Abstract
The expression of cell adhesion molecules (CAMs) in the choroid plexus was studied in normal brain and during experimental autoimmune encephalomyelitis (EAE) in the SJL/J mouse during inflammation induced by intracerebral injection of killed Corynebacterium parvum in the C3H/He mouse. Both ICAM-1 and VCAM-1, but not MAdCAM-1, were constitutively expressed on choroid plexus epithelium but not on the fenestrated capillary endothelial cells within the choroid plexus. During EAE, we observed an up-regulation of ICAM-1 and VCAM-1 and de novo expression of MAdCAM-1 on choroid plexus epithelial cells. In contrast, endothelial cells in the choroid plexus were not induced to express any of the investigated CAMs. In in situ hybridization analysis we demonstrated that ICAM-1, VCAM-1, and MAdCAM-1 were locally synthesized and that the amount of their mRNAs increased in the inflamed choroid plexus. In vitro, primary choroid plexus epithelial cells could be induced to express ICAM-1, VCAM-1, and MAdCAM-1 on their surface after treatment with proinflammatory cytokines such as tumor necrosis factor-alpha, interleukin-1, interferon-gamma, and lipopolysaccharide. To investigate the functional status of the expressed CAMs we performed Stamper-Woodruff binding assays on frozen sections of inflamed and naive brains. ICAM-1, VCAM-1, and MAdCAM-1 expressed in choroid plexus epithelial cells mediated binding of lymphocytes via their known ligands LFA-1 and alpha4-integrin, respectively. The expression of ICAM-1, VCAM-1, and MAdCAM-1 on choroid plexus epithelial cells together with the lack of their expression on the fenestrated choroid plexus endothelium raises the possibility that the epithelial blood-cerebrospinal-fluid barrier plays an important role in the immunosurveillance of the central nervous system.
Full text
PDF











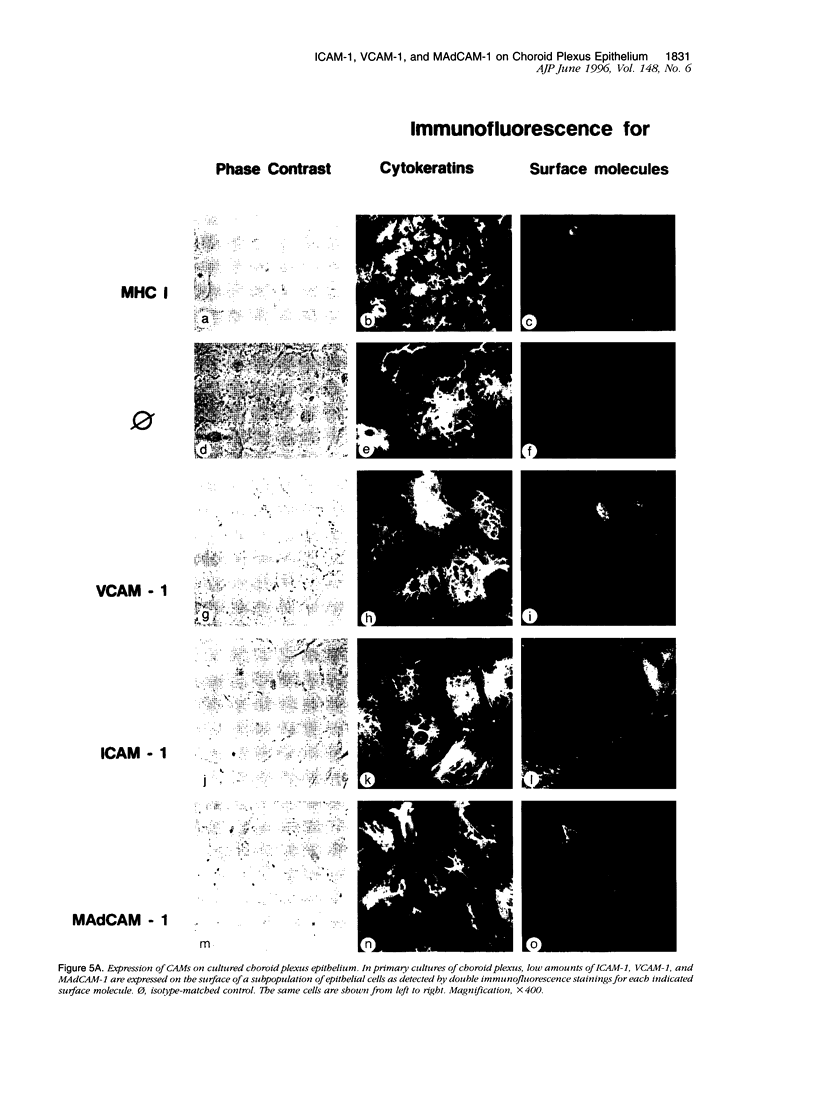







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen D. J., Highison G. J., Werneck H., Gentry G. Morphological specializations of the ventricular surface of the choroidal epithelium and associated epiplexus cells. Prog Clin Biol Res. 1981;59B:11–20. [PubMed] [Google Scholar]
- Andrew D. P., Berlin C., Honda S., Yoshino T., Hamann A., Holzmann B., Kilshaw P. J., Butcher E. C. Distinct but overlapping epitopes are involved in alpha 4 beta 7-mediated adhesion to vascular cell adhesion molecule-1, mucosal addressin-1, fibronectin, and lymphocyte aggregation. J Immunol. 1994 Nov 1;153(9):3847–3861. [PubMed] [Google Scholar]
- Archelos J. J., Jung S., Mäurer M., Schmied M., Lassmann H., Tamatani T., Miyasaka M., Toyka K. V., Hartung H. P. Inhibition of experimental autoimmune encephalomyelitis by an antibody to the intercellular adhesion molecule ICAM-1. Ann Neurol. 1993 Aug;34(2):145–154. doi: 10.1002/ana.410340209. [DOI] [PubMed] [Google Scholar]
