Abstract
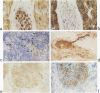
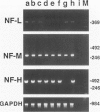
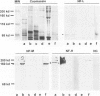
Autoantibodies against both striated muscle proteins, particularly titin, and the acetylcholine receptor are a hallmark of thymoma-associated myasthenia gravis. However, the stimulus for these responses remains enigmatic as whole titin is not detectable in these tumors. This study reports that in thymomas with cortical differentiation many of the neoplastic epithelial cells expressed low and medium molecular weight neurofilaments detected with several antibodies (on selections and blots) and at the RNA level (by reverse transcriptase polymerase chain reaction). Moreover, higher molecular weight forms sharing at least one epitope with titin were detectable slightly less frequently, as were the more strongly phosphorylated epitopes. In stark contrast, in medullary and mixed thymomas, and especially in the normal thymus, immunoreactivity with anti-neurofilament antibodies was rare. This aberrant overexpression of a titin epitope by epithelial cells with antigen-presenting phenotype in an inappropriate cortical microenvironment suggests that they might autosensitize maturing T cells there and so initiate anti-titin autoimmunity in these patients.
Full text
PDF

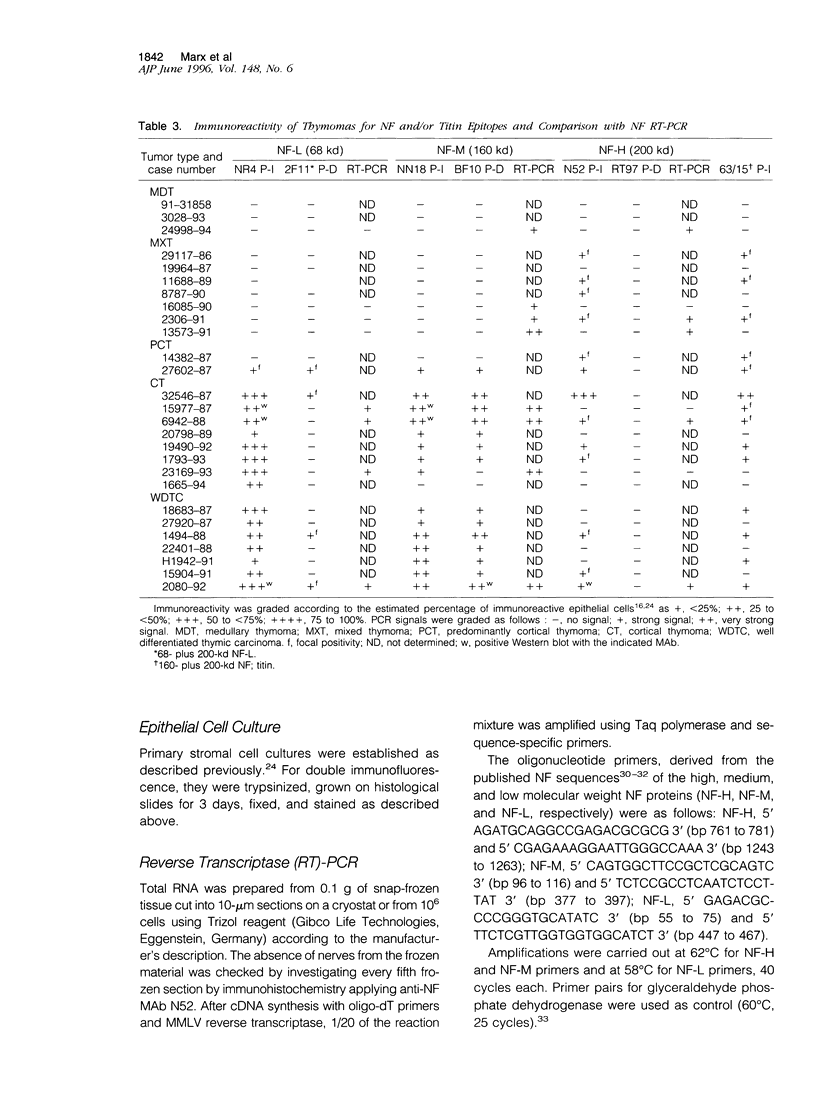
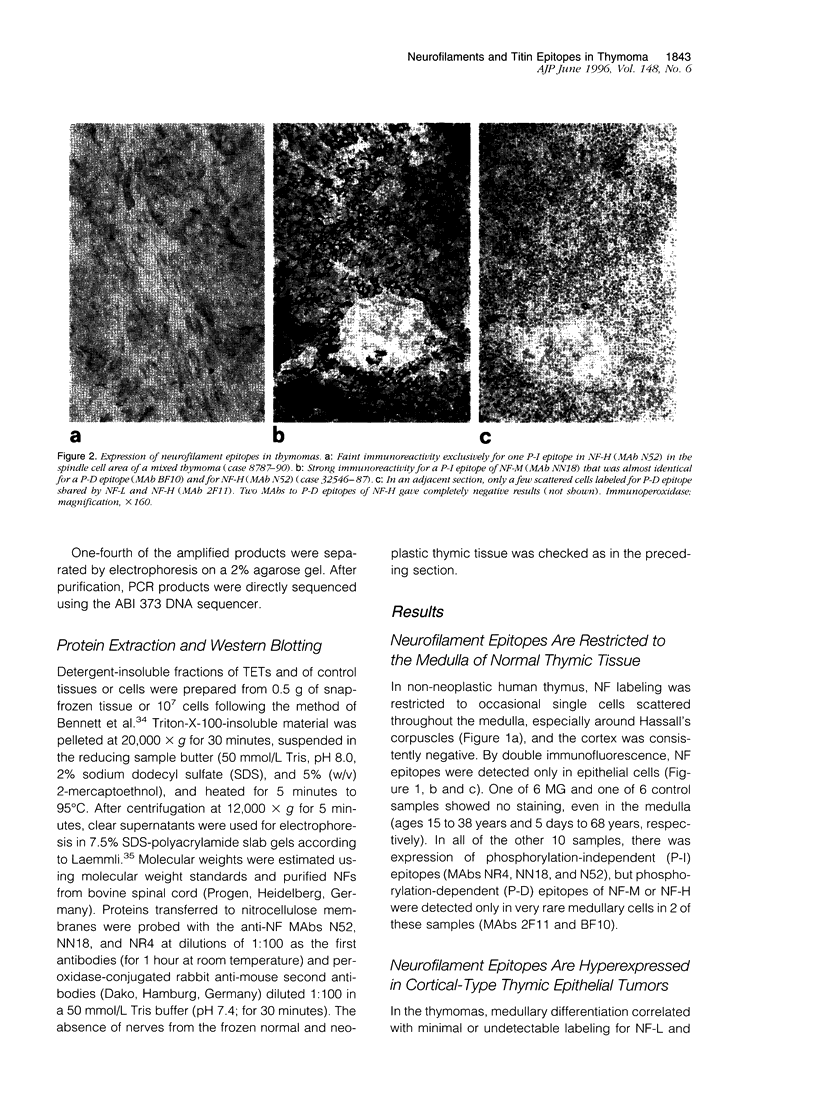
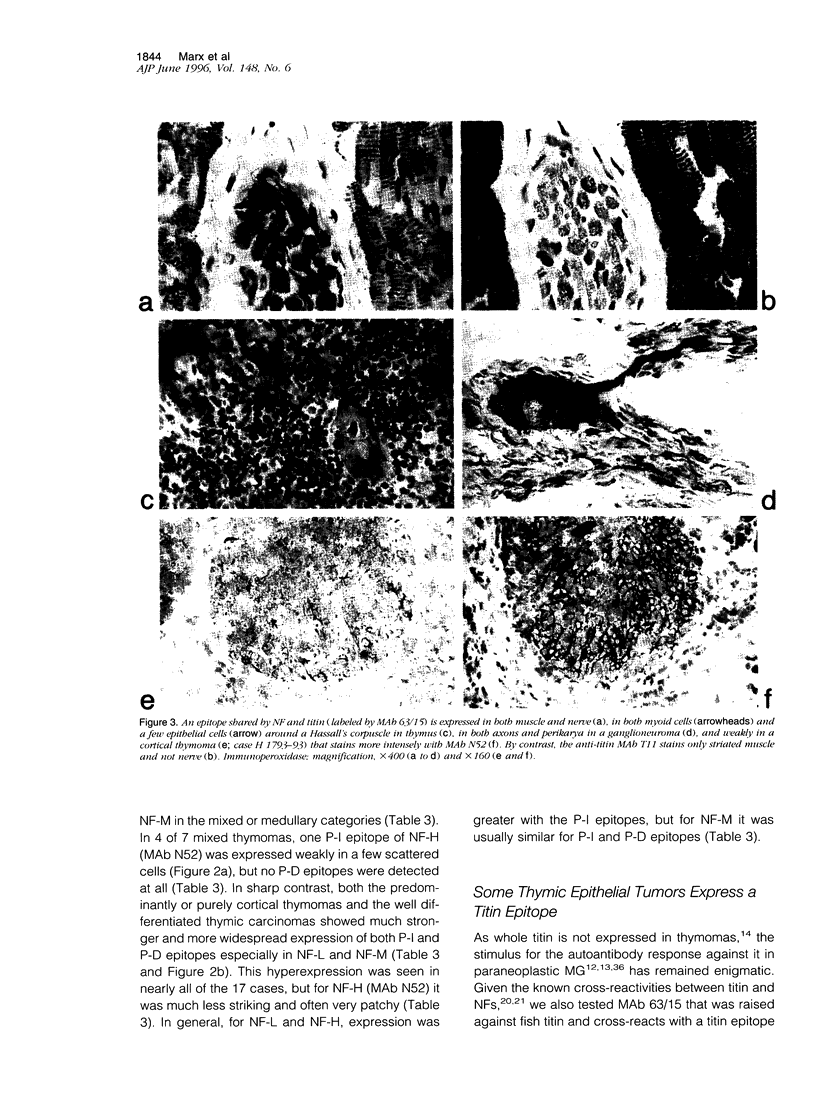

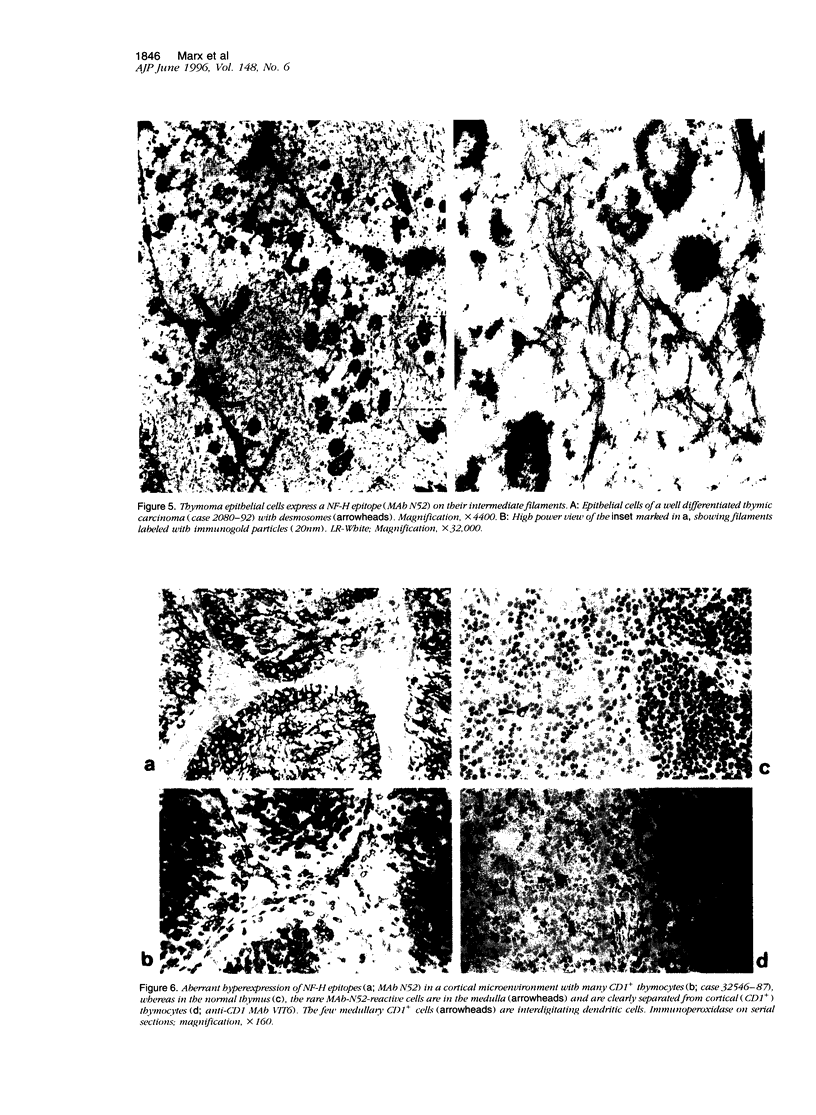



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aarli J. A., Stefansson K., Marton L. S., Wollmann R. L. Patients with myasthenia gravis and thymoma have in their sera IgG autoantibodies against titin. Clin Exp Immunol. 1990 Nov;82(2):284–288. doi: 10.1111/j.1365-2249.1990.tb05440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderton B. H., Breinburg D., Downes M. J., Green P. J., Tomlinson B. E., Ulrich J., Wood J. N., Kahn J. Monoclonal antibodies show that neurofibrillary tangles and neurofilaments share antigenic determinants. Nature. 1982 Jul 1;298(5869):84–86. doi: 10.1038/298084a0. [DOI] [PubMed] [Google Scholar]
