Abstract
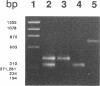
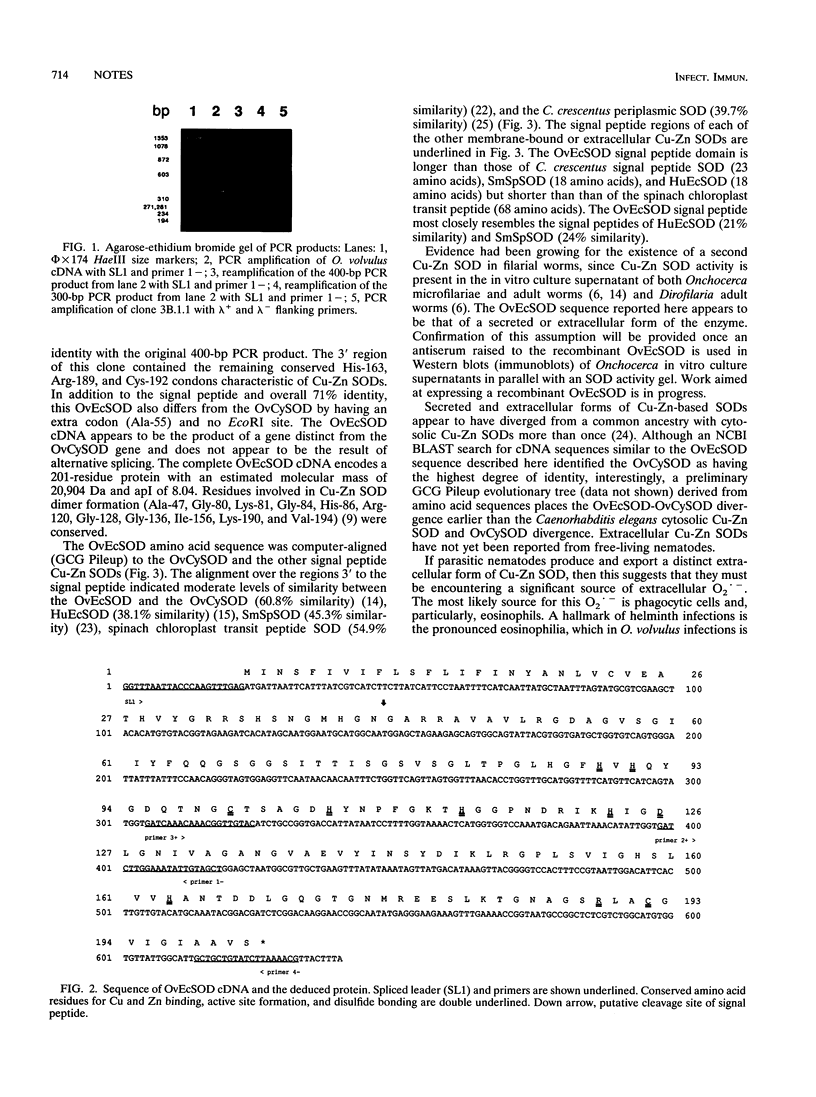
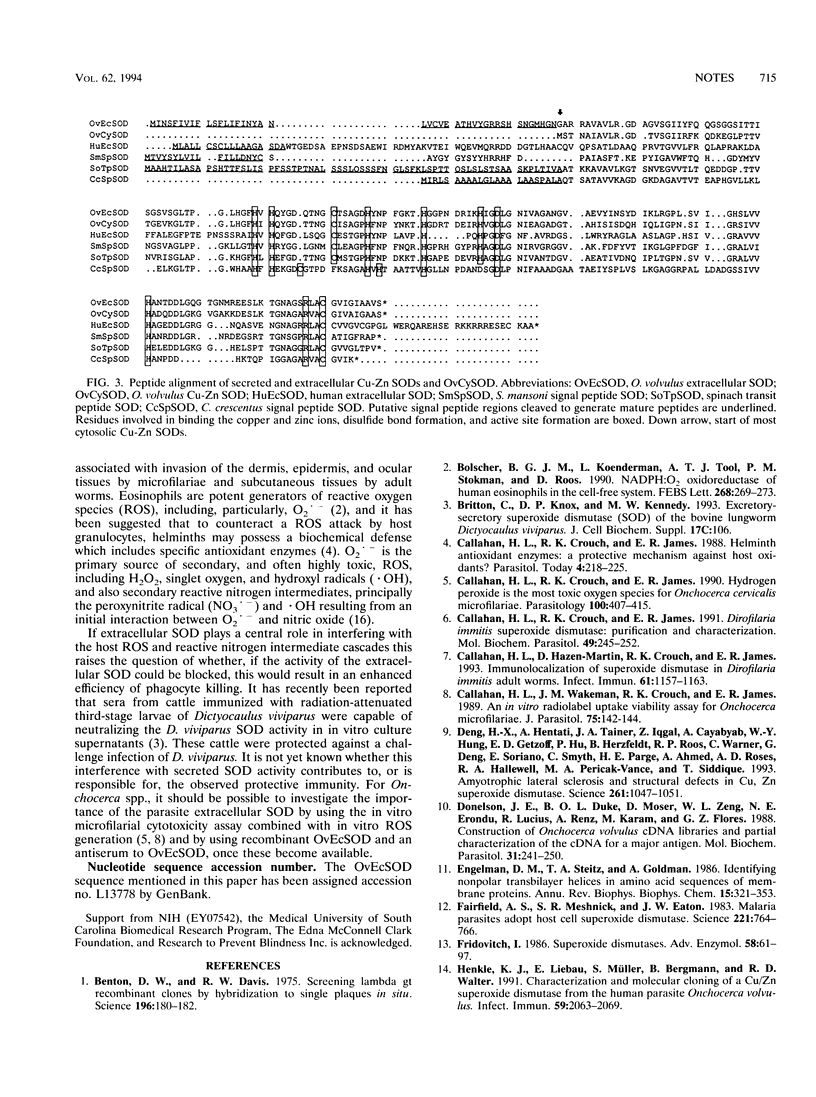
Onchocerca volvulus, a human parasitic nematode, is the third leading cause of preventable blindness worldwide. This study describes the molecular cloning of a novel superoxide dismutase (SOD) from the parasite. This putative O. volvulus extracellular SOD (OvEcSOD) is 628 nucleotides (nt) long, including a 22-nt 5' spliced leader (SL1) and a portion encoding an N-terminal hydrophobic 42-amino-acid signal peptide. The remainder of the cDNA shares 71% identity with an O. volvulus cytosolic SOD sequence and is 3 nt longer. All residues involved in metal ion binding, active site formation, folding, and dimer formation in SODs are conserved. Data indicate the OvEcSOD and O. volvulus cytosolic SOD are separate gene products and that the OvEcSOD appears to possess the characteristics of a membrane-bound or secreted enzyme which may be involved in the parasite defense against phagocyte-generated reactive oxygen species.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Bolscher B. G., Koenderman L., Tool A. T., Stokman P. M., Roos D. NADPH:O2 oxidoreductase of human eosinophils in the cell-free system. FEBS Lett. 1990 Jul 30;268(1):269–273. doi: 10.1016/0014-5793(90)81025-j. [DOI] [PubMed] [Google Scholar]
- Callahan H. L., Crouch R. K., James E. R. Dirofilaria immitis superoxide dismutase: purification and characterization. Mol Biochem Parasitol. 1991 Dec;49(2):245–251. doi: 10.1016/0166-6851(91)90068-h. [DOI] [PubMed] [Google Scholar]
- Callahan H. L., Crouch R. K., James E. R. Helminth anti-oxidant enzymes: a protective mechanism against host oxidants? Parasitol Today. 1988 Aug;4(8):218–225. doi: 10.1016/0169-4758(88)90162-7. [DOI] [PubMed] [Google Scholar]
- Callahan H. L., Crouch R. K., James E. R. Hydrogen peroxide is the most toxic oxygen species for Onchocerca cervicalis microfilariae. Parasitology. 1990 Jun;100(Pt 3):407–415. doi: 10.1017/s0031182000078690. [DOI] [PubMed] [Google Scholar]