- Archelos J. J., Jung S., Mäurer M., Schmied M., Lassmann H., Tamatani T., Miyasaka M., Toyka K. V., Hartung H. P. Inhibition of experimental autoimmune encephalomyelitis by an antibody to the intercellular adhesion molecule ICAM-1. Ann Neurol. 1993 Aug;34(2):145–154. doi: 10.1002/ana.410340209. [DOI] [PubMed] [Google Scholar]
- Bargatze R. F., Wu N. W., Weissman I. L., Butcher E. C. High endothelial venule binding as a predictor of the dissemination of passaged murine lymphomas. J Exp Med. 1987 Oct 1;166(4):1125–1131. doi: 10.1084/jem.166.4.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron J. L., Madri J. A., Ruddle N. H., Hashim G., Janeway C. A., Jr Surface expression of alpha 4 integrin by CD4 T cells is required for their entry into brain parenchyma. J Exp Med. 1993 Jan 1;177(1):57–68. doi: 10.1084/jem.177.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton R. W., Rothlein R., Ksiazek J., Kennedy C. The effect of anti-intercellular adhesion molecule-1 on phorbol-ester-induced rabbit lung inflammation. J Immunol. 1989 Aug 15;143(4):1278–1282. [PubMed] [Google Scholar]
- Berlin C., Berg E. L., Briskin M. J., Andrew D. P., Kilshaw P. J., Holzmann B., Weissman I. L., Hamann A., Butcher E. C. Alpha 4 beta 7 integrin mediates lymphocyte binding to the mucosal vascular addressin MAdCAM-1. Cell. 1993 Jul 16;74(1):185–195. doi: 10.1016/0092-8674(93)90305-a. [DOI] [PubMed] [Google Scholar]
- Breier G., Albrecht U., Sterrer S., Risau W. Expression of vascular endothelial growth factor during embryonic angiogenesis and endothelial cell differentiation. Development. 1992 Feb;114(2):521–532. doi: 10.1242/dev.114.2.521. [DOI] [PubMed] [Google Scholar]
- Briskin M. J., McEvoy L. M., Butcher E. C. MAdCAM-1 has homology to immunoglobulin and mucin-like adhesion receptors and to IgA1. Nature. 1993 Jun 3;363(6428):461–464. doi: 10.1038/363461a0. [DOI] [PubMed] [Google Scholar]
- Campanero M. R., Pulido R., Ursa M. A., Rodríguez-Moya M., de Landázuri M. O., Sánchez-Madrid F. An alternative leukocyte homotypic adhesion mechanism, LFA-1/ICAM-1-independent, triggered through the human VLA-4 integrin. J Cell Biol. 1990 Jun;110(6):2157–2165. doi: 10.1083/jcb.110.6.2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter S. J., McCarthy L. E., Borison H. L. Electron microscopic study of the epiplexus (Kolmer) cells of the cat choroid plexus. Z Zellforsch Mikrosk Anat. 1970;110(4):471–486. doi: 10.1007/BF00330099. [DOI] [PubMed] [Google Scholar]
- Coomber B. L., Stewart P. A. Morphometric analysis of CNS microvascular endothelium. Microvasc Res. 1985 Jul;30(1):99–115. doi: 10.1016/0026-2862(85)90042-1. [DOI] [PubMed] [Google Scholar]
- Cosimi A. B., Conti D., Delmonico F. L., Preffer F. I., Wee S. L., Rothlein R., Faanes R., Colvin R. B. In vivo effects of monoclonal antibody to ICAM-1 (CD54) in nonhuman primates with renal allografts. J Immunol. 1990 Jun 15;144(12):4604–4612. [PubMed] [Google Scholar]
