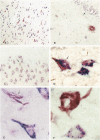
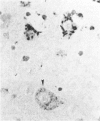
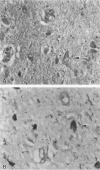
Abstract
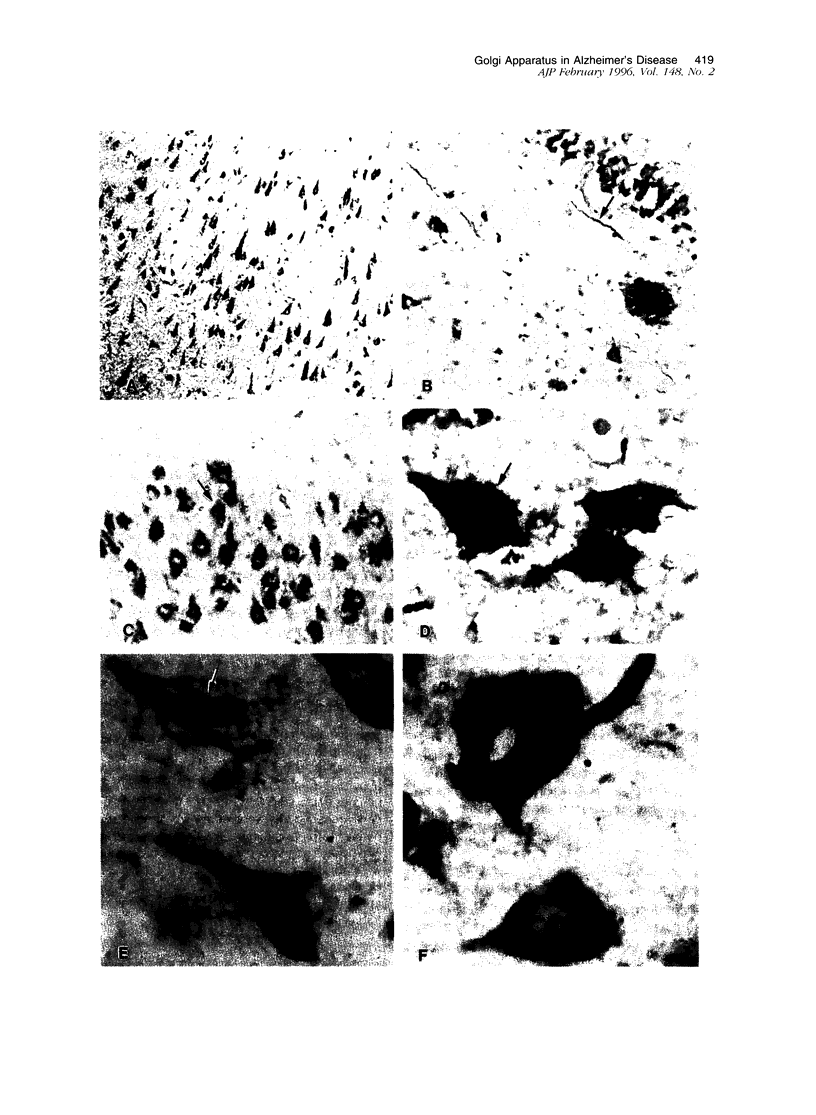
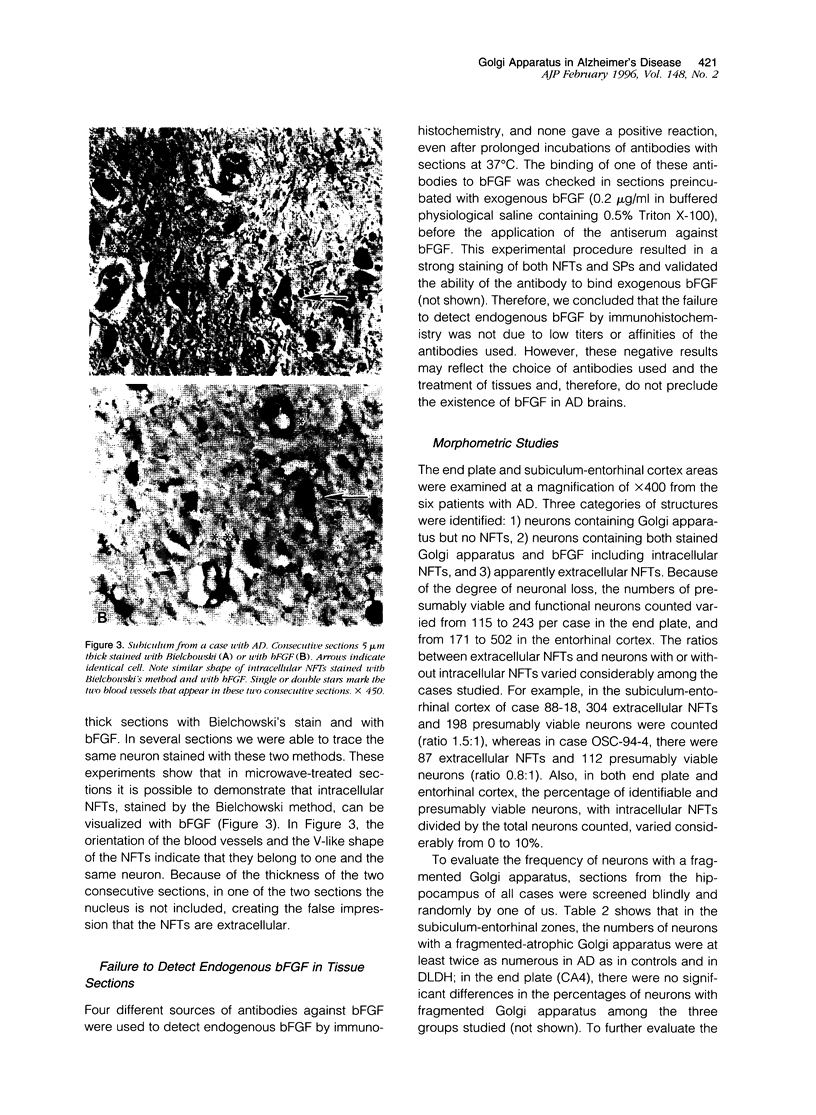
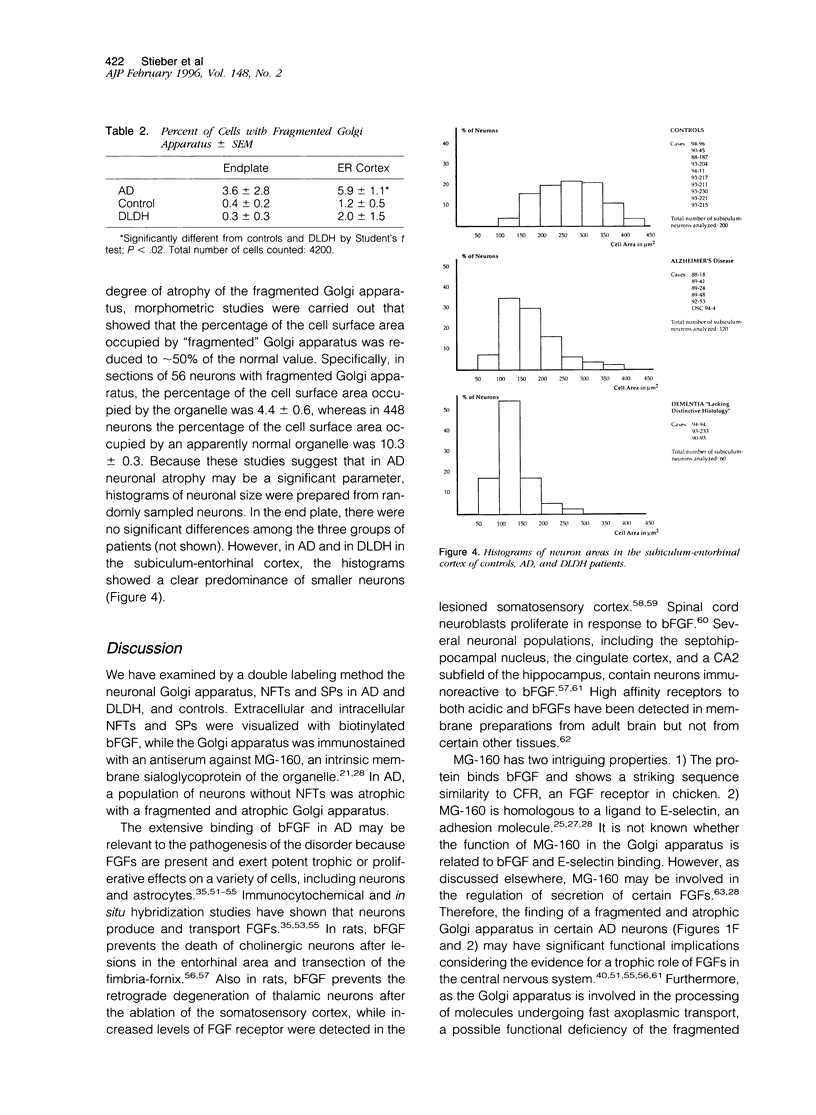
Recent immunocytochemical and morphometric studies in amyotrophic lateral sclerosis, Alzheimer's disease (AD), and aging indicate that the neuronal Golgi apparatus is a reliable index of activity or degeneration. To further evaluate a possible role of the Golgi apparatus in the pathogenesis of AD, we examined by double labeling the neuronal Golgi apparatus, neurofibrillary tangles (NFTs), and senile plaques (SPs) in the hippocampus of six cases of AD, and in 13 controls including three cases of a rare form of dementia lacking distinctive histopathological features. The Golgi apparatus was visualized with a polyclonal antiserum against MG-160, a membrane sialoglycoprotein of the organelle, and NFTs and SPs were visualized with biotinylated basic fibroblast growth factor (bFGF). Only a rare SP contained a few small immunostained elements of the Golgi apparatus. Neurons with intracellular NFTs, labeled with biotinylated bFGF, contained intensely labeled but deformed Golgi apparatus, which was displaced by the NFTs and coalesced into larger irregular granules. In contrast, a population of neurons without NFTs displayed fragmentation of the Golgi apparatus, ie, the organelle appeared in the form of small round, disconnected, and dispersed elements instead of the normal perinuclear network of irregular or linear profiles which often extended into the proximal segments of dendrites. In addition, the fragmented neuronal Golgi apparatus was atrophic as the percentage of the cell surface area occupied by the organelle was 4.4 +/- 0.6% SD, whereas in neurons with a normal Golgi apparatus the percentage of the cell surface area occupied by the organelle was 10.3 +/- 0.3% SD. The results of this study suggest that in AD the Golgi apparatus of a population of neurons without NFTs is involved in the pathogenesis of the disease. Considering the role of the Golgi apparatus in the processing of polypeptides destined for fast axoplasmic transports, the fragmentation of the organelle may be associated with functional and structural impairments of axons and presynaptic terminals.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amara J. F., Cheng S. H., Smith A. E. Intracellular protein trafficking defects in human disease. Trends Cell Biol. 1992 May;2(5):145–149. doi: 10.1016/0962-8924(92)90101-r. [DOI] [PubMed] [Google Scholar]
