Abstract
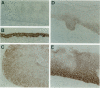
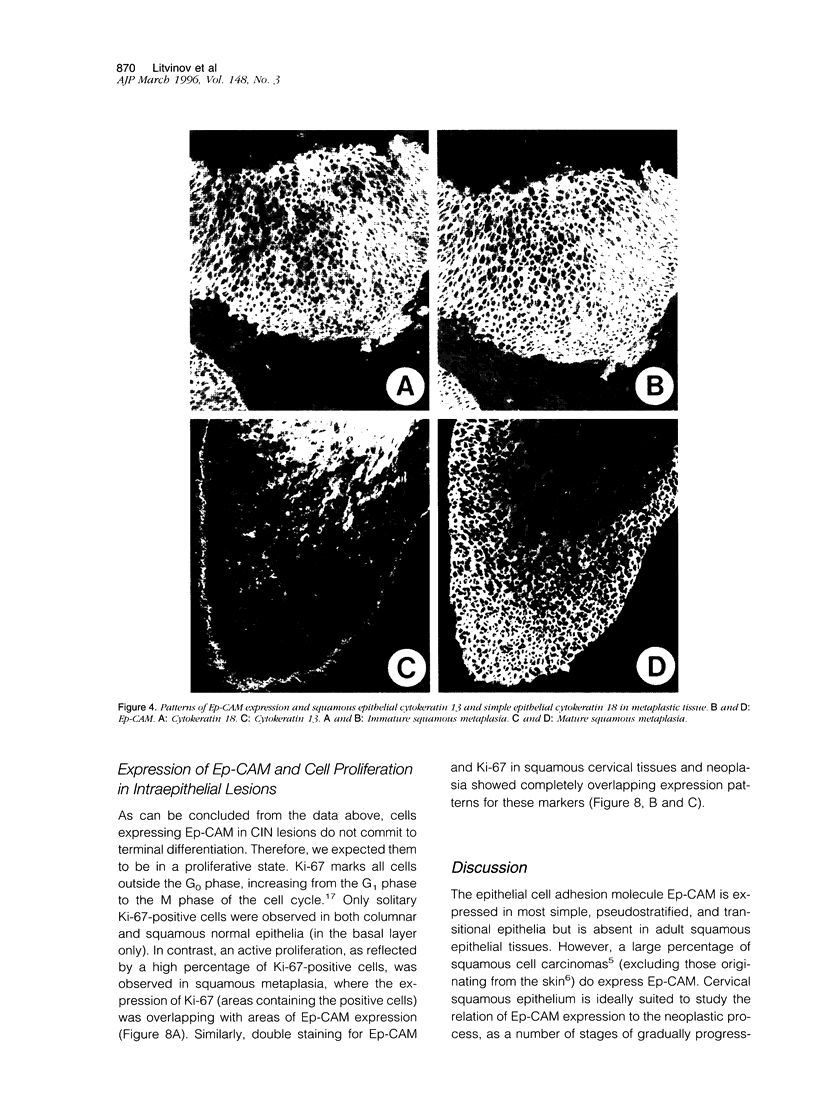
Ep-CAM, an epithelial adhesion molecule, is absent in normal squamous epithelia but can be detected in some squamous carcinomas. Using a panel of monoclonal antibodies to keratinocyte differentiation and proliferation markers, we investigated the association of EP-CAM expression with differentiation-related and/or neoplastic changes in cervical epithelium. Normal endocervical glandular epithelium (Both columnar and reserve cells) appeared strongly positive for EP-CAM, whereas ectocervical squamous epithelial cells did not express this molecule. Expression of Ep-CAM (in basal cells) was sometimes observed in morphologically normal ectocervical tissue but only in areas bordering cervical intraepithelial neoplasia (CIN) lesions. At the early stages of neoplasia the expression of Ep-CAM was regularly present in squamous epithelium, in general consistent with the areas of atypical, undifferentiated cells. Thus, in CIN grades I and II, the basal/suprabasal layers of the epithelia were positive, whereas in CIN grade III lesions, up to 100% of the cells over the whole thickness of the epithelium sometimes excluding the very upper layers, expressed Ep-CAM. A clear increase, not only in number of positive cells but also in levels of Ep-CAM expression (intensity) was observed during progression from CIN I to CIN III. Expression of Ep-CAM in ectocervical lesions did not coincide with a reappearance of the simple epithelium cytokeratins (CK8 and CK18). On the other hand, expression of Ep-CAM in atypical cells of CIN lesions correlated with the disappearance of CK13, which normally marks cells undergoing squamous differentiation. As was shown with Ki-67, a marker for proliferating cell populations, the areas of Ep-CAM expression were also the areas of enhanced proliferation. Cells expressing Ep-CAM did not express involucrin, a marker for cells committed to terminal differentiation. In the majority of both squamous and adenocarcinomas of the cervix a strong expression of Ep-CAM was observed, although some decrease in the expression (both the intensity and the number of positive cells), as compared with CIN III lesions, was observed in the areas of squamous differentiation. This study demonstrates that the expression of Ep-CAM in cervical squamous epithelium is associated with abnormal proliferation of cell populations that are not committed to terminal differentiation.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bosari S., Roncalli M., Viale G., Bossi P., Coggi G. p53 immunoreactivity in inflammatory and neoplastic diseases of the uterine cervix. J Pathol. 1993 Apr;169(4):425–430. doi: 10.1002/path.1711690407. [DOI] [PubMed] [Google Scholar]
