Abstract
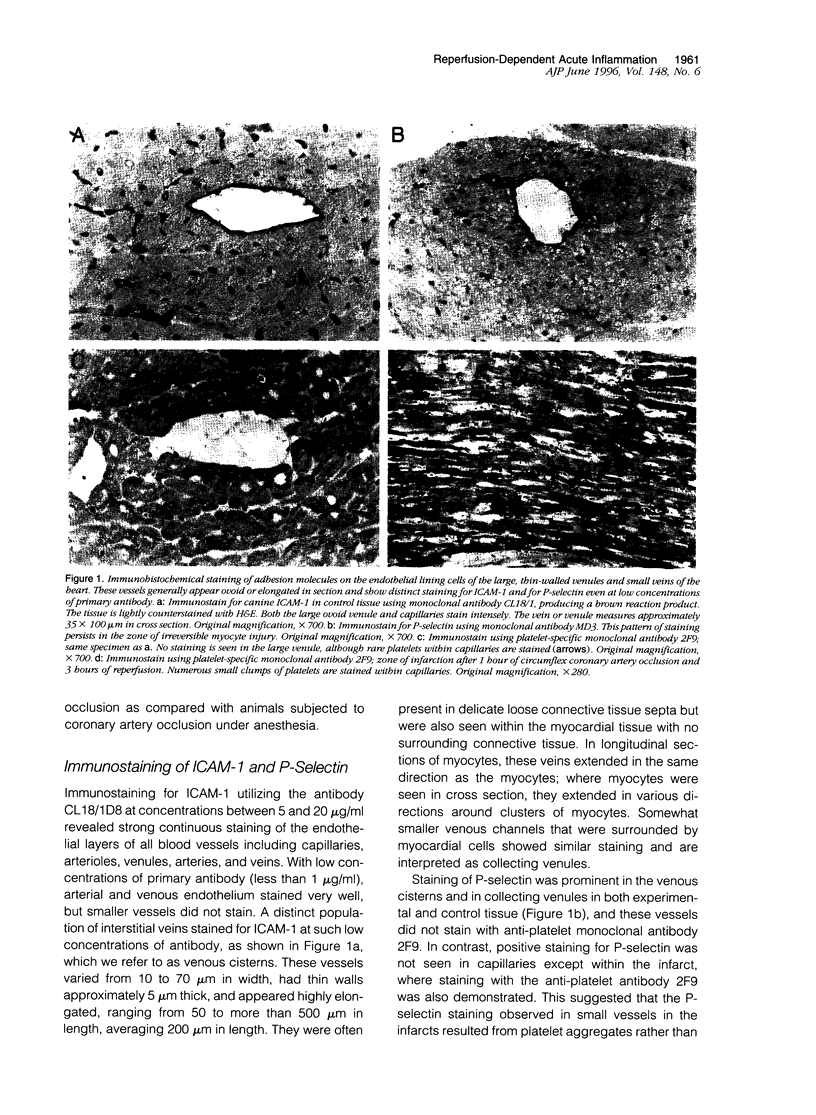
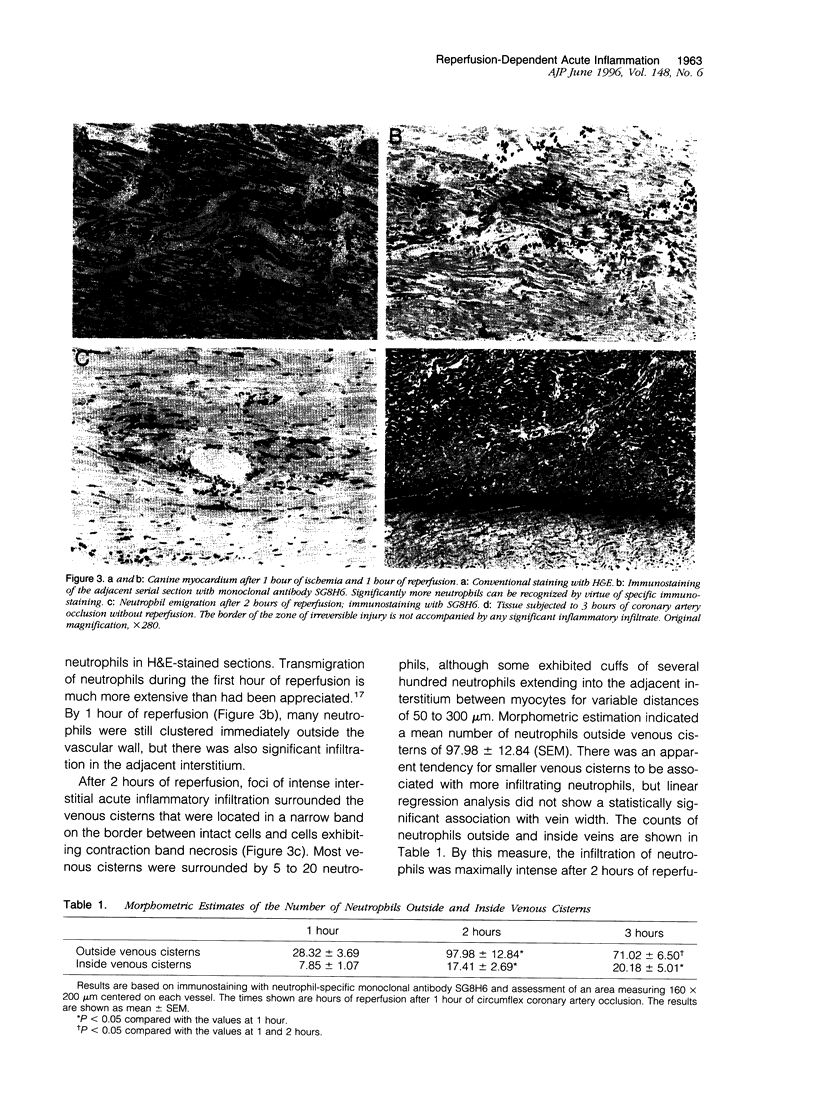
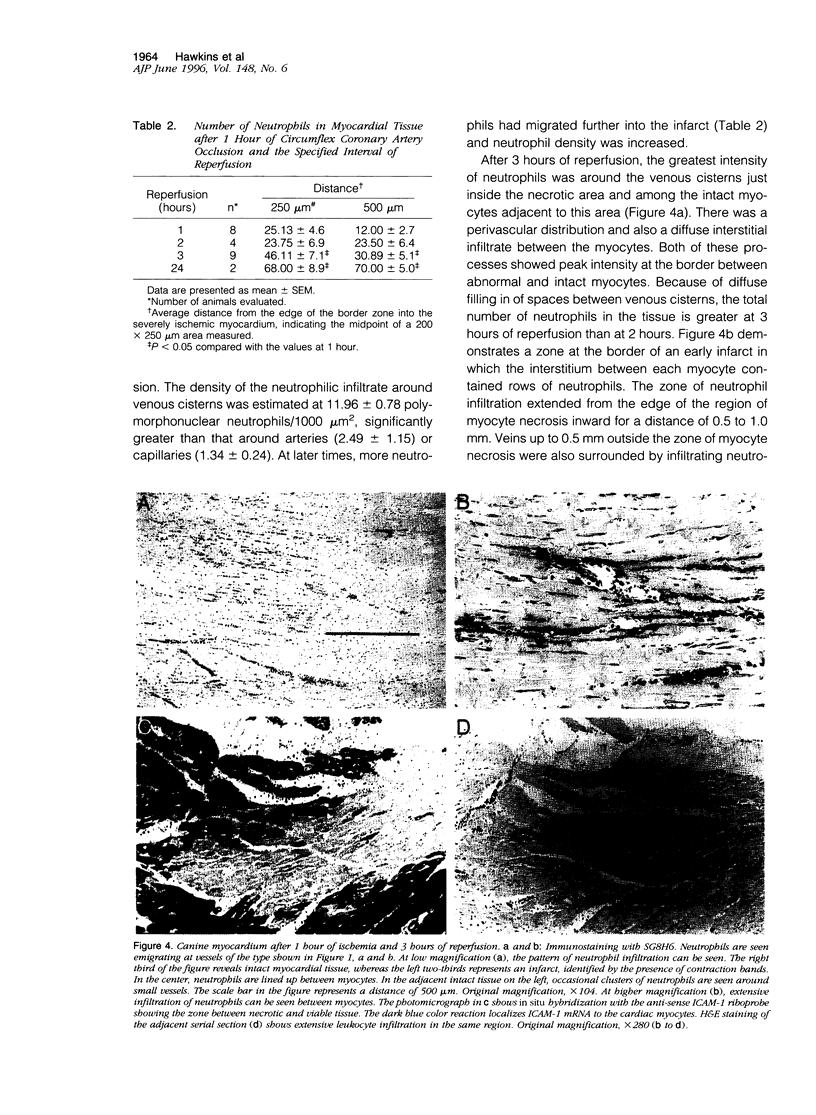
Reperfusion of the infarcted canine myocardium after 1 hour of ischemia is associated with an acute inflammatory infiltrate at the border of the infarct. In this paper, we demonstrate that early margination and emigration of neutrophils originate in thin-walled (approximately 5 micrometers) venous cisterns that average 200 micrometers in length and vary from 10 to 70 micrometers in width and show strong constitutive expression of both ICAM-1 and P-selectin; this class of vessels (venous cisterns) appears to be a unique feature in heart. A monoclonal antibody (SG8H6) with specificity for canine neutrophils was developed that allowed much more sensitive immunohistochemical detection of neutrophils in tissue and allowed us to follow tissue infiltration with time. Samples from 1 hour of reperfusion revealed dense margination and substantial emigration of neutrophils associated with the venous cisterns and collecting venules. By 2 hours, there was intense local emigration to the extravascular space between cardiac myocytes. By 3 hours, the infiltrate extended deeper into the infarct, and there was a continuous border zone of neutrophil infiltration that overlapped a region where intact cardiac myocytes strongly expressed ICAM-1 mRNA and extended into the necrotic tissue. At later times, neutrophil migration into infarcted tissue continued to progress. Neutrophil transmigration into reperfused myocardium is more extensive than previously described, and its extravascular distribution during early reperfusion is primarily in the viable border zone of the myocardium where myocyte ICAM-1 mRNA is found. These data are compatible with the hypothesis that extravascular neutrophils may participate in reperfusion injury.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albertine K. H., Weyrich A. S., Ma X. L., Lefer D. J., Becker L. C., Lefer A. M. Quantification of neutrophil migration following myocardial ischemia and reperfusion in cats and dogs. J Leukoc Biol. 1994 May;55(5):557–566. doi: 10.1002/jlb.55.5.557. [DOI] [PubMed] [Google Scholar]