- Anderson K. J., Dam D., Lee S., Cotman C. W. Basic fibroblast growth factor prevents death of lesioned cholinergic neurons in vivo. Nature. 1988 Mar 24;332(6162):360–361. doi: 10.1038/332360a0. [DOI] [PubMed] [Google Scholar]
- Antoine J. C., Maurice M., Feldmann G., Avrameas S. In vivo and in vitro effects of colchicine and vinblastine on the secretory process of antibody-producing cells. J Immunol. 1980 Nov;125(5):1939–1949. [PubMed] [Google Scholar]
- Baird A. Fibroblast growth factors: activities and significance of non-neurotrophin neurotrophic growth factors. Curr Opin Neurobiol. 1994 Feb;4(1):78–86. doi: 10.1016/0959-4388(94)90035-3. [DOI] [PubMed] [Google Scholar]
- Baird A., Schubert D., Ling N., Guillemin R. Receptor- and heparin-binding domains of basic fibroblast growth factor. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2324–2328. doi: 10.1073/pnas.85.7.2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondareff W., Harrington C. R., Wischik C. M., Hauser D. L., Roth M. Absence of abnormal hyperphosphorylation of tau in intracellular tangles in Alzheimer's disease. J Neuropathol Exp Neurol. 1995 Sep;54(5):657–663. doi: 10.1097/00005072-199509000-00007. [DOI] [PubMed] [Google Scholar]
- Burgess W. H., Maciag T. The heparin-binding (fibroblast) growth factor family of proteins. Annu Rev Biochem. 1989;58:575–606. doi: 10.1146/annurev.bi.58.070189.003043. [DOI] [PubMed] [Google Scholar]
- Burrus L. W., Zuber M. E., Lueddecke B. A., Olwin B. B. Identification of a cysteine-rich receptor for fibroblast growth factors. Mol Cell Biol. 1992 Dec;12(12):5600–5609. doi: 10.1128/mcb.12.12.5600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croul S., Mezitis S. G., Stieber A., Chen Y. J., Gonatas J. O., Goud B., Gonatas N. K. Immunocytochemical visualization of the Golgi apparatus in several species, including human, and tissues with an antiserum against MG-160, a sialoglycoprotein of rat Golgi apparatus. J Histochem Cytochem. 1990 Jul;38(7):957–963. doi: 10.1177/38.7.2355176. [DOI] [PubMed] [Google Scholar]
- Cummings B. J., Su J. H., Cotman C. W. Neuritic involvement within bFGF immunopositive plaques of Alzheimer's disease. Exp Neurol. 1993 Dec;124(2):315–325. doi: 10.1006/exnr.1993.1202. [DOI] [PubMed] [Google Scholar]
- Davies D. C., Horwood N., Isaacs S. L., Mann D. M. The effect of age and Alzheimer's disease on pyramidal neuron density in the individual fields of the hippocampal formation. Acta Neuropathol. 1992;83(5):510–517. doi: 10.1007/BF00310028. [DOI] [PubMed] [Google Scholar]