- Bennett G. S., Hollander B. A., Laskowska D. Expression and phosphorylation of the mid-sized neurofilament protein NF-M during chick spinal cord neurogenesis. J Neurosci Res. 1988 Oct-Dec;21(2-4):376–390. doi: 10.1002/jnr.490210229. [DOI] [PubMed] [Google Scholar]
- Carden M. J., Schlaepfer W. W., Lee V. M. The structure, biochemical properties, and immunogenicity of neurofilament peripheral regions are determined by phosphorylation state. J Biol Chem. 1985 Aug 15;260(17):9805–9817. [PubMed] [Google Scholar]
- Chin S. S., Liem R. K. Expression of rat neurofilament proteins NF-L and NF-M in transfected non-neuronal cells. Eur J Cell Biol. 1989 Dec;50(2):475–490. [PubMed] [Google Scholar]
- Chin S. S., Liem R. K. Transfected rat high-molecular-weight neurofilament (NF-H) coassembles with vimentin in a predominantly nonphosphorylated form. J Neurosci. 1990 Nov;10(11):3714–3726. doi: 10.1523/JNEUROSCI.10-11-03714.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cikes N., Momoi M. Y., Williams C. L., Howard F. M., Jr, Hoagland H. C., Whittingham S., Lennon V. A. Striational autoantibodies: quantitative detection by enzyme immunoassay in myasthenia gravis, thymoma, and recipients of D-penicillamine or allogeneic bone marrow. Mayo Clin Proc. 1988 May;63(5):474–481. doi: 10.1016/s0025-6196(12)65645-6. [DOI] [PubMed] [Google Scholar]
- Dardenne M., Savino W., Bach J. F. Thymomatous epithelial cells and skeletal muscle share a common epitope defined by a monoclonal antibody. Am J Pathol. 1987 Jan;126(1):194–198. [PMC free article] [PubMed] [Google Scholar]
- Franke F. E., Schachenmayr W., Osborn M., Altmannsberger M. Unexpected immunoreactivities of intermediate filament antibodies in human brain and brain tumors. Am J Pathol. 1991 Jul;139(1):67–79. [PMC free article] [PubMed] [Google Scholar]