- Anderson W. D., Anderson B. G., Seguin R. J. Microvasculature of the bear heart demonstrated by scanning electron microscopy. Acta Anat (Basel) 1988;131(4):305–313. doi: 10.1159/000146533. [DOI] [PubMed] [Google Scholar]
- BROWN R. E. THE PATTERN OF THE MICROCIRCULATORY BED IN THE VENTRICULAR MYOCARDIUM OF DOMESTIC MAMMALS. Am J Anat. 1965 Mar;116:355–374. doi: 10.1002/aja.1001160203. [DOI] [PubMed] [Google Scholar]
- Bassingthwaighte J. B., Yipintsoi T., Harvey R. B. Microvasculature of the dog left ventricular myocardium. Microvasc Res. 1974 Mar;7(2):229–249. doi: 10.1016/0026-2862(74)90008-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doré M., Hawkins H. K., Entman M. L., Smith C. W. Production of a monoclonal antibody against canine GMP-140 (P-selectin) and studies of its vascular distribution in canine tissues. Vet Pathol. 1993 May;30(3):213–222. doi: 10.1177/030098589303000301. [DOI] [PubMed] [Google Scholar]
- Doré M., Korthuis R. J., Granger D. N., Entman M. L., Smith C. W. P-selectin mediates spontaneous leukocyte rolling in vivo. Blood. 1993 Aug 15;82(4):1308–1316. [PubMed] [Google Scholar]
- Doré M., Simon S. I., Hughes B. J., Entman M. L., Smith C. W. P-selectin- and CD18-mediated recruitment of canine neutrophils under conditions of shear stress. Vet Pathol. 1995 May;32(3):258–268. doi: 10.1177/030098589503200307. [DOI] [PubMed] [Google Scholar]
- Dreyer W. J., Michael L. H., Nguyen T., Smith C. W., Anderson D. C., Entman M. L., Rossen R. D. Kinetics of C5a release in cardiac lymph of dogs experiencing coronary artery ischemia-reperfusion injury. Circ Res. 1992 Dec;71(6):1518–1524. doi: 10.1161/01.res.71.6.1518. [DOI] [PubMed] [Google Scholar]
- Dreyer W. J., Michael L. H., West M. S., Smith C. W., Rothlein R., Rossen R. D., Anderson D. C., Entman M. L. Neutrophil accumulation in ischemic canine myocardium. Insights into time course, distribution, and mechanism of localization during early reperfusion. Circulation. 1991 Jul;84(1):400–411. doi: 10.1161/01.cir.84.1.400. [DOI] [PubMed] [Google Scholar]
- Dreyer W. J., Smith C. W., Michael L. H., Rossen R. D., Hughes B. J., Entman M. L., Anderson D. C. Canine neutrophil activation by cardiac lymph obtained during reperfusion of ischemic myocardium. Circ Res. 1989 Dec;65(6):1751–1762. doi: 10.1161/01.res.65.6.1751. [DOI] [PubMed] [Google Scholar]
