Abstract
Atherosclerotic arteries are well known to exhibit impaired endothelium-dependent relaxation (EDR), but the exact mechanism of this impairment remains unclear. Recently, endothelial constitutive nitric oxide synthase (ECNOS), which generates nitric oxide and mediates EDR, was cloned, and ECNOS mRNA expression was reported to be modified by various cytokines, lipoproteins, and shear stress. To investigate the expression of ECNOS mRNA and protein in atherosclerotic arteries with impaired EDR, thoracic aortas isolated from Watanabe heritable hyperlipidemic (WHHL) rabbits were examined by using in situ hybridization and immunohistochemistry. Compared with thoracic aortas from Japanese White rabbits, WHHL aortas exhibited significantly impaired EDRs, although both in situ hybridization and immunohistochemistry exhibited enhanced expression of ECNOS mRNA and protein in WHHL aortas. There was no significant relationship between expression of ECNOS mRNA and protein of endothelial cells and age of the examined WHHL aortas. These data suggest that the mechanism of impaired EDR in atherosclerotic arteries is not due to the decrease in ECNOS mRNA and protein.
Full text
PDF


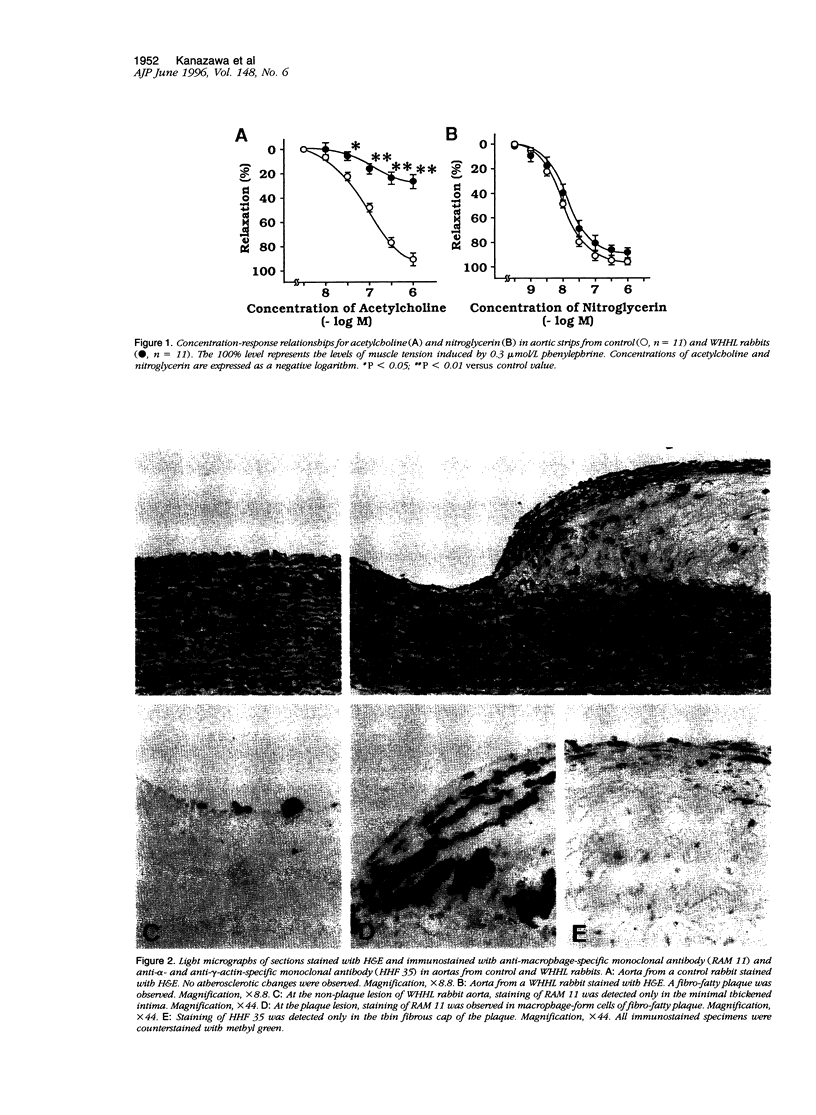

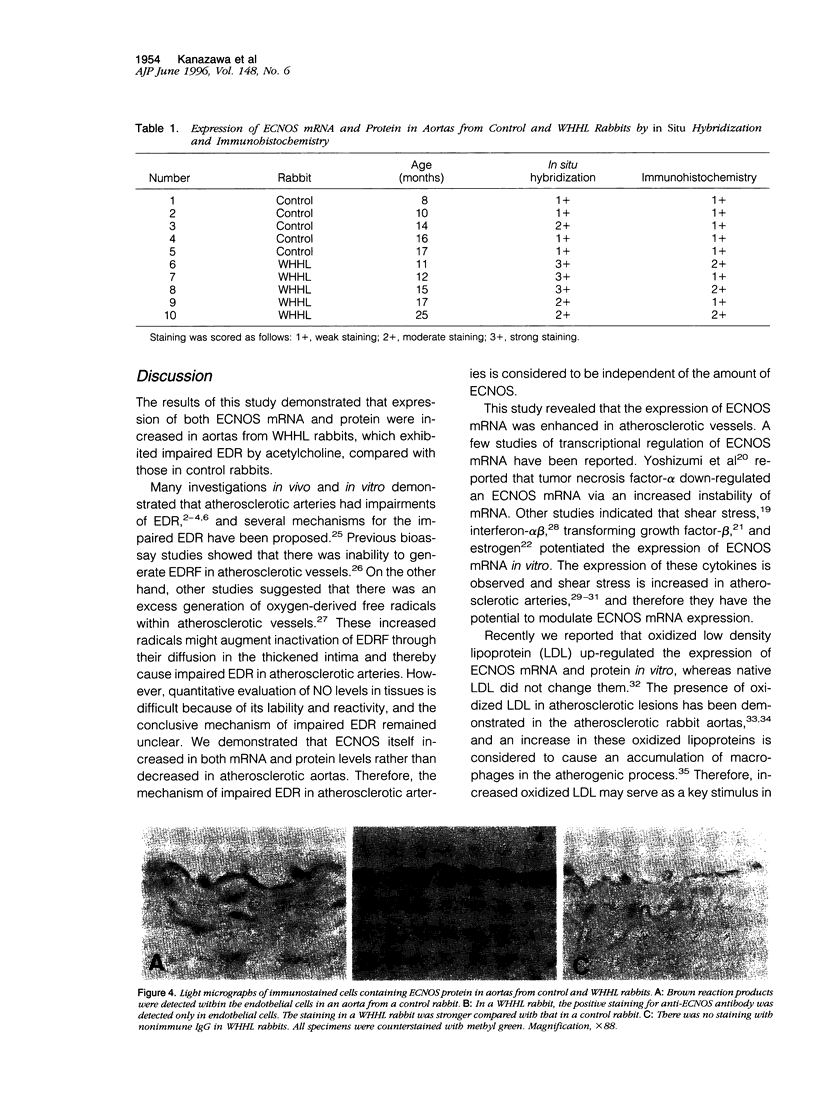


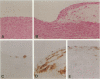
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barath P., Fishbein M. C., Cao J., Berenson J., Helfant R. H., Forrester J. S. Tumor necrosis factor gene expression in human vascular intimal smooth muscle cells detected by in situ hybridization. Am J Pathol. 1990 Sep;137(3):503–509. [PMC free article] [PubMed] [Google Scholar]
- Boyd H. C., Gown A. M., Wolfbauer G., Chait A. Direct evidence for a protein recognized by a monoclonal antibody against oxidatively modified LDL in atherosclerotic lesions from a Watanabe heritable hyperlipidemic rabbit. Am J Pathol. 1989 Nov;135(5):815–825. [PMC free article] [PubMed] [Google Scholar]
- Bredt D. S., Hwang P. M., Glatt C. E., Lowenstein C., Reed R. R., Snyder S. H. Cloned and expressed nitric oxide synthase structurally resembles cytochrome P-450 reductase. Nature. 1991 Jun 27;351(6329):714–718. doi: 10.1038/351714a0. [DOI] [PubMed] [Google Scholar]