- Fujii Y., Monden Y., Nakahara K., Hashimoto J., Kawashima Y. Antibody to acetylcholine receptor in myasthenia gravis: production by lymphocytes from thymus or thymoma. Neurology. 1984 Sep;34(9):1182–1186. doi: 10.1212/wnl.34.9.1182. [DOI] [PubMed] [Google Scholar]
- Fürst D. O., Osborn M., Nave R., Weber K. The organization of titin filaments in the half-sarcomere revealed by monoclonal antibodies in immunoelectron microscopy: a map of ten nonrepetitive epitopes starting at the Z line extends close to the M line. J Cell Biol. 1988 May;106(5):1563–1572. doi: 10.1083/jcb.106.5.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautel M., Lakey A., Barlow D. P., Holmes Z., Scales S., Leonard K., Labeit S., Mygland A., Gilhus N. E., Aarli J. A. Titin antibodies in myasthenia gravis: identification of a major immunogenic region of titin. Neurology. 1993 Aug;43(8):1581–1585. doi: 10.1212/wnl.43.8.1581. [DOI] [PubMed] [Google Scholar]
- Geuder K. I., Marx A., Witzemann V., Schalke B., Kirchner T., Müller-Hermelink H. K. Genomic organization and lack of transcription of the nicotinic acetylcholine receptor subunit genes in myasthenia gravis-associated thymoma. Lab Invest. 1992 Apr;66(4):452–458. [PubMed] [Google Scholar]
- Gilhus N. E., Aarli J. A., Christensson B., Matre R. Rabbit antiserum to a citric acid extract of human skeletal muscle staining thymomas from myasthenia gravis patients. J Neuroimmunol. 1984 Nov;7(1):55–64. doi: 10.1016/s0165-5728(84)80006-5. [DOI] [PubMed] [Google Scholar]
- Gilhus N. E., Willcox N., Harcourt G., Nagvekar N., Beeson D., Vincent A., Newsom-Davis J. Antigen presentation by thymoma epithelial cells from myasthenia gravis patients to potentially pathogenic T cells. J Neuroimmunol. 1995 Jan;56(1):65–76. doi: 10.1016/0165-5728(94)00134-a. [DOI] [PubMed] [Google Scholar]
