Abstract
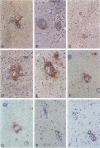
In an adoptive transfer model of experimental allergic encephalomyelitis, stimulation of lymph node cells with proteolipid protein and recombinant murine interleukin (rmIL)-12 before cell transfer accelerated the onset and exacerbates clinical disease. In vitro stimulation with proteolipid protein in the presence of rmIL-12 was associated with an increase in interferon-gamma-producing cells and a decrease in IL-4-producing cells, indicating a preferential expansion of Th1 effector cells. This was supported by the finding that severe disease with rapid onset could be transferred with as few as 10 x 10(6) rmIL-12-stimulated lymph node cells. Immunohistochemical analysis confirmed that the accelerated onset of disease after in vitro stimulation with rmIL-12 coincided with an acute inflammatory response in the central nervous system. At peak disease, both control and rmIL-12 treatment groups exhibited extensive cellular infiltration with characteristic perivascular cuffing. No notable differences in either the cellular composition or cytokine expression within the lesions were seen between groups. However, the frequency of macrophages that stained positively for inducible nitric oxide synthase was increased in animals challenged with rmIL-12-treated lymph node cells. The results suggest that, in addition to promoting the preferential expansion of interferon-gamma-producing cells by rmIL-12 in vitro, secondary in vivo effects leading to macrophage activation and inducible nitric oxide synthase expression may contribute to the severe and protracted course of central nervous system inflammation in this model.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baron J. L., Madri J. A., Ruddle N. H., Hashim G., Janeway C. A., Jr Surface expression of alpha 4 integrin by CD4 T cells is required for their entry into brain parenchyma. J Exp Med. 1993 Jan 1;177(1):57–68. doi: 10.1084/jem.177.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell I. L., Samimi A., Chiang C. S. Expression of the inducible nitric oxide synthase. Correlation with neuropathology and clinical features in mice with lymphocytic choriomeningitis. J Immunol. 1994 Oct 15;153(8):3622–3629. [PubMed] [Google Scholar]
- Cash E., Minty A., Ferrara P., Caput D., Fradelizi D., Rott O. Macrophage-inactivating IL-13 suppresses experimental autoimmune encephalomyelitis in rats. J Immunol. 1994 Nov 1;153(9):4258–4267. [PubMed] [Google Scholar]
- Ding A. H., Nathan C. F., Stuehr D. J. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J Immunol. 1988 Oct 1;141(7):2407–2412. [PubMed] [Google Scholar]
- Duong T. T., St Louis J., Gilbert J. J., Finkelman F. D., Strejan G. H. Effect of anti-interferon-gamma and anti-interleukin-2 monoclonal antibody treatment on the development of actively and passively induced experimental allergic encephalomyelitis in the SJL/J mouse. J Neuroimmunol. 1992 Feb;36(2-3):105–115. doi: 10.1016/0165-5728(92)90042-j. [DOI] [PubMed] [Google Scholar]
- Heinzel F. P., Schoenhaut D. S., Rerko R. M., Rosser L. E., Gately M. K. Recombinant interleukin 12 cures mice infected with Leishmania major. J Exp Med. 1993 May 1;177(5):1505–1509. doi: 10.1084/jem.177.5.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huitinga I., van Rooijen N., de Groot C. J., Uitdehaag B. M., Dijkstra C. D. Suppression of experimental allergic encephalomyelitis in Lewis rats after elimination of macrophages. J Exp Med. 1990 Oct 1;172(4):1025–1033. doi: 10.1084/jem.172.4.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs C. A., Baker P. E., Roux E. R., Picha K. S., Toivola B., Waugh S., Kennedy M. K. Experimental autoimmune encephalomyelitis is exacerbated by IL-1 alpha and suppressed by soluble IL-1 receptor. J Immunol. 1991 May 1;146(9):2983–2989. [PubMed] [Google Scholar]
- Kennedy M. K., Torrance D. S., Picha K. S., Mohler K. M. Analysis of cytokine mRNA expression in the central nervous system of mice with experimental autoimmune encephalomyelitis reveals that IL-10 mRNA expression correlates with recovery. J Immunol. 1992 Oct 1;149(7):2496–2505. [PubMed] [Google Scholar]
- Kuchroo V. K., Das M. P., Brown J. A., Ranger A. M., Zamvil S. S., Sobel R. A., Weiner H. L., Nabavi N., Glimcher L. H. B7-1 and B7-2 costimulatory molecules activate differentially the Th1/Th2 developmental pathways: application to autoimmune disease therapy. Cell. 1995 Mar 10;80(5):707–718. doi: 10.1016/0092-8674(95)90349-6. [DOI] [PubMed] [Google Scholar]