- Eckenstein F., Woodward W. R., Nishi R. Differential localization and possible functions of aFGF and bFGF in the central and peripheral nervous systems. Ann N Y Acad Sci. 1991;638:348–360. doi: 10.1111/j.1749-6632.1991.tb49045.x. [DOI] [PubMed] [Google Scholar]
- Farquhar M. G., Palade G. E. The Golgi apparatus (complex)-(1954-1981)-from artifact to center stage. J Cell Biol. 1981 Dec;91(3 Pt 2):77s–103s. doi: 10.1083/jcb.91.3.77s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farquhar M. G. Progress in unraveling pathways of Golgi traffic. Annu Rev Cell Biol. 1985;1:447–488. doi: 10.1146/annurev.cb.01.110185.002311. [DOI] [PubMed] [Google Scholar]
- Gonatas J. O., Mezitis S. G., Stieber A., Fleischer B., Gonatas N. K. MG-160. A novel sialoglycoprotein of the medial cisternae of the Golgi apparatus [published eeratum appears in J Biol Chem 1989 Mar 5;264(7):4264]. J Biol Chem. 1989 Jan 5;264(1):646–653. [PubMed] [Google Scholar]
- Gonatas J. O., Mourelatos Z., Stieber A., Lane W. S., Brosius J., Gonatas N. K. MG-160, a membrane sialoglycoprotein of the medial cisternae of the rat Golgi apparatus, binds basic fibroblast growth factor and exhibits a high level of sequence identity to a chicken fibroblast growth factor receptor. J Cell Sci. 1995 Feb;108(Pt 2):457–467. doi: 10.1242/jcs.108.2.457. [DOI] [PubMed] [Google Scholar]
- Gonatas N. K. Axonic and synaptic lesions in neuropsychiatric disorders. Nature. 1967 Apr 22;214(5086):352–355. doi: 10.1038/214352a0. [DOI] [PubMed] [Google Scholar]
- Gonatas N. K. Rous-Whipple Award Lecture. Contributions to the physiology and pathology of the Golgi apparatus. Am J Pathol. 1994 Oct;145(4):751–761. [PMC free article] [PubMed] [Google Scholar]
- Gonatas N. K., Stieber A., Mourelatos Z., Chen Y., Gonatas J. O., Appel S. H., Hays A. P., Hickey W. F., Hauw J. J. Fragmentation of the Golgi apparatus of motor neurons in amyotrophic lateral sclerosis. Am J Pathol. 1992 Mar;140(3):731–737. [PMC free article] [PubMed] [Google Scholar]
- Gómez-Pinilla F., Lee J. W., Cotman C. W. Basic FGF in adult rat brain: cellular distribution and response to entorhinal lesion and fimbria-fornix transection. J Neurosci. 1992 Jan;12(1):345–355. doi: 10.1523/JNEUROSCI.12-01-00345.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerschlag R., Stone G. C., Bolen F. A., Lindsey J. D., Ellisman M. H. Evidence that all newly synthesized proteins destined for fast axonal transport pass through the Golgi apparatus. J Cell Biol. 1982 Jun;93(3):568–575. doi: 10.1083/jcb.93.3.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasmin B. J., Antony C., Changeux J. P., Cartaud J. Nerve-dependent plasticity of the Golgi complex in skeletal muscle fibres: compartmentalization within the subneural sarcoplasm. Eur J Neurosci. 1995 Mar 1;7(3):470–479. doi: 10.1111/j.1460-9568.1995.tb00343.x. [DOI] [PubMed] [Google Scholar]