- Crone C., Olesen S. P. Electrical resistance of brain microvascular endothelium. Brain Res. 1982 Jun 3;241(1):49–55. doi: 10.1016/0006-8993(82)91227-6. [DOI] [PubMed] [Google Scholar]
- Das P. K., de Boer O. J., Visser A., Verhagen C. E., Bos J. D., Pals S. T. Differential expression of ICAM-1, E-selectin and VCAM-1 by endothelial cells in psoriasis and contact dermatitis. Acta Derm Venereol Suppl (Stockh) 1994;186:21–22. [PubMed] [Google Scholar]
- Deckert-Schlüter M., Schlüter D., Hof H., Wiestler O. D., Lassmann H. Differential expression of ICAM-1, VCAM-1 and their ligands LFA-1, Mac-1, CD43, VLA-4, and MHC class II antigens in murine Toxoplasma encephalitis: a light microscopic and ultrastructural immunohistochemical study. J Neuropathol Exp Neurol. 1994 Sep;53(5):457–468. doi: 10.1097/00005072-199409000-00005. [DOI] [PubMed] [Google Scholar]
- Engelhardt B., Conley F. K., Butcher E. C. Cell adhesion molecules on vessels during inflammation in the mouse central nervous system. J Neuroimmunol. 1994 May;51(2):199–208. doi: 10.1016/0165-5728(94)90082-5. [DOI] [PubMed] [Google Scholar]
- Engelhardt B., Conley F. K., Butcher E. C. Cell adhesion molecules on vessels during inflammation in the mouse central nervous system. J Neuroimmunol. 1994 May;51(2):199–208. doi: 10.1016/0165-5728(94)90082-5. [DOI] [PubMed] [Google Scholar]
- Freedman A. S., Munro J. M., Rice G. E., Bevilacqua M. P., Morimoto C., McIntyre B. W., Rhynhart K., Pober J. S., Nadler L. M. Adhesion of human B cells to germinal centers in vitro involves VLA-4 and INCAM-110. Science. 1990 Aug 31;249(4972):1030–1033. doi: 10.1126/science.1697696. [DOI] [PubMed] [Google Scholar]
- Gallatin W. M., Weissman I. L., Butcher E. C. A cell-surface molecule involved in organ-specific homing of lymphocytes. Nature. 1983 Jul 7;304(5921):30–34. doi: 10.1038/304030a0. [DOI] [PubMed] [Google Scholar]
- Ge A. Z., Butcher E. C. Cloning and expression of a cDNA encoding mouse endoglin, an endothelial cell TGF-beta ligand. Gene. 1994 Jan 28;138(1-2):201–206. doi: 10.1016/0378-1119(94)90808-7. [DOI] [PubMed] [Google Scholar]
- Griffiths C. E., Nickoloff B. J. Keratinocyte intercellular adhesion molecule-1 (ICAM-1) expression precedes dermal T lymphocytic infiltration in allergic contact dermatitis (Rhus dermatitis). Am J Pathol. 1989 Dec;135(6):1045–1053. [PMC free article] [PubMed] [Google Scholar]
- Hallmann R., Mayer D. N., Berg E. L., Broermann R., Butcher E. C. Novel mouse endothelial cell surface marker is suppressed during differentiation of the blood brain barrier. Dev Dyn. 1995 Apr;202(4):325–332. doi: 10.1002/aja.1002020402. [DOI] [PubMed] [Google Scholar]
- Hauser S. L., Reinherz E. L., Hoban C. J., Schlossman S. F., Weiner H. L. CSF cells in multiple sclerosis: monoclonal antibody analysis and relationship to peripheral blood T-cell subsets. Neurology. 1983 May;33(5):575–579. doi: 10.1212/wnl.33.5.575. [DOI] [PubMed] [Google Scholar]