- Callahan H. L., Hazen-Martin D., Crouch R. K., James E. R. Immunolocalization of superoxide dismutase in Dirofilaria immitis adult worms. Infect Immun. 1993 Mar;61(3):1157–1163. doi: 10.1128/iai.61.3.1157-1163.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan H. L., Wakeman J. M., Crouch R. K., James E. R. An in vitro radiolabel uptake viability assay for Onchocerca microfilariae. J Parasitol. 1989 Feb;75(1):142–144. [PubMed] [Google Scholar]
- Deng H. X., Hentati A., Tainer J. A., Iqbal Z., Cayabyab A., Hung W. Y., Getzoff E. D., Hu P., Herzfeldt B., Roos R. P. Amyotrophic lateral sclerosis and structural defects in Cu,Zn superoxide dismutase. Science. 1993 Aug 20;261(5124):1047–1051. doi: 10.1126/science.8351519. [DOI] [PubMed] [Google Scholar]
- Donelson J. E., Duke B. O., Moser D., Zeng W. L., Erondu N. E., Lucius R., Renz A., Karam M., Flores G. Z. Construction of Onchocerca volvulus cDNA libraries and partial characterization of the cDNA for a major antigen. Mol Biochem Parasitol. 1988 Dec;31(3):241–250. doi: 10.1016/0166-6851(88)90154-5. [DOI] [PubMed] [Google Scholar]
- Engelman D. M., Steitz T. A., Goldman A. Identifying nonpolar transbilayer helices in amino acid sequences of membrane proteins. Annu Rev Biophys Biophys Chem. 1986;15:321–353. doi: 10.1146/annurev.bb.15.060186.001541. [DOI] [PubMed] [Google Scholar]
- Fairfield A. S., Meshnick S. R., Eaton J. W. Malaria parasites adopt host cell superoxide dismutase. Science. 1983 Aug 19;221(4612):764–766. doi: 10.1126/science.6348944. [DOI] [PubMed] [Google Scholar]
- Fridovich I. Superoxide dismutases. Adv Enzymol Relat Areas Mol Biol. 1986;58:61–97. doi: 10.1002/9780470123041.ch2. [DOI] [PubMed] [Google Scholar]