- Johnston P. A., Stieber A., Gonatas N. K. A hypothesis on the traffic of MG160, a medial Golgi sialoglycoprotein, from the trans-Golgi network to the Golgi cisternae. J Cell Sci. 1994 Mar;107(Pt 3):529–537. doi: 10.1242/jcs.107.3.529. [DOI] [PubMed] [Google Scholar]
- KIDD M. Paired helical filaments in electron microscopy of Alzheimer's disease. Nature. 1963 Jan 12;197:192–193. doi: 10.1038/197192b0. [DOI] [PubMed] [Google Scholar]
- Kato T., Sasaki H., Katagiri T., Sasaki H., Koiwai K., Youki H., Totsuka S., Ishii T. The binding of basic fibroblast growth factor to Alzheimer's neurofibrillary tangles and senile plaques. Neurosci Lett. 1991 Jan 14;122(1):33–36. doi: 10.1016/0304-3940(91)90186-w. [DOI] [PubMed] [Google Scholar]
- Kiefer P., Peters G., Dickson C. Retention of fibroblast growth factor 3 in the Golgi complex may regulate its export from cells. Mol Cell Biol. 1993 Sep;13(9):5781–5793. doi: 10.1128/mcb.13.9.5781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopman D. S., Mastri A. R., Frey W. H., 2nd, Sung J. H., Rustan T. Dementia lacking distinctive histologic features: a common non-Alzheimer degenerative dementia. Neurology. 1990 Feb;40(2):251–256. doi: 10.1212/wnl.40.2.251. [DOI] [PubMed] [Google Scholar]
- Kosik K. S. The Alzheimer's disease sphinx: a riddle with plaques and tangles. J Cell Biol. 1994 Dec;127(6 Pt 1):1501–1504. doi: 10.1083/jcb.127.6.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacy P. E., Howell S. L., Young D. A., Fink C. J. New hypothesis of insulin secretion. Nature. 1968 Sep 14;219(5159):1177–1179. doi: 10.1038/2191177a0. [DOI] [PubMed] [Google Scholar]
- Ledoux D., Mereau A., Jaye M., Miskulin M., Barritault D., Courty J. Adult brain but not kidney, liver, lung, intestine, and stomach membrane preparations contain detectable amounts of high-affinity receptors to acidic and basic growth factors. Ann N Y Acad Sci. 1991;638:397–399. doi: 10.1111/j.1749-6632.1991.tb49051.x. [DOI] [PubMed] [Google Scholar]
- Lucassen P. J., Ravid R., Gonatas N. K., Swaab D. F. Activation of the human supraoptic and paraventricular nucleus neurons with aging and in Alzheimer's disease as judged from increasing size of the Golgi apparatus. Brain Res. 1993 Dec 31;632(1-2):105–113. doi: 10.1016/0006-8993(93)91144-h. [DOI] [PubMed] [Google Scholar]