- Hohlfeld R. Myasthenia gravis and thymoma: paraneoplastic failure of neuromuscular transmission. Lab Invest. 1990 Mar;62(3):241–243. [PubMed] [Google Scholar]
- Jones S. M., Williams R. C., Jr Phosphate content of mammalian neurofilaments. J Biol Chem. 1982 Sep 10;257(17):9902–9905. [PubMed] [Google Scholar]
- Julien J. P., Grosveld F., Yazdanbaksh K., Flavell D., Meijer D., Mushynski W. The structure of a human neurofilament gene (NF-L): a unique exon-intron organization in the intermediate filament gene family. Biochim Biophys Acta. 1987 Jun 6;909(1):10–20. doi: 10.1016/0167-4781(87)90041-8. [DOI] [PubMed] [Google Scholar]
- Julien J. P., Mushynski W. E. Multiple phosphorylation sites in mammalian neurofilament polypeptides. J Biol Chem. 1982 Sep 10;257(17):10467–10470. [PubMed] [Google Scholar]
- Kirchner T., Schalke B., Buchwald J., Ritter M., Marx A., Müller-Hermelink H. K. Well-differentiated thymic carcinoma. An organotypical low-grade carcinoma with relationship to cortical thymoma. Am J Surg Pathol. 1992 Dec;16(12):1153–1169. [PubMed] [Google Scholar]
- Kirchner T., Tzartos S., Hoppe F., Schalke B., Wekerle H., Müller-Hermelink H. K. Pathogenesis of myasthenia gravis. Acetylcholine receptor-related antigenic determinants in tumor-free thymuses and thymic epithelial tumors. Am J Pathol. 1988 Feb;130(2):268–280. [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lees J. F., Shneidman P. S., Skuntz S. F., Carden M. J., Lazzarini R. A. The structure and organization of the human heavy neurofilament subunit (NF-H) and the gene encoding it. EMBO J. 1988 Jul;7(7):1947–1955. doi: 10.1002/j.1460-2075.1988.tb03032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx A., Geuder K. I., Altmannsberger M., Schömig E., Kirchner T., Müller-Hermelink H. K. Die Bedeutung neuronaler Acetylcholinrezeptoren als Differenzierungsantigene in Neuroblastomen und Paragangliomen. Verh Dtsch Ges Pathol. 1990;74:354–358. [PubMed] [Google Scholar]