- Engler R. L., Schmid-Schönbein G. W., Pavelec R. S. Leukocyte capillary plugging in myocardial ischemia and reperfusion in the dog. Am J Pathol. 1983 Apr;111(1):98–111. [PMC free article] [PubMed] [Google Scholar]
- Entman M. L., Smith C. W. Postreperfusion inflammation: a model for reaction to injury in cardiovascular disease. Cardiovasc Res. 1994 Sep;28(9):1301–1311. doi: 10.1093/cvr/28.9.1301. [DOI] [PubMed] [Google Scholar]
- Entman M. L., Youker K., Shappell S. B., Siegel C., Rothlein R., Dreyer W. J., Schmalstieg F. C., Smith C. W. Neutrophil adherence to isolated adult canine myocytes. Evidence for a CD18-dependent mechanism. J Clin Invest. 1990 May;85(5):1497–1506. doi: 10.1172/JCI114596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entman M. L., Youker K., Shoji T., Kukielka G., Shappell S. B., Taylor A. A., Smith C. W. Neutrophil induced oxidative injury of cardiac myocytes. A compartmented system requiring CD11b/CD18-ICAM-1 adherence. J Clin Invest. 1992 Oct;90(4):1335–1345. doi: 10.1172/JCI115999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddard-Finegold J., Michael L. H. Cerebral blood flow and experimental intraventricular hemorrhage. Pediatr Res. 1984 Jan;18(1):7–11. [PubMed] [Google Scholar]
- Heymann M. A., Payne B. D., Hoffman J. I., Rudolph A. M. Blood flow measurements with radionuclide-labeled particles. Prog Cardiovasc Dis. 1977 Jul-Aug;20(1):55–79. doi: 10.1016/s0033-0620(77)80005-4. [DOI] [PubMed] [Google Scholar]
- Jones D. A., Abbassi O., McIntire L. V., McEver R. P., Smith C. W. P-selectin mediates neutrophil rolling on histamine-stimulated endothelial cells. Biophys J. 1993 Oct;65(4):1560–1569. doi: 10.1016/S0006-3495(93)81195-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukielka G. L., Hawkins H. K., Michael L., Manning A. M., Youker K., Lane C., Entman M. L., Smith C. W., Anderson D. C. Regulation of intercellular adhesion molecule-1 (ICAM-1) in ischemic and reperfused canine myocardium. J Clin Invest. 1993 Sep;92(3):1504–1516. doi: 10.1172/JCI116729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukielka G. L., Smith C. W., LaRosa G. J., Manning A. M., Mendoza L. H., Daly T. J., Hughes B. J., Youker K. A., Hawkins H. K., Michael L. H. Interleukin-8 gene induction in the myocardium after ischemia and reperfusion in vivo. J Clin Invest. 