Abstract
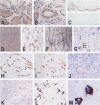
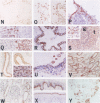
We investigated the distribution of the high-affinity neurotrophin receptors TrkA, TrkB, and TrkC in a wide range of normal non-neuronal tissues of adult human by immunohistochemical methods. Trk immunoreactivity (IR) was detected at various levels in all tissues examined, except for the heart and liver. The gastric parietal cells showed strong TrkA and TrkC IR and all of the Trks had IR for the putative intestinal neuroendocrine cells. In the pancreas, TrkA and TrkC IR was detected in the sub-intralobular ducts, whereas TrkB IR was found specifically in the alpha-islet cells. The lymph node and spleen exhibited TrkB IR in monocytes/macrophages. The adrenal cortex showed selective TrkA IR with TrkC IR in the medulla. In the reproductive system, TrkA IR was detected in the prostatic epithelial cells, TrkC in the ovarian theca and granulosa cells, TrkA and TrkC in the secretory-phase endometrium, and TrkA in the mammary ducts. The kidney showed strong TrkA and TrkC IR in it tubules, but no Trk receptors were present in the glomeruli. In the skin, TrkA and TrkB/TrkC were present in the basal and granular layers of the epidermis, respectively.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altar C. A., Criden M. R., Lindsay R. M., DiStefano P. S. Characterization and topography of high-affinity 125I-neurotrophin-3 binding to mammalian brain. J Neurosci. 1993 Feb;13(2):733–743. doi: 10.1523/JNEUROSCI.13-02-00733.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker D. L., Molenaar W. M., Trojanowski J. Q., Evans A. E., Ross A. H., Rorke L. B., Packer R. J., Lee V. M., Pleasure D. Nerve growth factor receptor expression in peripheral and central neuroectodermal tumors, other pediatric brain tumors, and during development of the adrenal gland. Am J Pathol. 1991 Jul;139(1):115–122. [PMC free article] [PubMed] [Google Scholar]
- Barbacid M. The Trk family of neurotrophin receptors. J Neurobiol. 1994 Nov;25(11):1386–1403. doi: 10.1002/neu.480251107. [DOI] [PubMed] [Google Scholar]
- Barker P. A., Lomen-Hoerth C., Gensch E. M., Meakin S. O., Glass D. J., Shooter E. M. Tissue-specific alternative splicing generates two isoforms of the trkA receptor. J Biol Chem. 1993 Jul 15;268(20):15150–15157. [PubMed] [Google Scholar]
- Berkemeier L. R., Winslow J. W., Kaplan D. R., Nikolics K., Goeddel D. V., Rosenthal A. Neurotrophin-5: a novel neurotrophic factor that activates trk and trkB. Neuron. 1991 Nov;7(5):857–866. doi: 10.1016/0896-6273(91)90287-a. [DOI] [PubMed] [Google Scholar]
- Chao M. V. Neurotrophin receptors: a window into neuronal differentiation. Neuron. 1992 Oct;9(4):583–593. doi: 10.1016/0896-6273(92)90023-7. [DOI] [PubMed] [Google Scholar]
- Cordon-Cardo C., Tapley P., Jing S. Q., Nanduri V., O'Rourke E., Lamballe F., Kovary K., Klein R., Jones K. R., Reichardt L. F. The trk tyrosine protein kinase mediates the mitogenic properties of nerve growth factor and neurotrophin-3. Cell. 1991 Jul 12;66(1):173–183. doi: 10.1016/0092-8674(91)90149-s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Marco E., Mathor M., Bondanza S., Cutuli N., Marchisio P. C., Cancedda R., De Luca M. Nerve growth factor binds to normal human keratinocytes through high and low affinity receptors and stimulates their growth by a novel autocrine loop. J Biol Chem. 1993 Oct 25;268(30):22838–22846. [PubMed] [Google Scholar]
- Djakiew D., Pflug B., Dionne C., Onoda M. Postnatal expression of nerve growth factor receptors in the rat testis. Biol Reprod. 1994 Aug;51(2):214–221. doi: 10.1095/biolreprod51.2.214. [DOI] [PubMed] [Google Scholar]