- Marx A., Kirchner T., Greiner A., Müller-Hermelink H. K., Schalke B., Osborn M. Neurofilament epitopes in thymoma and antiaxonal autoantibodies in myasthenia gravis. Lancet. 1992 Mar 21;339(8795):707–708. doi: 10.1016/0140-6736(92)90601-x. [DOI] [PubMed] [Google Scholar]
- Marx A., Kirchner T., Hoppe F., O'Connor R., Schalke B., Tzartos S., Müller-Hermelink H. K. Proteins with epitopes of the acetylcholine receptor in epithelial cell cultures of thymomas in myasthenia gravis. Am J Pathol. 1989 Apr;134(4):865–877. [PMC free article] [PubMed] [Google Scholar]
- Marx A., O'Connor R., Geuder K. I., Hoppe F., Schalke B., Tzartos S., Kalies I., Kirchner T., Müller-Hermelink H. K. Characterization of a protein with an acetylcholine receptor epitope from myasthenia gravis-associated thymomas. Lab Invest. 1990 Mar;62(3):279–286. [PubMed] [Google Scholar]
- Marx A., Osborn M., Tzartos S., Geuder K. I., Schalke B., Nix W., Kirchner T., Müller-Hermelink H. K. A striational muscle antigen and myasthenia gravis-associated thymomas share an acetylcholine-receptor epitope. Dev Immunol. 1992;2(2):77–84. doi: 10.1155/1992/86853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masaoka A., Monden Y., Nakahara K., Tanioka T. Follow-up study of thymomas with special reference to their clinical stages. Cancer. 1981 Dec 1;48(11):2485–2492. doi: 10.1002/1097-0142(19811201)48:11<2485::aid-cncr2820481123>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Mencarelli C., Magi B., Marzocchi B., Armellini D., Pallini V. Evolution of the "titin epitope" in neurofilament proteins. Comp Biochem Physiol B. 1991;100(4):741–744. doi: 10.1016/0305-0491(91)90283-j. [DOI] [PubMed] [Google Scholar]
- Miettinen M., Clark R., Lehto V. P., Virtanen I., Damjanov I. Intermediate-filament proteins in parathyroid glands and parathyroid adenomas. Arch Pathol Lab Med. 1985 Nov;109(11):986–989. [PubMed] [Google Scholar]