1995 Jan;95(1):89–103. doi: 10.1172/JCI117680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence M. B., Springer T. A. Leukocytes roll on a selectin at physiologic flow rates: distinction from and prerequisite for adhesion through integrins. Cell. 1991 May 31;65(5):859–873. doi: 10.1016/0092-8674(91)90393-d. [DOI] [PubMed] [Google Scholar]
- Lefer A. M. Platelet activating factor (PAF) and its role in cardiac injury. Prog Clin Biol Res. 1989;301:53–60. [PubMed] [Google Scholar]
- Lefer D. J., Shandelya S. M., Serrano C. V., Jr, Becker L. C., Kuppusamy P., Zweier J. L. Cardioprotective actions of a monoclonal antibody against CD-18 in myocardial ischemia-reperfusion injury. Circulation. 1993 Oct;88(4 Pt 1):1779–1787. doi: 10.1161/01.cir.88.4.1779. [DOI] [PubMed] [Google Scholar]
- Ma X. L., Tsao P. S., Lefer A. M. Antibody to CD-18 exerts endothelial and cardiac protective effects in myocardial ischemia and reperfusion. J Clin Invest. 1991 Oct;88(4):1237–1243. doi: 10.1172/JCI115427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X. L., Weyrich A. S., Lefer D. J., Buerke M., Albertine K. H., Kishimoto T. K., Lefer A. M. Monoclonal antibody to L-selectin attenuates neutrophil accumulation and protects ischemic reperfused cat myocardium. Circulation. 1993 Aug;88(2):649–658. doi: 10.1161/01.cir.88.2.649. [DOI] [PubMed] [Google Scholar]
- Michael L. H., Zhang Z., Hartley C. J., Bolli R., Taylor A. A., Entman M. L. Thromboxane B2 in cardiac lymph. Effect of superoxide dismutase and catalase during myocardial ischemia and reperfusion. Circ Res. 1990 Apr;66(4):1040–1044. doi: 10.1161/01.res.66.4.1040. [DOI] [PubMed] [Google Scholar]
- Mullane K. M., Read N., Salmon J. A., Moncada S. Role of leukocytes in acute myocardial infarction in anesthetized dogs: relationship to myocardial salvage by anti-inflammatory drugs. J Pharmacol Exp Ther. 1984 Feb;228(2):510–522. [PubMed] [Google Scholar]
- REYNOLDS S. R., KIRSCH M., BING R. J. Functional capillary beds in the beating, KCl-arrested and KCl-arrested-perfused myocardium of the dog. Circ Res. 1958 Sep;6(5):600–611. doi: 10.1161/01.res.6.5.600. [DOI] [PubMed] [Google Scholar]
