Abstract
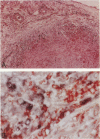
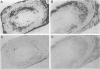
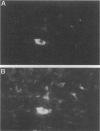
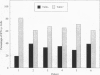
Giant cell vasculitis is an arteritis that predominantly affects medium- and large-sized arteries. Genetic risk factors and clonal expansion of selected CD4+ T cell specificities in the vascular lesions support the model that giant cell arteritis is a T-cell-driven disease. Interferon (IFN)-gamma production in the tissue is intimately associated with the formation of the inflammatory infiltrates. Antigens inducing stimulation of T cells are unknown. To provide indirect evidence for the type and the tissue localization of the antigen, we examined CD4+ T cells in the lesions that secrete IFN-gamma. Temporal artery specimens from patients with giant cell arteritis were analyzed bu two-color immunohistochemistry applying antibodies to T cell markers. IFN-gamma, the interleukin-2 receptor alpha-chain (CD25) and talin, a cytoskeletal protein that is reorganized in T cells interacting with antigen-presenting cells. Proliferating cells in the lesions were identified through the expression of the Ki-67 nuclear antigen. More than 90% of tissue-infiltrating IFN-gamma-producing cells were CD4+ CD45RO+. They represented a minute subset (2 to 4%) of tissue-infiltrating T cells. IFN-gamma+ T cells aggregated in the adventitial layer of the inflamed artery where they were either diffusely distributed or arranged in clusters. The majority of IFN-gamma-secreting T cells expressed CD25. IFN-gamma+ T cells included a fraction of cells that had reorganized the cytoskeletal protein talin, indicating an interaction of the T cell receptor and an antigen-presenting cell. A subset of IFN-gamma-expressing T cells was undergoing proliferation in the tissue. IFN-gamma-producing T cells in vasculitic lesions of giant cell arteritis express several markers that identify them as T cells that have recently been stimulated through their antigen-specific receptor. These putatively disease-relevant T cells represent only a very minor fraction of tissue-infiltrating cells. Their preferential accumulation in the adventitia is most compatible with the model that they contact the relevant antigen primarily in this particular region of the artery. Their regulatory function appears to extend into the inner media and intima where pathological changes in giant cell arteritis are most pronounced.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arai K. I., Lee F., Miyajima A., Miyatake S., Arai N., Yokota T. Cytokines: coordinators of immune and inflammatory responses. Annu Rev Biochem. 1990;59:783–836. doi: 10.1146/annurev.bi.59.070190.004031. [DOI] [PubMed] [Google Scholar]
- Banks P. M., Cohen M. D., Ginsburg W. W., Hunder G. G. Immunohistologic and cytochemical studies of temporal arteritis. Arthritis Rheum. 1983 Oct;26(10):1201–1207. doi: 10.1002/art.1780261005. [DOI] [PubMed] [Google Scholar]
- Cohen J. J., Duke R. C., Fadok V. A., Sellins K. S. Apoptosis and programmed cell death in immunity. Annu Rev Immunol. 1992;10:267–293. doi: 10.1146/annurev.iy.10.040192.001411. [DOI] [PubMed] [Google Scholar]
- Crabtree G. R. Contingent genetic regulatory events in T lymphocyte activation. Science. 1989 Jan 20;243(4889):355–361. doi: 10.1126/science.2783497. [DOI] [PubMed] [Google Scholar]
- Folkman J., Klagsbrun M. Angiogenic factors. Science. 1987 Jan 23;235(4787):442–447. doi: 10.1126/science.2432664. [DOI] [PubMed] [Google Scholar]
- Gerdes J., Li L., Schlueter C., Duchrow M., Wohlenberg C., Gerlach C., Stahmer I., Kloth S., Brandt E., Flad H. D. Immunobiochemical and molecular biologic characterization of the cell proliferation-associated nuclear antigen that is defined by monoclonal antibody Ki-67. Am J Pathol. 1991 Apr;138(4):867–873. [PMC free article] [PubMed] [Google Scholar]
- Haqqi T. M., Anderson G. D., Banerjee S., David C. S. Restricted heterogeneity in T-cell antigen receptor V beta gene usage in the lymph nodes and arthritic joints of mice. Proc Natl Acad Sci U S A. 1992 Feb 15;89(4):1253–1255. doi: 10.1073/pnas.89.4.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell M. D., Diveley J. P., Lundeen K. A., Esty A., Winters S. T., Carlo D. J., Brostoff S. W. Limited T-cell receptor beta-chain heterogeneity among interleukin 2 receptor-positive synovial T cells suggests a role for superantigen in rheumatoid arthritis. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10921–10925. doi: 10.1073/pnas.88.23.10921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunder G. G., Michet C. J. Giant cell arteritis and polymyalgia rheumatica. Clin Rheum Dis. 1985 Dec;11(3):471–483. [PubMed] [Google Scholar]