- Kuchroo V. K., Martin C. A., Greer J. M., Ju S. T., Sobel R. A., Dorf M. E. Cytokines and adhesion molecules contribute to the ability of myelin proteolipid protein-specific T cell clones to mediate experimental allergic encephalomyelitis. J Immunol. 1993 Oct 15;151(8):4371–4382. [PubMed] [Google Scholar]
- Kuchroo V. K., Martin C. A., Greer J. M., Ju S. T., Sobel R. A., Dorf M. E. Cytokines and adhesion molecules contribute to the ability of myelin proteolipid protein-specific T cell clones to mediate experimental allergic encephalomyelitis. J Immunol. 1993 Oct 15;151(8):4371–4382. [PubMed] [Google Scholar]
- Manetti R., Parronchi P., Giudizi M. G., Piccinni M. P., Maggi E., Trinchieri G., Romagnani S. Natural killer cell stimulatory factor (interleukin 12 [IL-12]) induces T helper type 1 (Th1)-specific immune responses and inhibits the development of IL-4-producing Th cells. J Exp Med. 1993 Apr 1;177(4):1199–1204. doi: 10.1084/jem.177.4.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill J. E., Ignarro L. J., Sherman M. P., Melinek J., Lane T. E. Microglial cell cytotoxicity of oligodendrocytes is mediated through nitric oxide. J Immunol. 1993 Aug 15;151(4):2132–2141. [PubMed] [Google Scholar]
- Merrill J. E., Kono D. H., Clayton J., Ando D. G., Hinton D. R., Hofman F. M. Inflammatory leukocytes and cytokines in the peptide-induced disease of experimental allergic encephalomyelitis in SJL and B10.PL mice. Proc Natl Acad Sci U S A. 1992 Jan 15;89(2):574–578. doi: 10.1073/pnas.89.2.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris S. C., Madden K. B., Adamovicz J. J., Gause W. C., Hubbard B. R., Gately M. K., Finkelman F. D. Effects of IL-12 on in vivo cytokine gene expression and Ig isotype selection. J Immunol. 1994 Feb 1;152(3):1047–1056. [PubMed] [Google Scholar]
- Pettinelli C. B., Fritz R. B., Chou C. H., McFarlin D. E. Encephalitogenic activity of guinea pig myelin basic protein in the SJL mouse. J Immunol. 1982 Sep;129(3):1209–1211. [PubMed] [Google Scholar]
- Pettinelli C. B., McFarlin D. E. Adoptive transfer of experimental allergic encephalomyelitis in SJL/J mice after in vitro activation of lymph node cells by myelin basic protein: requirement for Lyt 1+ 2- T lymphocytes. J Immunol. 1981 Oct;127(4):1420–1423. [PubMed] [Google Scholar]
- Racke M. K., Bonomo A., Scott D. E., Cannella B., Levine A., Raine C. S., Shevach E. M., Röcken M. Cytokine-induced immune deviation as a therapy for inflammatory autoimmune disease. J Exp Med. 1994 Nov 1;180(5):1961–1966. doi: 10.1084/jem.180.5.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rott O., Fleischer B., Cash E. Interleukin-10 prevents experimental allergic encephalomyelitis in rats. Eur J Immunol. 1994 Jun;24(6):1434–1440. doi: 10.1002/eji.1830240629. [DOI] [PubMed] [Google Scholar]
- Ruddle N. H., Bergman C. M., McGrath K. M., Lingenheld E. G., Grunnet M. L., Padula S. J., Clark R. B. An antibody to lymphotoxin and tumor necrosis factor prevents transfer of experimental allergic encephalomyelitis. J Exp Med. 1990 Oct 1;172(4):1193–1200. doi: 10.1084/jem.172.4.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sypek J. P., Chung C. L., Mayor S. E., Subramanyam J. M., Goldman S. J., Sieburth D. S., Wolf S. F., Schaub R. G. Resolution of cutaneous leishmaniasis: interleukin 12 initiates a protective T helper type 1 immune response. J Exp Med. 1993 Jun 1;177(6):1797–1802. doi: 10.1084/jem.177.6.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuohy V. K., Sobel R. A., Lees M. B. Myelin proteolipid protein-induced experimental allergic encephalomyelitis. Variations of disease expression in different strains of mice. J Immunol. 1988 Mar 15;140(6):1868–1873. [PubMed] [Google Scholar]
- Wolf S. F., Temple P. A., Kobayashi M., Young D., Dicig M., Lowe L., Dzialo R., Fitz L., Ferenz C., Hewick R. M. Cloning of cDNA for natural killer cell stimulatory factor, a heterodimeric cytokine with multiple biologic effects on T and natural killer cells. J Immunol. 1991 May 1;146(9):3074–3081. [PubMed] [Google Scholar]
- Yednock T. A., Cannon C., Fritz L. C., Sanchez-Madrid F., Steinman L., Karin N. Prevention of experimental autoimmune encephalomyelitis by antibodies against alpha 4 beta 1 integrin. Nature. 1992 Mar 5;356(6364):63–66. doi: 10.1038/356063a0. [DOI] [PubMed] [Google Scholar]