- Donovan M. J., Miranda R. C., Kraemer R., McCaffrey T. A., Tessarollo L., Mahadeo D., Sharif S., Kaplan D. R., Tsoulfas P., Parada L. Neurotrophin and neurotrophin receptors in vascular smooth muscle cells. Regulation of expression in response to injury. Am J Pathol. 1995 Aug;147(2):309–324. [PMC free article] [PubMed] [Google Scholar]
- Durbeej M., Söderström S., Ebendal T., Birchmeier C., Ekblom P. Differential expression of neurotrophin receptors during renal development. Development. 1993 Dec;119(4):977–989. doi: 10.1242/dev.119.4.977. [DOI] [PubMed] [Google Scholar]
- Eide F. F., Lowenstein D. H., Reichardt L. F. Neurotrophins and their receptors--current concepts and implications for neurologic disease. Exp Neurol. 1993 Jun;121(2):200–214. doi: 10.1006/exnr.1993.1087. [DOI] [PubMed] [Google Scholar]
- Hannestad J., Levanti M. B., Vega J. A. Distribution of neurotrophin receptors in human palatine tonsils: an immunohistochemical study. J Neuroimmunol. 1995 May;58(2):131–137. doi: 10.1016/0165-5728(95)00014-s. [DOI] [PubMed] [Google Scholar]
- Hempstead B. L., Martin-Zanca D., Kaplan D. R., Parada L. F., Chao M. V. High-affinity NGF binding requires coexpression of the trk proto-oncogene and the low-affinity NGF receptor. Nature. 1991 Apr 25;350(6320):678–683. doi: 10.1038/350678a0. [DOI] [PubMed] [Google Scholar]
- Hoehner J. C., Olsen L., Sandstedt B., Kaplan D. R., Påhlman S. Association of neurotrophin receptor expression and differentiation in human neuroblastoma. Am J Pathol. 1995 Jul;147(1):102–113. [PMC free article] [PubMed] [Google Scholar]
- Ip N. Y., Ibáez C. F., Nye S. H., McClain J., Jones P. F., Gies D. R., Belluscio L., Le Beau M. M., Espinosa R., 3rd, Squinto S. P. Mammalian neurotrophin-4: structure, chromosomal localization, tissue distribution, and receptor specificity. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):3060–3064. doi: 10.1073/pnas.89.7.3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanaka-Gantenbein C., Tazi A., Czernichow P., Scharfmann R. In vivo presence of the high affinity nerve growth factor receptor Trk-A in the rat pancreas: differential localization during pancreatic development. Endocrinology. 1995 Feb;136(2):761–769. doi: 10.1210/endo.136.2.7835308. [DOI] [PubMed] [Google Scholar]
- Kaplan D. R., Hempstead B. L., Martin-Zanca D., Chao M. V., Parada L. F. The trk proto-oncogene product: a signal transducing receptor for nerve growth factor. Science. 1991 Apr 26;252(5005):554–558. doi: 10.1126/science.1850549. [DOI] [PubMed] [Google Scholar]
- Kaplan D. R., Martin-Zanca D., Parada L. F. Tyrosine phosphorylation and tyrosine kinase activity of the trk proto-oncogene product induced by NGF. Nature. 1991 Mar 14;350(6314):158–160. doi: 10.1038/350158a0. [DOI] [PubMed] [Google Scholar]
- Kaplan D. R., Stephens R. M. Neurotrophin signal transduction by the Trk receptor. J Neurobiol. 1994 Nov;25(11):1404–1417. doi: 10.1002/neu.480251108. [DOI] [PubMed] [Google Scholar]
- Klein R., Conway D., Parada L. F., Barbacid M. The trkB tyrosine protein kinase gene codes for a second neurogenic receptor that lacks the catalytic kinase domain. Cell. 1990 May 18;61(4):647–656. doi: 10.1016/0092-8674(90)90476-u. [DOI] [PubMed] [Google Scholar]
- Klein R., Lamballe F., Bryant S., Barbacid M. The trkB tyrosine protein kinase is a receptor for neurotrophin-4. Neuron. 1992 May;8(5):947–956. doi: 10.1016/0896-6273(92)90209-v. [DOI] [PubMed] [Google Scholar]