- Kupfer A., Burn P., Singer S. J. The PMA-induced specific association of LFA-1 and talin in intact cloned T helper cells. J Mol Cell Immunol. 1990;4(6):317–325. [PubMed] [Google Scholar]
- Kupfer A., Swain S. L., Singer S. J. The specific direct interaction of helper T cells and antigen-presenting B cells. II. Reorientation of the microtubule organizing center and reorganization of the membrane-associated cytoskeleton inside the bound helper T cells. J Exp Med. 1987 Jun 1;165(6):1565–1580. doi: 10.1084/jem.165.6.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupfer H., Monks C. R., Kupfer A. Small splenic B cells that bind to antigen-specific T helper (Th) cells and face the site of cytokine production in the Th cells selectively proliferate: immunofluorescence microscopic studies of Th-B antigen-presenting cell interactions. J Exp Med. 1994 May 1;179(5):1507–1515. doi: 10.1084/jem.179.5.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lie J. T. Illustrated histopathologic classification criteria for selected vasculitis syndromes. American College of Rheumatology Subcommittee on Classification of Vasculitis. Arthritis Rheum. 1990 Aug;33(8):1074–1087. doi: 10.1002/art.1780330804. [DOI] [PubMed] [Google Scholar]
- Minami Y., Kono T., Miyazaki T., Taniguchi T. The IL-2 receptor complex: its structure, function, and target genes. Annu Rev Immunol. 1993;11:245–268. doi: 10.1146/annurev.iy.11.040193.001333. [DOI] [PubMed] [Google Scholar]
- Moskophidis D., Lechner F., Pircher H., Zinkernagel R. M. Virus persistence in acutely infected immunocompetent mice by exhaustion of antiviral cytotoxic effector T cells. Nature. 1993 Apr 22;362(6422):758–761. doi: 10.1038/362758a0. [DOI] [PubMed] [Google Scholar]
- Paliard X., West S. G., Lafferty J. A., Clements J. R., Kappler J. W., Marrack P., Kotzin B. L. Evidence for the effects of a superantigen in rheumatoid arthritis. Science. 1991 Jul 19;253(5017):325–329. doi: 10.1126/science.1857971. [DOI] [PubMed] [Google Scholar]
- Paulnock D. M. Macrophage activation by T cells. Curr Opin Immunol. 1992 Jun;4(3):344–349. doi: 10.1016/0952-7915(92)90087-u. [DOI] [PubMed] [Google Scholar]
- Pober J. S., Cotran R. S. Immunologic interactions of T lymphocytes with vascular endothelium. Adv Immunol. 1991;50:261–302. doi: 10.1016/s0065-2776(08)60827-5. [DOI] [PubMed] [Google Scholar]
- Roberts A. B., McCune B. K., Sporn M. B. TGF-beta: regulation of extracellular matrix. Kidney Int. 1992 Mar;41(3):557–559. doi: 10.1038/ki.1992.81. [DOI] [PubMed] [Google Scholar]
- Ross R., Raines E. W., Bowen-Pope D. F. The biology of platelet-derived growth factor. Cell. 1986 Jul 18;46(2):155–169. doi: 10.1016/0092-8674(86)90733-6. [DOI] [PubMed] [Google Scholar]
- Steinman R. M. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- Wagner A. D., Goronzy J. J., Weyand C. M. Functional profile of tissue-infiltrating and circulating CD68+ cells in giant cell arteritis. Evidence for two components of the disease. J Clin Invest. 1994 Sep;94(3):1134–1140. doi: 10.1172/JCI117428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb S., Morris C., Sprent J. Extrathymic tolerance of mature T cells: clonal elimination as a consequence of immunity. Cell. 1990 Dec 21;63(6):1249–1256. doi: 10.1016/0092-8674(90)90420-j. [DOI] [PubMed] [Google Scholar]
- Weyand C. M., Goronzy J. J. Giant cell arteritis as an antigen-driven disease. Rheum Dis Clin North Am. 1995 Nov;21(4):1027–1039. [PubMed] [Google Scholar]
- Weyand C. M., Hicok K. C., Hunder G. G., Goronzy J. J. The HLA-DRB1 locus as a genetic component in giant cell arteritis. Mapping of a disease-linked sequence motif to the antigen binding site of the HLA-DR molecule. J Clin Invest. 1992 Dec;90(6):2355–2361. doi: 10.1172/JCI116125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weyand C. M., Hicok K. C., Hunder G. G., Goronzy J. J. Tissue cytokine patterns in patients with polymyalgia rheumatica and giant cell arteritis. Ann Intern Med. 1994 Oct 1;121(7):484–491. doi: 10.7326/0003-4819-121-7-199410010-00003. [DOI] [PubMed] [Google Scholar]
- Weyand C. M., Schönberger J., Oppitz U., Hunder N. N., Hicok K. C., Goronzy J. J. Distinct vascular lesions in giant cell arteritis share identical T cell clonotypes. J Exp Med. 1994 Mar 1;179(3):951–960. doi: 10.1084/jem.179.3.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams W. V., Fang Q., Demarco D., VonFeldt J., Zurier R. B., Weiner D. B. Restricted heterogeneity of T cell receptor transcripts in rheumatoid synovium. J Clin Invest. 1992 Aug;90(2):326–333. doi: 10.1172/JCI115866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamvil S. S., Steinman L. The T lymphocyte in experimental allergic encephalomyelitis. Annu Rev Immunol. 1990;8:579–621. doi: 10.1146/annurev.iy.08.040190.003051. [DOI] [PubMed] [Google Scholar]