- Lucassen P. J., Salehi A., Pool C. W., Gonatas N. K., Swaab D. F. Activation of vasopressin neurons in aging and Alzheimer's disease. J Neuroendocrinol. 1994 Dec;6(6):673–679. doi: 10.1111/j.1365-2826.1994.tb00634.x. [DOI] [PubMed] [Google Scholar]
- Masliah E., Terry R. The role of synaptic proteins in the pathogenesis of disorders of the central nervous system. Brain Pathol. 1993 Jan;3(1):77–85. doi: 10.1111/j.1750-3639.1993.tb00728.x. [DOI] [PubMed] [Google Scholar]
- Matsuo E. S., Shin R. W., Billingsley M. L., Van deVoorde A., O'Connor M., Trojanowski J. Q., Lee V. M. Biopsy-derived adult human brain tau is phosphorylated at many of the same sites as Alzheimer's disease paired helical filament tau. Neuron. 1994 Oct;13(4):989–1002. doi: 10.1016/0896-6273(94)90264-x. [DOI] [PubMed] [Google Scholar]
- Morrison R. S. Suppression of basic fibroblast growth factor expression by antisense oligodeoxynucleotides inhibits the growth of transformed human astrocytes. J Biol Chem. 1991 Jan 15;266(2):728–734. [PubMed] [Google Scholar]
- Mourelatos Z., Adler H., Hirano A., Donnenfeld H., Gonatas J. O., Gonatas N. K. Fragmentation of the Golgi apparatus of motor neurons in amyotrophic lateral sclerosis revealed by organelle-specific antibodies. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4393–4395. doi: 10.1073/pnas.87.11.4393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mourelatos Z., Hirano A., Rosenquist A. C., Gonatas N. K. Fragmentation of the Golgi apparatus of motor neurons in amyotrophic lateral sclerosis (ALS). Clinical studies in ALS of Guam and experimental studies in deafferented neurons and in beta,beta'-iminodipropionitrile axonopathy. Am J Pathol. 1994 Jun;144(6):1288–1300. [PMC free article] [PubMed] [Google Scholar]
- Mourelatos Z., Yachnis A., Rorke L., Mikol J., Gonatas N. K. The Golgi apparatus of motor neurons in amyotrophic lateral sclerosis. Ann Neurol. 1993 Jun;33(6):608–615. doi: 10.1002/ana.410330609. [DOI] [PubMed] [Google Scholar]
- Perry G., Siedlak S. L., Richey P., Kawai M., Cras P., Kalaria R. N., Galloway P. G., Scardina J. M., Cordell B., Greenberg B. D. Association of heparan sulfate proteoglycan with the neurofibrillary tangles of Alzheimer's disease. J Neurosci. 1991 Nov;11(11):3679–3683. doi: 10.1523/JNEUROSCI.11-11-03679.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBBINS E., GONATAS N. K. HISTOCHEMICAL AND ULTRASTRUCTURAL STUDIES ON HELA CELL CULTURES EXPOSED TO SPINDLE INHIBITORS WITH SPECIAL REFERENCE TO THE INTERPHASE CELL. J Histochem Cytochem. 1964 Sep;12:704–711. doi: 10.1177/12.9.704. [DOI] [PubMed] [Google Scholar]