- Hession C., Moy P., Tizard R., Chisholm P., Williams C., Wysk M., Burkly L., Miyake K., Kincade P., Lobb R. Cloning of murine and rat vascular cell adhesion molecule-1. Biochem Biophys Res Commun. 1992 Feb 28;183(1):163–169. doi: 10.1016/0006-291x(92)91623-x. [DOI] [PubMed] [Google Scholar]
- Hickey W. F., Hsu B. L., Kimura H. T-lymphocyte entry into the central nervous system. J Neurosci Res. 1991 Feb;28(2):254–260. doi: 10.1002/jnr.490280213. [DOI] [PubMed] [Google Scholar]
- Hickey W. F. Migration of hematogenous cells through the blood-brain barrier and the initiation of CNS inflammation. Brain Pathol. 1991 Jan;1(2):97–105. doi: 10.1111/j.1750-3639.1991.tb00646.x. [DOI] [PubMed] [Google Scholar]
- Hänninen A., Taylor C., Streeter P. R., Stark L. S., Sarte J. M., Shizuru J. A., Simell O., Michie S. A. Vascular addressins are induced on islet vessels during insulitis in nonobese diabetic mice and are involved in lymphoid cell binding to islet endothelium. J Clin Invest. 1993 Nov;92(5):2509–2515. doi: 10.1172/JCI116859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegsmann J., Keyszer G. M., Geiler T., Bräuer R., Gay R. E., Gay S. Expression of vascular cell adhesion molecule-1 mRNA and protein in rheumatoid synovium demonstrated by in situ hybridization and immunohistochemistry. Lab Invest. 1995 Feb;72(2):209–214. [PubMed] [Google Scholar]
- Maxwell W. L., Hardy I. G., Watt C., McGadey J., Graham D. I., Adams J. H., Gennarelli T. A. Changes in the choroid plexus, responses by intrinsic epiplexus cells and recruitment from monocytes after experimental head acceleration injury in the non-human primate. Acta Neuropathol. 1992;84(1):78–84. doi: 10.1007/BF00427218. [DOI] [PubMed] [Google Scholar]
- Maxwell W. L., McGadey J. Response of intraventricular macrophages after a penetrant cerebral lesion. J Anat. 1988 Oct;160:145–155. [PMC free article] [PubMed] [Google Scholar]
- Miyake K., Medina K., Ishihara K., Kimoto M., Auerbach R., Kincade P. W. A VCAM-like adhesion molecule on murine bone marrow stromal cells mediates binding of lymphocyte precursors in culture. J Cell Biol. 1991 Aug;114(3):557–565. doi: 10.1083/jcb.114.3.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake K., Weissman I. L., Greenberger J. S., Kincade P. W. Evidence for a role of the integrin VLA-4 in lympho-hemopoiesis. J Exp Med. 1991 Mar 1;173(3):599–607. doi: 10.1084/jem.173.3.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathanson J. A., Chun L. L. Immunological function of the blood-cerebrospinal fluid barrier. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1684–1688. doi: 10.1073/pnas.86.5.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill J. K., Butter C., Baker D., Gschmeissner S. E., Kraal G., Butcher E. C., Turk J. L. Expression of vascular addressins and ICAM-1 by endothelial cells in the spinal cord during chronic relapsing experimental allergic encephalomyelitis in the Biozzi AB/H mouse. Immunology. 1991 Apr;72(4):520–525. [PMC free article] [PubMed] [Google Scholar]