- Klein R., Nanduri V., Jing S. A., Lamballe F., Tapley P., Bryant S., Cordon-Cardo C., Jones K. R., Reichardt L. F., Barbacid M. The trkB tyrosine protein kinase is a receptor for brain-derived neurotrophic factor and neurotrophin-3. Cell. 1991 Jul 26;66(2):395–403. doi: 10.1016/0092-8674(91)90628-c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamballe F., Klein R., Barbacid M. trkC, a new member of the trk family of tyrosine protein kinases, is a receptor for neurotrophin-3. Cell. 1991 Sep 6;66(5):967–979. doi: 10.1016/0092-8674(91)90442-2. [DOI] [PubMed] [Google Scholar]
- Laurenzi M. A., Barbany G., Timmusk T., Lindgren J. A., Persson H. Expression of mRNA encoding neurotrophins and neurotrophin receptors in rat thymus, spleen tissue and immunocompetent cells. Regulation of neurotrophin-4 mRNA expression by mitogens and leukotriene B4. Eur J Biochem. 1994 Aug 1;223(3):733–741. doi: 10.1111/j.1432-1033.1994.tb19047.x. [DOI] [PubMed] [Google Scholar]
- Lobach D. F., Haynes B. F. Ontogeny of the human thymus during fetal development. J Clin Immunol. 1987 Mar;7(2):81–97. doi: 10.1007/BF00916002. [DOI] [PubMed] [Google Scholar]
- Lomen-Hoerth C., Shooter E. M. Widespread neurotrophin receptor expression in the immune system and other nonneuronal rat tissues. J Neurochem. 1995 Apr;64(4):1780–1789. doi: 10.1046/j.1471-4159.1995.64041780.x. [DOI] [PubMed] [Google Scholar]
- Maness L. M., Kastin A. J., Weber J. T., Banks W. A., Beckman B. S., Zadina J. E. The neurotrophins and their receptors: structure, function, and neuropathology. Neurosci Biobehav Rev. 1994 Spring;18(1):143–159. doi: 10.1016/0149-7634(94)90043-4. [DOI] [PubMed] [Google Scholar]
- Martin-Zanca D., Barbacid M., Parada L. F. Expression of the trk proto-oncogene is restricted to the sensory cranial and spinal ganglia of neural crest origin in mouse development. Genes Dev. 1990 May;4(5):683–694. doi: 10.1101/gad.4.5.683. [DOI] [PubMed] [Google Scholar]
- Miranda R. C., Sohrabji F., Toran-Allerand D. Interactions of estrogen with the neurotrophins and their receptors during neural development. Horm Behav. 1994 Dec;28(4):367–375. doi: 10.1006/hbeh.1994.1033. [DOI] [PubMed] [Google Scholar]
- Ojeda S. R., Dissen G. A., Junier M. P. Neurotrophic factors and female sexual development. Front Neuroendocrinol. 1992 Apr;13(2):120–162. [PubMed] [Google Scholar]
- Paus R., Lüftl M., Czarnetzki B. M. Nerve growth factor modulates keratinocyte proliferation in murine skin organ culture. Br J Dermatol. 1994 Feb;130(2):174–180. doi: 10.1111/j.1365-2133.1994.tb02896.x. [DOI] [PubMed] [Google Scholar]
- Pescarmona E., Pisacane A., Pignatelli E., Baroni C. D. Expression of epidermal and nerve growth factor receptors in human thymus and thymomas. Histopathology. 1993 Jul;23(1):39–44. doi: 10.1111/j.1365-2559.1993.tb01181.x. [DOI] [PubMed] [Google Scholar]
- Pflug B. R., Dionne C., Kaplan D. R., Lynch J., Djakiew D. Expression of a Trk high affinity nerve growth factor receptor in the human prostate. Endocrinology. 1995 Jan;136(1):262–268. doi: 10.1210/endo.136.1.7828539. [DOI] [PubMed] [Google Scholar]
- Pulford K. A., Rigney E. M., Micklem K. J., Jones M., Stross W. P., Gatter K. C., Mason D. Y. KP1: a new monoclonal antibody that detects a monocyte/macrophage associated antigen in routinely processed tissue sections. J Clin Pathol. 1989 Apr;42(4):414–421. doi: 10.1136/jcp.42.4.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rindi G., Buffa R., Sessa F., Tortora O., Solcia E. Chromogranin A, B and C immunoreactivities of mammalian endocrine cells. Distribution, distinction from costored hormones/prohormones and relationship with the argyrophil component of secretory granules. Histochemistry. 1986;85(1):19–28. doi: 10.1007/BF00508649. [DOI] [PubMed] [Google Scholar]