- Romson J. L., Hook B. G., Rigot V. H., Schork M. A., Swanson D. P., Lucchesi B. R. The effect of ibuprofen on accumulation of indium-111-labeled platelets and leukocytes in experimental myocardial infarction. Circulation. 1982 Nov;66(5):1002–1011. doi: 10.1161/01.cir.66.5.1002. [DOI] [PubMed] [Google Scholar]
- Rossen R. D., Michael L. H., Kagiyama A., Savage H. E., Hanson G., Reisberg M. A., Moake J. N., Kim S. H., Self D., Weakley S. Mechanism of complement activation after coronary artery occlusion: evidence that myocardial ischemia in dogs causes release of constituents of myocardial subcellular origin that complex with human C1q in vivo. Circ Res. 1988 Mar;62(3):572–584. doi: 10.1161/01.res.62.3.572. [DOI] [PubMed] [Google Scholar]
- Rot A. Endothelial cell binding of NAP-1/IL-8: role in neutrophil emigration. Immunol Today. 1992 Aug;13(8):291–294. doi: 10.1016/0167-5699(92)90039-A. [DOI] [PubMed] [Google Scholar]
- SOMMERS H. M., JENNINGS R. B. EXPERIMENTAL ACUTE MYOCARDIAL INFARCTION; HISTOLOGIC AND HISTOCHEMICAL STUDIES OF EARLY MYOCARDIAL INFARCTS INDUCED BY TEMPORARY OR PERMANENT OCCLUSION OF A CORONARY ARTERY. Lab Invest. 1964 Dec;13:1491–1503. [PubMed] [Google Scholar]
- Smith C. W., Entman M. L., Lane C. L., Beaudet A. L., Ty T. I., Youker K., Hawkins H. K., Anderson D. C. Adherence of neutrophils to canine cardiac myocytes in vitro is dependent on intercellular adhesion molecule-1. J Clin Invest. 1991 Oct;88(4):1216–1223. doi: 10.1172/JCI115424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. W., Rothlein R., Hughes B. J., Mariscalco M. M., Rudloff H. E., Schmalstieg F. C., Anderson D. C. Recognition of an endothelial determinant for CD 18-dependent human neutrophil adherence and transendothelial migration. J Clin Invest. 1988 Nov;82(5):1746–1756. doi: 10.1172/JCI113788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl G. L., Terashita Z., Lefer A. M. Role of platelet activating factor in propagation of cardiac damage during myocardial ischemia. J Pharmacol Exp Ther. 1988 Mar;244(3):898–904. [PubMed] [Google Scholar]
- Thakur M. L., Gottschalk A., Zaret B. L. Imaging experimental myocardial infarction with indium-111-labeled autologous leukocytes: effects of infarct age and residual regional myocardial blood flow. Circulation. 1979 Aug;60(2):297–305. doi: 10.1161/01.cir.60.2.297. [DOI] [PubMed] [Google Scholar]