- Myers M. W., Lazzarini R. A., Lee V. M., Schlaepfer W. W., Nelson D. L. The human mid-size neurofilament subunit: a repeated protein sequence and the relationship of its gene to the intermediate filament gene family. EMBO J. 1987 Jun;6(6):1617–1626. doi: 10.1002/j.1460-2075.1987.tb02409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller-Hermelink H. K., Marino M., Palestro G. Pathology of thymic epithelial tumors. Curr Top Pathol. 1986;75:207–268. doi: 10.1007/978-3-642-82480-7_7. [DOI] [PubMed] [Google Scholar]
- Müller-Hermelink H. K., Marx A., Geuder K., Kirchner T. The pathological basis of thymoma-associated myasthenia gravis. Ann N Y Acad Sci. 1993 Jun 21;681:56–65. doi: 10.1111/j.1749-6632.1993.tb22869.x. [DOI] [PubMed] [Google Scholar]
- NASTUK W. L., PLESCIA O. J., OSSERMAN K. E. Changes in serum complement activity in patients with myasthenia gravis. Proc Soc Exp Biol Med. 1960 Oct;105:177–184. doi: 10.3181/00379727-105-26050. [DOI] [PubMed] [Google Scholar]
- Oosterhuis H. J., Kuks J. B. Myasthenia gravis and myasthenic syndromes. Curr Opin Neurol Neurosurg. 1992 Oct;5(5):638–644. [PubMed] [Google Scholar]
- Osborn M., Marx A., Kirchner T., Tzartos S. J., Plessman U., Weber K. A shared epitope in the acetylcholine receptor-alpha subunit and fast troponin I of skeletal muscle. Is it important for myasthenia gravis? Am J Pathol. 1992 May;140(5):1215–1223. [PMC free article] [PubMed] [Google Scholar]
- Pleasure S. J., Lee V. M., Nelson D. L. Site-specific phosphorylation of the middle molecular weight human neurofilament protein in transfected non-neuronal cells. J Neurosci. 1990 Jul;10(7):2428–2437. doi: 10.1523/JNEUROSCI.10-07-02428.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw G., Osborn M., Weber K. Reactivity of a panel of neurofilament antibodies on phosphorylated and dephosphorylated neurofilaments. Eur J Cell Biol. 1986 Oct;42(1):1–9. [PubMed] [Google Scholar]