- Bredt D. S., Snyder S. H. Isolation of nitric oxide synthetase, a calmodulin-requiring enzyme. Proc Natl Acad Sci U S A. 1990 Jan;87(2):682–685. doi: 10.1073/pnas.87.2.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fort P., Marty L., Piechaczyk M., el Sabrouty S., Dani C., Jeanteur P., Blanchard J. M. Various rat adult tissues express only one major mRNA species from the glyceraldehyde-3-phosphate-dehydrogenase multigenic family. Nucleic Acids Res. 1985 Mar 11;13(5):1431–1442. doi: 10.1093/nar/13.5.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freiman P. C., Mitchell G. G., Heistad D. D., Armstrong M. L., Harrison D. G. Atherosclerosis impairs endothelium-dependent vascular relaxation to acetylcholine and thrombin in primates. Circ Res. 1986 Jun;58(6):783–789. doi: 10.1161/01.res.58.6.783. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Förstermann U., Mügge A., Alheid U., Haverich A., Frölich J. C. Selective attenuation of endothelium-mediated vasodilation in atherosclerotic human coronary arteries. Circ Res. 1988 Feb;62(2):185–190. doi: 10.1161/01.res.62.2.185. [DOI] [PubMed] [Google Scholar]
- Girerd X. J., Hirsch A. T., Cooke J. P., Dzau V. J., Creager M. A. L-arginine augments endothelium-dependent vasodilation in cholesterol-fed rabbits. Circ Res. 1990 Dec;67(6):1301–1308. doi: 10.1161/01.res.67.6.1301. [DOI] [PubMed] [Google Scholar]
- Guerra R., Jr, Brotherton A. F., Goodwin P. J., Clark C. R., Armstrong M. L., Harrison D. G. Mechanisms of abnormal endothelium-dependent vascular relaxation in atherosclerosis: implications for altered autocrine and paracrine functions of EDRF. Blood Vessels. 1989;26(5):300–314. doi: 10.1159/000158779. [DOI] [PubMed] [Google Scholar]
- Haberland M. E., Fong D., Cheng L. Malondialdehyde-altered protein occurs in atheroma of Watanabe heritable hyperlipidemic rabbits. Science. 1988 Jul 8;241(4862):215–218. doi: 10.1126/science.2455346. [DOI] [PubMed] [Google Scholar]
- Hirata K., Akita H., Yokoyama M., Watanabe Y. Impaired vasodilatory response to atrial natriuretic peptide during atherosclerosis progression. Arterioscler Thromb. 1992 Jan;12(1):99–105. doi: 10.1161/01.atv.12.1.99. [DOI] [PubMed] [Google Scholar]
- Hirata K., Miki N., Kuroda Y., Sakoda T., Kawashima S., Yokoyama M. Low concentration of oxidized low-density lipoprotein and lysophosphatidylcholine upregulate constitutive nitric oxide synthase mRNA expression in bovine aortic endothelial cells. Circ Res. 1995 Jun;76(6):958–962. doi: 10.1161/01.res.76.6.958. [DOI] [PubMed] [Google Scholar]
- Inoue N., Hirata K., Yamada M., Hamamori Y., Matsuda Y., Akita H., Yokoyama M. Lysophosphatidylcholine inhibits bradykinin-induced phosphoinositide hydrolysis and calcium transients in cultured bovine aortic endothelial cells. Circ Res. 1992 Dec;71(6):1410–1421. doi: 10.1161/01.res.71.6.1410. [DOI] [PubMed] [Google Scholar]