- Weisman H. F., Bartow T., Leppo M. K., Marsh H. C., Jr, Carson G. R., Concino M. F., Boyle M. P., Roux K. H., Weisfeldt M. L., Fearon D. T. Soluble human complement receptor type 1: in vivo inhibitor of complement suppressing post-ischemic myocardial inflammation and necrosis. Science. 1990 Jul 13;249(4965):146–151. doi: 10.1126/science.2371562. [DOI] [PubMed] [Google Scholar]
- Weiss E. S., Ahmed S. A., Thakur M. L., Welch M. J., Coleman R. E., Sobel B. E. Imaging of the inflammatory response in ischemic canine myocardium with 111indium-labeled leukocytes. Am J Cardiol. 1977 Aug;40(2):195–199. doi: 10.1016/0002-9149(77)90008-x. [DOI] [PubMed] [Google Scholar]
- Weyrich A. S., Ma X. Y., Lefer D. J., Albertine K. H., Lefer A. M. In vivo neutralization of P-selectin protects feline heart and endothelium in myocardial ischemia and reperfusion injury. J Clin Invest. 1993 Jun;91(6):2620–2629. doi: 10.1172/JCI116501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki T., Seko Y., Tamatani T., Miyasaka M., Yagita H., Okumura K., Nagai R., Yazaki Y. Expression of intercellular adhesion molecule-1 in rat heart with ischemia/reperfusion and limitation of infarct size by treatment with antibodies against cell adhesion molecules. Am J Pathol. 1993 Aug;143(2):410–418. [PMC free article] [PubMed] [Google Scholar]
- Youker K. A., Hawkins H. K., Kukielka G. L., Perrard J. L., Michael L. H., Ballantyne C. M., Smith C. W., Entman M. L. Molecular evidence for induction of intracellular adhesion molecule-1 in the viable border zone associated with ischemia-reperfusion injury of the dog heart. Circulation. 1994 Jun;89(6):2736–2746. doi: 10.1161/01.cir.89.6.2736. [DOI] [PubMed] [Google Scholar]
- Youker K., Smith C. W., Anderson D. C., Miller D., Michael L. H., Rossen R. D., Entman M. L. Neutrophil adherence to isolated adult cardiac myocytes. Induction by cardiac lymph collected during ischemia and reperfusion. J Clin Invest. 1992 Feb;89(2):602–609. doi: 10.1172/JCI115626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman G. A., McIntyre T. M., Mehra M., Prescott S. M. Endothelial cell-associated platelet-activating factor: a novel mechanism for signaling intercellular adhesion. J Cell Biol. 1990 Feb;110(2):529–540. doi: 10.1083/jcb.110.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]