- Ray J., Gage F. H. Spinal cord neuroblasts proliferate in response to basic fibroblast growth factor. J Neurosci. 1994 Jun;14(6):3548–3564. doi: 10.1523/JNEUROSCI.14-06-03548.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regeur L., Jensen G. B., Pakkenberg H., Evans S. M., Pakkenberg B. No global neocortical nerve cell loss in brains from patients with senile dementia of Alzheimer's type. Neurobiol Aging. 1994 May-Jun;15(3):347–352. doi: 10.1016/0197-4580(94)90030-2. [DOI] [PubMed] [Google Scholar]
- Rifkin D. B., Moscatelli D. Recent developments in the cell biology of basic fibroblast growth factor. J Cell Biol. 1989 Jul;109(1):1–6. doi: 10.1083/jcb.109.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salehi A., Heyn S., Gonatas N. K., Swaab D. F. Decreased protein synthetic activity of the hypothalamic tuberomamillary nucleus in Alzheimer's disease as suggested by smaller Golgi apparatus. Neurosci Lett. 1995 Jun 23;193(1):29–32. doi: 10.1016/0304-3940(95)11659-k. [DOI] [PubMed] [Google Scholar]
- Salehi A., Lucassen P. J., Pool C. W., Gonatas N. K., Ravid R., Swaab D. F. Decreased neuronal activity in the nucleus basalis of Meynert in Alzheimer's disease as suggested by the size of the Golgi apparatus. Neuroscience. 1994 Apr;59(4):871–880. doi: 10.1016/0306-4522(94)90291-7. [DOI] [PubMed] [Google Scholar]
- Salehi A., Ravid R., Gonatas N. K., Swaab D. F. Decreased activity of hippocampal neurons in Alzheimer's disease is not related to the presence of neurofibrillary tangles. J Neuropathol Exp Neurol. 1995 Sep;54(5):704–709. doi: 10.1097/00005072-199509000-00013. [DOI] [PubMed] [Google Scholar]
- Salehi A., Van de Nes J. A., Hofman M. A., Gonatas N. K., Swaab D. F. Early cytoskeletal changes as shown by Alz-50 are not accompanied by decreased neuronal activity. Brain Res. 1995 Apr 24;678(1-2):28–39. doi: 10.1016/0006-8993(95)00138-g. [DOI] [PubMed] [Google Scholar]
- Sensenbrenner M. The neurotrophic activity of fibroblast growth factors. Prog Neurobiol. 1993 Dec;41(6):683–704. doi: 10.1016/0301-0082(93)90031-m. [DOI] [PubMed] [Google Scholar]
- Shi S. R., Key M. E., Kalra K. L. Antigen retrieval in formalin-fixed, paraffin-embedded tissues: an enhancement method for immunohistochemical staining based on microwave oven heating of tissue sections. J Histochem Cytochem. 1991 Jun;39(6):741–748. doi: 10.1177/39.6.1709656. [DOI] [PubMed] [Google Scholar]
- Siedlak S. L., Cras P., Kawai M., Richey P., Perry G. Basic fibroblast growth factor binding is a marker for extracellular neurofibrillary tangles in Alzheimer disease. J Histochem Cytochem. 1991 Jul;39(7):899–904. doi: 10.1177/39.7.1865106. [DOI] [PubMed] [Google Scholar]