- Inoue N., Venema R. C., Sayegh H. S., Ohara Y., Murphy T. J., Harrison D. G. Molecular regulation of the bovine endothelial cell nitric oxide synthase by transforming growth factor-beta 1. Arterioscler Thromb Vasc Biol. 1995 Aug;15(8):1255–1261. doi: 10.1161/01.atv.15.8.1255. [DOI] [PubMed] [Google Scholar]
- Jayakody L., Senaratne M., Thomson A., Kappagoda T. Endothelium-dependent relaxation in experimental atherosclerosis in the rabbit. Circ Res. 1987 Feb;60(2):251–264. doi: 10.1161/01.res.60.2.251. [DOI] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Moyer C. F., Sajuthi D., Tulli H., Williams J. K. Synthesis of IL-1 alpha and IL-1 beta by arterial cells in atherosclerosis. Am J Pathol. 1991 Apr;138(4):951–960. [PMC free article] [PubMed] [Google Scholar]
- Myers P. R., Minor R. L., Jr, Guerra R., Jr, Bates J. N., Harrison D. G. Vasorelaxant properties of the endothelium-derived relaxing factor more closely resemble S-nitrosocysteine than nitric oxide. Nature. 1990 May 10;345(6271):161–163. doi: 10.1038/345161a0. [DOI] [PubMed] [Google Scholar]
- Mügge A., Elwell J. H., Peterson T. E., Hofmeyer T. G., Heistad D. D., Harrison D. G. Chronic treatment with polyethylene-glycolated superoxide dismutase partially restores endothelium-dependent vascular relaxations in cholesterol-fed rabbits. Circ Res. 1991 Nov;69(5):1293–1300. doi: 10.1161/01.res.69.5.1293. [DOI] [PubMed] [Google Scholar]
- Nishida K., Harrison D. G., Navas J. P., Fisher A. A., Dockery S. P., Uematsu M., Nerem R. M., Alexander R. W., Murphy T. J. Molecular cloning and characterization of the constitutive bovine aortic endothelial cell nitric oxide synthase. J Clin Invest. 1992 Nov;90(5):2092–2096. doi: 10.1172/JCI116092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunokawa Y., Ishida N., Tanaka S. Cloning of inducible nitric oxide synthase in rat vascular smooth muscle cells. Biochem Biophys Res Commun. 1993 Feb 26;191(1):89–94. doi: 10.1006/bbrc.1993.1188. [DOI] [PubMed] [Google Scholar]
- Ohara Y., Peterson T. E., Harrison D. G. Hypercholesterolemia increases endothelial superoxide anion production. J Clin Invest. 1993 Jun;91(6):2546–2551. doi: 10.1172/JCI116491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Pollock J. S., Förstermann U., Mitchell J. A., Warner T. D., Schmidt H. H., Nakane M., Murad F. Purification and characterization of particulate endothelium-derived relaxing factor synthase from cultured and native bovine aortic endothelial cells. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10480–10484. doi: 10.1073/pnas.88.23.10480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock J. S., Nakane M., Buttery L. D., Martinez A., Springall D., Polak J. M., Förstermann U., Murad F. Characterization and localization of endothelial nitric oxide synthase using specific monoclonal antibodies. Am J Physiol. 1993 Nov;265(5 Pt 1):C1379–C1387. doi: 10.1152/ajpcell.1993.265.5.C1379. [DOI] [PubMed] [Google Scholar]
- Ross R. Rous-Whipple Award Lecture. Atherosclerosis: a defense mechanism gone awry. Am J Pathol. 1993 Oct;143(4):987–1002. [PMC free article] [PubMed] [Google Scholar]
- Sreeharan N., Jayakody R. L., Senaratne M. P., Thomson A. B., Kappagoda C. T. Endothelium-dependent relaxation and experimental atherosclerosis in the rabbit aorta. Can J Physiol Pharmacol. 1986 Nov;64(11):1451–1453. doi: 10.1139/y86-246. [DOI] [PubMed] [Google Scholar]
- Verbeuren T. J., Jordaens F. H., Zonnekeyn L. L., Van Hove C. E., Coene M. C., Herman A. G. Effect of hypercholesterolemia on vascular reactivity in the rabbit. I. Endothelium-dependent and endothelium-independent contractions and relaxations in isolated arteries of control and hypercholesterolemic rabbits. Circ Res. 1986 Apr;58(4):552–564. doi: 10.1161/01.res.58.4.552. [DOI] [PubMed] [Google Scholar]
- Weiner C. P., Lizasoain I., Baylis S. A., Knowles R. G., Charles I. G., Moncada S. Induction of calcium-dependent nitric oxide synthases by sex hormones. Proc Natl Acad Sci U S A. 1994 May 24;91(11):5212–5216. doi: 10.1073/pnas.91.11.5212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Q. W., Cho H. J., Calaycay J., Mumford R. A., Swiderek K. M., Lee T. D., Ding A., Troso T., Nathan C. Cloning and characterization of inducible nitric oxide synthase from mouse macrophages. Science. 1992 Apr 10;256(5054):225–228. doi: 10.1126/science.1373522. [DOI] [PubMed] [Google Scholar]
- Yokota T., Shimokado K., Kosaka C., Sasaguri T., Masuda J., Ogata J. Mitogenic activity of interferon gamma on growth-arrested human vascular smooth muscle cells. Arterioscler Thromb. 1992 Dec;12(12):1393–1401. doi: 10.1161/01.atv.12.12.1393. [DOI] [PubMed] [Google Scholar]
- Yoshizumi M., Perrella M. A., Burnett J. C., Jr, Lee M. E. Tumor necrosis factor downregulates an endothelial nitric oxide synthase mRNA by shortening its half-life. Circ Res. 1993 Jul;73(1):205–209. doi: 10.1161/01.res.73.1.205. [DOI] [PubMed] [Google Scholar]
- Yui Y., Hattori R., Kosuga K., Eizawa H., Hiki K., Kawai C. Purification of nitric oxide synthase from rat macrophages. J Biol Chem. 1991 Jul 5;266(19):12544–12547. [PubMed] [Google Scholar]