- Henkle K. J., Liebau E., Müller S., Bergmann B., Walter R. D. Characterization and molecular cloning of a Cu/Zn superoxide dismutase from the human parasite Onchocerca volvulus. Infect Immun. 1991 Jun;59(6):2063–2069. doi: 10.1128/iai.59.6.2063-2069.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjalmarsson K., Marklund S. L., Engström A., Edlund T. Isolation and sequence of complementary DNA encoding human extracellular superoxide dismutase. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6340–6344. doi: 10.1073/pnas.84.18.6340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogg N., Darley-Usmar V. M., Wilson M. T., Moncada S. Production of hydroxyl radicals from the simultaneous generation of superoxide and nitric oxide. Biochem J. 1992 Jan 15;281(Pt 2):419–424. doi: 10.1042/bj2810419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong Z., LoVerde P. T., Thakur A., Hammarskjöld M. L., Rekosh D. Schistosoma mansoni: a Cu/Zn superoxide dismutase is glycosylated when expressed in mammalian cells and localizes to a subtegumental region in adult schistosomes. Exp Parasitol. 1993 Mar;76(2):101–114. doi: 10.1006/expr.1993.1012. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Leid R. W., Suquet C. M. A superoxide dismutase of metacestodes of Taenia taeniaeformis. Mol Biochem Parasitol. 1986 Mar;18(3):301–311. doi: 10.1016/0166-6851(86)90087-3. [DOI] [PubMed] [Google Scholar]
- Rhoads M. L. Trichinella spiralis: identification and purification of superoxide dismutase. Exp Parasitol. 1983 Aug;56(1):41–54. doi: 10.1016/0014-4894(83)90095-4. [DOI] [PubMed] [Google Scholar]
- Smith M. W., Doolittle R. F. A comparison of evolutionary rates of the two major kinds of superoxide dismutase. J Mol Evol. 1992 Feb;34(2):175–184. doi: 10.1007/BF00182394. [DOI] [PubMed] [Google Scholar]
- Steinman H. M., Ely B. Copper-zinc superoxide dismutase of Caulobacter crescentus: cloning, sequencing, and mapping of the gene and periplasmic location of the enzyme. J Bacteriol. 1990 Jun;172(6):2901–2910. doi: 10.1128/jb.172.6.2901-2910.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]