi: 10.1523/JNEUROSCI.11-09-02759.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masliah E., Ellisman M., Carragher B., Mallory M., Young S., Hansen L., DeTeresa R., Terry R. D. Three-dimensional analysis of the relationship between synaptic pathology and neuropil threads in Alzheimer disease. J Neuropathol Exp Neurol. 1992 Jul;51(4):404–414. doi: 10.1097/00005072-199207000-00003. [DOI] [PubMed] [Google Scholar]
- Masliah E., Fagan A. M., Terry R. D., DeTeresa R., Mallory M., Gage F. H. Reactive synaptogenesis assessed by synaptophysin immunoreactivity is associated with GAP-43 in the dentate gyrus of the adult rat. Exp Neurol. 1991 Aug;113(2):131–142. doi: 10.1016/0014-4886(91)90169-d. [DOI] [PubMed] [Google Scholar]
- Masliah E., Hansen L., Albright T., Mallory M., Terry R. D. Immunoelectron microscopic study of synaptic pathology in Alzheimer's disease. Acta Neuropathol. 1991;81(4):428–433. doi: 10.1007/BF00293464. [DOI] [PubMed] [Google Scholar]
- Masliah E., Iimoto D. S., Mallory M., Albright T., Hansen L., Saitoh T. Casein kinase II alteration precedes tau accumulation in tangle formation. Am J Pathol. 1992 Feb;140(2):263–268. [PMC free article] [PubMed] [Google Scholar]
- Masliah E., Mallory M., Hansen L., Alford M., Albright T., DeTeresa R., Terry R., Baudier J., Saitoh T. Patterns of aberrant sprouting in Alzheimer's disease. Neuron. 1991 May;6(5):729–739. doi: 10.1016/0896-6273(91)90170-5. [DOI] [PubMed] [Google Scholar]
- Masliah E., Mallory M., Hansen L., Alford M., DeTeresa R., Terry R. An antibody against phosphorylated neurofilaments identifies a subset of damaged association axons in Alzheimer's disease. Am J Pathol. 1993 Mar;142(3):871–882. [PMC free article] [PubMed] [Google Scholar]
- Masliah E., Mallory M., Hansen L., DeTeresa R., Alford M., Terry R. Synaptic and neuritic alterations during the progression of Alzheimer's disease. Neurosci Lett. 1994 Jun 6;174(1):67–72. doi: 10.1016/0304-3940(94)90121-x. [DOI] [PubMed] [Google Scholar]
- Masliah E., Terry R. D., Alford M., DeTeresa R., Hansen L. A. Cortical and subcortical patterns of synaptophysinlike immunoreactivity in Alzheimer's disease. Am J Pathol. 1991 Jan;138(1):235–246. [PMC free article] [PubMed] [Google Scholar]
- Masters C. L., Multhaup G., Simms G., Pottgiesser J., Martins R. N., Beyreuther K. Neuronal origin of a cerebral amyloid: neurofibrillary tangles of Alzheimer's disease contain the same protein as the amyloid of plaque cores and blood vessels. EMBO J. 1985 Nov;4(11):2757–2763. doi: 10.1002/j.1460-2075.1985.tb04000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mujumdar R. B., Ernst L. A., Mujumdar S. R., Waggoner A. S. Cyanine dye labeling reagents containing isothiocyanate groups. Cytometry. 1989 Jan;10(1):11–19. doi: 10.1002/cyto.990100104. [DOI] [PubMed] [Google Scholar]
- Nakajo S., Tsukada K., Omata K., Nakamura Y., Nakaya K. A new brain-specific 14-kDa protein is a phosphoprotein. Its complete amino acid sequence and evidence for phosphorylation. Eur J Biochem. 1993 Nov 1;217(3):1057–1063. doi: 10.1111/j.1432-1033.1993.tb18337.x. [DOI] [PubMed] [Google Scholar]