- Reese T. S., Karnovsky M. J. Fine structural localization of a blood-brain barrier to exogenous peroxidase. J Cell Biol. 1967 Jul;34(1):207–217. doi: 10.1083/jcb.34.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimphanitchayakit V., Hatfull G. F., Grindley N. D. The 43 residue DNA binding domain of gamma delta resolvase binds adjacent major and minor grooves of DNA. Nucleic Acids Res. 1989 Feb 11;17(3):1035–1050. doi: 10.1093/nar/17.3.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risau W., Engelhardt B., Wekerle H. Immune function of the blood-brain barrier: incomplete presentation of protein (auto-)antigens by rat brain microvascular endothelium in vitro. J Cell Biol. 1990 May;110(5):1757–1766. doi: 10.1083/jcb.110.5.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruddle N. H., Bergman C. M., McGrath K. M., Lingenheld E. G., Grunnet M. L., Padula S. J., Clark R. B. An antibody to lymphotoxin and tumor necrosis factor prevents transfer of experimental allergic encephalomyelitis. J Exp Med. 1990 Oct 1;172(4):1193–1200. doi: 10.1084/jem.172.4.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasseville V. G., Newman W. A., Lackner A. A., Smith M. O., Lausen N. C., Beall D., Ringler D. J. Elevated vascular cell adhesion molecule-1 in AIDS encephalitis induced by simian immunodeficiency virus. Am J Pathol. 1992 Nov;141(5):1021–1030. [PMC free article] [PubMed] [Google Scholar]
- Sasseville V. G., Newman W., Brodie S. J., Hesterberg P., Pauley D., Ringler D. J. Monocyte adhesion to endothelium in simian immunodeficiency virus-induced AIDS encephalitis is mediated by vascular cell adhesion molecule-1/alpha 4 beta 1 integrin interactions. Am J Pathol. 1994 Jan;144(1):27–40. [PMC free article] [PubMed] [Google Scholar]
- Seron D., Cameron J. S., Haskard D. O. Expression of VCAM-1 in the normal and diseased kidney. Nephrol Dial Transplant. 1991;6(12):917–922. doi: 10.1093/ndt/6.12.917. [DOI] [PubMed] [Google Scholar]
- Sligh J. E., Jr, Ballantyne C. M., Rich S. S., Hawkins H. K., Smith C. W., Bradley A., Beaudet A. L. Inflammatory and immune responses are impaired in mice deficient in intercellular adhesion molecule 1. Proc Natl Acad Sci U S A. 1993 Sep 15;90(18):8529–8533. doi: 10.1073/pnas.90.18.8529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobel R. A., Mitchell M. E., Fondren G. Intercellular adhesion molecule-1 (ICAM-1) in cellular immune reactions in the human central nervous system. Am J Pathol. 1990 Jun;136(6):1309–1316. [PMC free article] [PubMed] [Google Scholar]
- Streeter P. R., Berg E. L., Rouse B. T., Bargatze R. F., Butcher E. C. A tissue-specific endothelial cell molecule involved in lymphocyte homing. Nature. 1988 Jan 7;331(6151):41–46. doi: 10.1038/331041a0. [DOI] [PubMed] [Google Scholar]