- Shi S. R., Key M. E., Kalra K. L. Antigen retrieval in formalin-fixed, paraffin-embedded tissues: an enhancement method for immunohistochemical staining based on microwave oven heating of tissue sections. J Histochem Cytochem. 1991 Jun;39(6):741–748. doi: 10.1177/39.6.1709656. [DOI] [PubMed] [Google Scholar]
- Smeyne R. J., Klein R., Schnapp A., Long L. K., Bryant S., Lewin A., Lira S. A., Barbacid M. Severe sensory and sympathetic neuropathies in mice carrying a disrupted Trk/NGF receptor gene. Nature. 1994 Mar 17;368(6468):246–249. doi: 10.1038/368246a0. [DOI] [PubMed] [Google Scholar]
- Sohrabji F., Greene L. A., Miranda R. C., Toran-Allerand C. D. Reciprocal regulation of estrogen and NGF receptors by their ligands in PC12 cells. J Neurobiol. 1994 Aug;25(8):974–988. doi: 10.1002/neu.480250807. [DOI] [PubMed] [Google Scholar]
- Sohrabji F., Miranda R. C., Toran-Allerand C. D. Estrogen differentially regulates estrogen and nerve growth factor receptor mRNAs in adult sensory neurons. J Neurosci. 1994 Feb;14(2):459–471. doi: 10.1523/JNEUROSCI.14-02-00459.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soppet D., Escandon E., Maragos J., Middlemas D. S., Reid S. W., Blair J., Burton L. E., Stanton B. R., Kaplan D. R., Hunter T. The neurotrophic factors brain-derived neurotrophic factor and neurotrophin-3 are ligands for the trkB tyrosine kinase receptor. Cell. 1991 May 31;65(5):895–903. doi: 10.1016/0092-8674(91)90396-g. [DOI] [PubMed] [Google Scholar]
- Timmusk T., Belluardo N., Metsis M., Persson H. Widespread and developmentally regulated expression of neurotrophin-4 mRNA in rat brain and peripheral tissues. Eur J Neurosci. 1993 Jun 1;5(6):605–613. doi: 10.1111/j.1460-9568.1993.tb00526.x. [DOI] [PubMed] [Google Scholar]
- Valenzuela D. M., Maisonpierre P. C., Glass D. J., Rojas E., Nuñez L., Kong Y., Gies D. R., Stitt T. N., Ip N. Y., Yancopoulos G. D. Alternative forms of rat TrkC with different functional capabilities. Neuron. 1993 May;10(5):963–974. doi: 10.1016/0896-6273(93)90211-9. [DOI] [PubMed] [Google Scholar]
- Vega J. A., Vazquez E., Naves F. J., Del Valle M. E., Calzada B., Represa J. J. Immunohistochemical localization of the high-affinity NGF receptor (gp140-trkA) in the adult human dorsal root and sympathetic ganglia and in the nerves and sensory corpuscles supplying digital skin. Anat Rec. 1994 Dec;240(4):579–588. doi: 10.1002/ar.1092400415. [DOI] [PubMed] [Google Scholar]
- Wheeler E. F., Bothwell M. Spatiotemporal patterns of expression of NGF and the low-affinity NGF receptor in rat embryos suggest functional roles in tissue morphogenesis and myogenesis. J Neurosci. 1992 Mar;12(3):930–945. doi: 10.1523/JNEUROSCI.12-03-00930.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiedenmann B., Waldherr R., Buhr H., Hille A., Rosa P., Huttner W. B. Identification of gastroenteropancreatic neuroendocrine cells in normal and neoplastic human tissue with antibodies against synaptophysin, chromogranin A, secretogranin I (chromogranin B), and secretogranin II. Gastroenterology. 1988 Nov;95(5):1364–1374. doi: 10.1016/0016-5085(88)90374-5. [DOI] [PubMed] [Google Scholar]
- Yaar M., Grossman K., Eller M., Gilchrest B. A. Evidence for nerve growth factor-mediated paracrine effects in human epidermis. J Cell Biol. 1991 Nov;115(3):821–828. doi: 10.1083/jcb.115.3.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X. F., Rush R. A. Localization of neurotrophin-3-like immunoreactivity in peripheral tissues of the rat. Brain Res. 1993 Sep 10;621(2):189–199. doi: 10.1016/0006-8993(93)90106-w. [DOI] [PubMed] [Google Scholar]