- Smith M. A., Taneda S., Richey P. L., Miyata S., Yan S. D., Stern D., Sayre L. M., Monnier V. M., Perry G. Advanced Maillard reaction end products are associated with Alzheimer disease pathology. Proc Natl Acad Sci U S A. 1994 Jun 7;91(12):5710–5714. doi: 10.1073/pnas.91.12.5710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow A. D., Mar H., Nochlin D., Kimata K., Kato M., Suzuki S., Hassell J., Wight T. N. The presence of heparan sulfate proteoglycans in the neuritic plaques and congophilic angiopathy in Alzheimer's disease. Am J Pathol. 1988 Dec;133(3):456–463. [PMC free article] [PubMed] [Google Scholar]
- Snow A. D., Sekiguchi R. T., Nochlin D., Kalaria R. N., Kimata K. Heparan sulfate proteoglycan in diffuse plaques of hippocampus but not of cerebellum in Alzheimer's disease brain. Am J Pathol. 1994 Feb;144(2):337–347. [PMC free article] [PubMed] [Google Scholar]
- Snow A. D., Sekiguchi R., Nochlin D., Fraser P., Kimata K., Mizutani A., Arai M., Schreier W. A., Morgan D. G. An important role of heparan sulfate proteoglycan (Perlecan) in a model system for the deposition and persistence of fibrillar A beta-amyloid in rat brain. Neuron. 1994 Jan;12(1):219–234. doi: 10.1016/0896-6273(94)90165-1. [DOI] [PubMed] [Google Scholar]
- St George-Hyslop P. H., Haines J. L., Farrer L. A., Polinsky R., Van Broeckhoven C., Goate A., McLachlan D. R., Orr H., Bruni A. C., Sorbi S. Genetic linkage studies suggest that Alzheimer's disease is not a single homogeneous disorder. Nature. 1990 Sep 13;347(6289):194–197. doi: 10.1038/347194a0. [DOI] [PubMed] [Google Scholar]
- Steegmaier M., Levinovitz A., Isenmann S., Borges E., Lenter M., Kocher H. P., Kleuser B., Vestweber D. The E-selectin-ligand ESL-1 is a variant of a receptor for fibroblast growth factor. Nature. 1995 Feb 16;373(6515):615–620. doi: 10.1038/373615a0. [DOI] [PubMed] [Google Scholar]
- Stieber A., Mourelatos Z., Chen Y. J., Le Douarin N., Gonatas N. K. MG160, a membrane protein of the Golgi apparatus which is homologous to a fibroblast growth factor receptor and to a ligand for E-selectin, is found only in the Golgi apparatus and appears early in chicken embryo development. Exp Cell Res. 1995 Aug;219(2):562–570. doi: 10.1006/excr.1995.1265. [DOI] [PubMed] [Google Scholar]
- Stopa E. G., Gonzalez A. M., Chorsky R., Corona R. J., Alvarez J., Bird E. D., Baird A. Basic fibroblast growth factor in Alzheimer's disease. Biochem Biophys Res Commun. 1990 Sep 14;171(2):690–696. doi: 10.1016/0006-291x(90)91201-3. [DOI] [PubMed] [Google Scholar]