- Namba Y., Tomonaga M., Kawasaki H., Otomo E., Ikeda K. Apolipoprotein E immunoreactivity in cerebral amyloid deposits and neurofibrillary tangles in Alzheimer's disease and kuru plaque amyloid in Creutzfeldt-Jakob disease. Brain Res. 1991 Feb 8;541(1):163–166. doi: 10.1016/0006-8993(91)91092-f. [DOI] [PubMed] [Google Scholar]
- Scheff S. W., DeKosky S. T., Price D. A. Quantitative assessment of cortical synaptic density in Alzheimer's disease. Neurobiol Aging. 1990 Jan-Feb;11(1):29–37. doi: 10.1016/0197-4580(90)90059-9. [DOI] [PubMed] [Google Scholar]
- Snow A. D., Sekiguchi R., Nochlin D., Fraser P., Kimata K., Mizutani A., Arai M., Schreier W. A., Morgan D. G. An important role of heparan sulfate proteoglycan (Perlecan) in a model system for the deposition and persistence of fibrillar A beta-amyloid in rat brain. Neuron. 1994 Jan;12(1):219–234. doi: 10.1016/0896-6273(94)90165-1. [DOI] [PubMed] [Google Scholar]
- Suzuki K., Terry R. D. Fine structural localization of acid phosphatase in senile plaques in Alzheimer's presenile dementia. Acta Neuropathol. 1967 May 5;8(3):276–284. doi: 10.1007/BF00688828. [DOI] [PubMed] [Google Scholar]
- Terry R. D., Masliah E., Salmon D. P., Butters N., DeTeresa R., Hill R., Hansen L. A., Katzman R. Physical basis of cognitive alterations in Alzheimer's disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol. 1991 Oct;30(4):572–580. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- Terry R. D., Peck A., DeTeresa R., Schechter R., Horoupian D. S. Some morphometric aspects of the brain in senile dementia of the Alzheimer type. Ann Neurol. 1981 Aug;10(2):184–192. doi: 10.1002/ana.410100209. [DOI] [PubMed] [Google Scholar]
- Uéda K., Fukushima H., Masliah E., Xia Y., Iwai A., Yoshimoto M., Otero D. A., Kondo J., Ihara Y., Saitoh T. Molecular cloning of cDNA encoding an unrecognized component of amyloid in Alzheimer disease. Proc Natl Acad Sci U S A. 1993 Dec 1;90(23):11282–11286. doi: 10.1073/pnas.90.23.11282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uéda K., Saitoh T., Mori H. Tissue-dependent alternative splicing of mRNA for NACP, the precursor of non-A beta component of Alzheimer's disease amyloid. Biochem Biophys Res Commun. 1994 Dec 15;205(2):1366–1372. doi: 10.1006/bbrc.1994.2816. [DOI] [PubMed] [Google Scholar]
- Wiedenmann B., Franke W. W. Identification and localization of synaptophysin, an integral membrane glycoprotein of Mr 38,000 characteristic of presynaptic vesicles. Cell. 1985 Jul;41(3):1017–1028. doi: 10.1016/s0092-8674(85)80082-9. [DOI] [PubMed] [Google Scholar]
- Wolozin B. L., Pruchnicki A., Dickson D. W., Davies P. A neuronal antigen in the brains of Alzheimer patients. Science. 1986 May 2;232(4750):648–650. doi: 10.1126/science.3083509. [DOI] [PubMed] [Google Scholar]
- Yoshimoto M., Iwai A., Kang D., Otero D. A., Xia Y., Saitoh T. NACP, the precursor protein of the non-amyloid beta/A4 protein (A beta) component of Alzheimer disease amyloid, binds A beta and stimulates A beta aggregation. Proc Natl Acad Sci U S A. 1995 Sep 26;92(20):9141–9145. doi: 10.1073/pnas.92.20.9141. [DOI] [PMC free article] [PubMed] [Google Scholar]