- Siara J., Rüdel R., Marx A. Absence of acetylcholine-induced current in epithelial cells from thymus glands and thymomas of myasthenia gravis patients. Neurology. 1991 Jan;41(1):128–131. doi: 10.1212/wnl.41.1.128. [DOI] [PubMed] [Google Scholar]
- Soifer D., Nicoletti V., Cabane K., Mack K., Poulos B. Expression of the neurofilament protein NF-H in L cells. J Neurosci Res. 1991 Sep;30(1):63–71. doi: 10.1002/jnr.490300108. [DOI] [PubMed] [Google Scholar]
- Sommer N., Willcox N., Harcourt G. C., Newsom-Davis J. Myasthenic thymus and thymoma are selectively enriched in acetylcholine receptor-reactive T cells. Ann Neurol. 1990 Sep;28(3):312–319. doi: 10.1002/ana.410280303. [DOI] [PubMed] [Google Scholar]
- Vincent A., Whiting P. J., Schluep M., Heidenreich F., Lang B., Roberts A., Willcox N., Newsom-Davis J. Antibody heterogeneity and specificity in myasthenia gravis. Ann N Y Acad Sci. 1987;505:106–120. doi: 10.1111/j.1749-6632.1987.tb51286.x. [DOI] [PubMed] [Google Scholar]
- Willcox N. Myasthenia gravis. Curr Opin Immunol. 1993 Dec;5(6):910–917. doi: 10.1016/0952-7915(93)90105-2. [DOI] [PubMed] [Google Scholar]
- Williams C. L., Hay J. E., Huiatt T. W., Lennon V. A. Paraneoplastic IgG striational autoantibodies produced by clonal thymic B cells and in serum of patients with myasthenia gravis and thymoma react with titin. Lab Invest. 1992 Mar;66(3):331–336. [PubMed] [Google Scholar]
- Yamasaki H., Bennett G. S., Itakura C., Mizutani M. Defective expression of neurofilament protein subunits in hereditary hypotrophic axonopathy of quail. Lab Invest. 1992 Jun;66(6):734–743. [PubMed] [Google Scholar]
- van der Geld H. W., Strauss A. J. Myasthenia gravis. Immunological relationship between striated muscle and thymus. Lancet. 1966 Jan 8;1(7428):57–60. doi: 10.1016/s0140-6736(66)92356-7. [DOI] [PubMed] [Google Scholar]