- Su J. H., Anderson A. J., Cummings B. J., Cotman C. W. Immunohistochemical evidence for apoptosis in Alzheimer's disease. Neuroreport. 1994 Dec 20;5(18):2529–2533. doi: 10.1097/00001756-199412000-00031. [DOI] [PubMed] [Google Scholar]
- Swaab D. F., Hofman M. A., Lucassen P. J., Salehi A., Uylings H. B. Neuronal atrophy, not cell death, is the main hallmark of Alzheimer's disease. Neurobiol Aging. 1994 May-Jun;15(3):369–380. doi: 10.1016/0197-4580(94)90037-x. [DOI] [PubMed] [Google Scholar]
- TERRY R. D., GONATAS N. K., WEISS M. ULTRASTRUCTURAL STUDIES IN ALZHEIMER'S PRESENILE DEMENTIA. Am J Pathol. 1964 Feb;44:269–297. [PMC free article] [PubMed] [Google Scholar]
- Tanzi R., Gaston S., Bush A., Romano D., Pettingell W., Peppercorn J., Paradis M., Gurubhagavatula S., Jenkins B., Wasco W. Genetic heterogeneity of gene defects responsible for familial Alzheimer disease. Genetica. 1993;91(1-3):255–263. doi: 10.1007/BF01436002. [DOI] [PubMed] [Google Scholar]
- Tascos N., Mourelatos Z., Gonatas N. K. On the significance and reproducibility of the fragmentation of the Golgi apparatus of motor neurons in human spinal cords. J Neuropathol Exp Neurol. 1995 May;54(3):331–338. doi: 10.1097/00005072-199505000-00006. [DOI] [PubMed] [Google Scholar]
- Tomlinson B. E., Blessed G., Roth M. Observations on the brains of demented old people. J Neurol Sci. 1970 Sep;11(3):205–242. doi: 10.1016/0022-510x(70)90063-8. [DOI] [PubMed] [Google Scholar]
- Torack R. M., Morris J. C. Mesolimbocortical dementia. A clinicopathologic case study of a putative disorder. Arch Neurol. 1986 Oct;43(10):1074–1078. doi: 10.1001/archneur.1986.00520100078018. [DOI] [PubMed] [Google Scholar]
- Turner J. R., Tartakoff A. M. The response of the Golgi complex to microtubule alterations: the roles of metabolic energy and membrane traffic in Golgi complex organization. J Cell Biol. 1989 Nov;109(5):2081–2088. doi: 10.1083/jcb.109.5.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuguchi T., Kohmura E., Yamada K., Wanaka A., Otsuki H., Sakaguchi T., Yamashita T., Tohyama M., Hayakawa T. Messenger RNA and protein expression of basic fibroblast growth factor receptor after cortical ablation. Brain Res Mol Brain Res. 1994 Aug;25(1-2):50–56. doi: 10.1016/0169-328x(94)90277-1. [DOI] [PubMed] [Google Scholar]
- Zagzag D., Miller D. C., Sato Y., Rifkin D. B., Burstein D. E. Immunohistochemical localization of basic fibroblast growth factor in astrocytomas. Cancer Res. 1990 Nov 15;50(22):7393–7398. [PubMed] [Google Scholar]