- Streeter P. R., Rouse B. T., Butcher E. C. Immunohistologic and functional characterization of a vascular addressin involved in lymphocyte homing into peripheral lymph nodes. J Cell Biol. 1988 Nov;107(5):1853–1862. doi: 10.1083/jcb.107.5.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarlow M. J., Jenkins R., Comis S. D., Osborne M. P., Stephens S., Stanley P., Crocker J. Ependymal cells of the choroid plexus express tumour necrosis factor-alpha. Neuropathol Appl Neurobiol. 1993 Aug;19(4):324–328. doi: 10.1111/j.1365-2990.1993.tb00447.x. [DOI] [PubMed] [Google Scholar]
- Tsutsumi M., Skinner M. K., Sanders-Bush E. Transferrin gene expression and synthesis by cultured choroid plexus epithelial cells. Regulation by serotonin and cyclic adenosine 3',5'-monophosphate. J Biol Chem. 1989 Jun 5;264(16):9626–9631. [PubMed] [Google Scholar]
- Vecchi A., Garlanda C., Lampugnani M. G., Resnati M., Matteucci C., Stoppacciaro A., Schnurch H., Risau W., Ruco L., Mantovani A. Monoclonal antibodies specific for endothelial cells of mouse blood vessels. Their application in the identification of adult and embryonic endothelium. Eur J Cell Biol. 1994 Apr;63(2):247–254. [PubMed] [Google Scholar]
- Wegner C. D., Gundel R. H., Reilly P., Haynes N., Letts L. G., Rothlein R. Intercellular adhesion molecule-1 (ICAM-1) in the pathogenesis of asthma. Science. 1990 Jan 26;247(4941):456–459. doi: 10.1126/science.1967851. [DOI] [PubMed] [Google Scholar]
- Willenborg D. O., Simmons R. D., Tamatani T., Miyasaka M. ICAM-1-dependent pathway is not critically involved in the inflammatory process of autoimmune encephalomyelitis or in cytokine-induced inflammation of the central nervous system. J Neuroimmunol. 1993 Jun;45(1-2):147–154. doi: 10.1016/0165-5728(93)90175-x. [DOI] [PubMed] [Google Scholar]
- Wilting J., Christ B. An experimental and ultrastructural study on the development of the avian choroid plexus. Cell Tissue Res. 1989 Mar;255(3):487–494. doi: 10.1007/BF00218783. [DOI] [PubMed] [Google Scholar]
- Wolburg H., Neuhaus J., Kniesel U., Krauss B., Schmid E. M., Ocalan M., Farrell C., Risau W. Modulation of tight junction structure in blood-brain barrier endothelial cells. Effects of tissue culture, second messengers and cocultured astrocytes. J Cell Sci. 1994 May;107(Pt 5):1347–1357. doi: 10.1242/jcs.107.5.1347. [DOI] [PubMed] [Google Scholar]
- Yednock T. A., Cannon C., Fritz L. C., Sanchez-Madrid F., Steinman L., Karin N. Prevention of experimental autoimmune encephalomyelitis by antibodies against alpha 4 beta 1 integrin. Nature. 1992 Mar 5;356(6364):63–66. doi: 10.1038/356063a0. [DOI] [PubMed] [Google Scholar]
- Zamvil S. S., Steinman L. The T lymphocyte in experimental allergic encephalomyelitis. Annu Rev Immunol. 1990;8:579–621. doi: 10.1146/annurev.iy.08.040190.003051. [DOI] [PubMed] [Google Scholar]