- Brown D. C., Cole D., Gatter K. C., Mason D. Y. Carcinoma of the cervix uteri: an assessment of tumour proliferation using the monoclonal antibody Ki67. Br J Cancer. 1988 Feb;57(2):178–181. doi: 10.1038/bjc.1988.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdes J., Lemke H., Baisch H., Wacker H. H., Schwab U., Stein H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol. 1984 Oct;133(4):1710–1715. [PubMed] [Google Scholar]
- Hodivala K. J., Watt F. M. Evidence that cadherins play a role in the downregulation of integrin expression that occurs during keratinocyte terminal differentiation. J Cell Biol. 1994 Feb;124(4):589–600. doi: 10.1083/jcb.124.4.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotchin N. A., Gandarillas A., Watt F. M. Regulation of cell surface beta 1 integrin levels during keratinocyte terminal differentiation. J Cell Biol. 1995 Mar;128(6):1209–1219. doi: 10.1083/jcb.128.6.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes D. E., Rebello G., al-Nafussi A. Integrin expression in squamous neoplasia of the cervix. J Pathol. 1994 Jun;173(2):97–104. doi: 10.1002/path.1711730205. [DOI] [PubMed] [Google Scholar]
- Inki P., Stenbäck F., Grenman S., Jalkanen M. Immunohistochemical localization of syndecan-1 in normal and pathological human uterine cervix. J Pathol. 1994 Apr;172(4):349–355. doi: 10.1002/path.1711720410. [DOI] [PubMed] [Google Scholar]
- Jones P. H., Watt F. M. Separation of human epidermal stem cells from transit amplifying cells on the basis of differences in integrin function and expression. Cell. 1993 May 21;73(4):713–724. doi: 10.1016/0092-8674(93)90251-k. [DOI] [PubMed] [Google Scholar]
- Levy R., Czernobilsky B., Geiger B. Subtyping of epithelial cells of normal and metaplastic human uterine cervix, using polypeptide-specific cytokeratin antibodies. Differentiation. 1988 Dec;39(3):185–196. doi: 10.1111/j.1432-0436.1988.tb00093.x. [DOI] [PubMed] [Google Scholar]
- Linnenbach A. J., Seng B. A., Wu S., Robbins S., Scollon M., Pyrc J. J., Druck T., Huebner K. Retroposition in a family of carcinoma-associated antigen genes. Mol Cell Biol. 1993 Mar;13(3):1507–1515. doi: 10.1128/mcb.13.3.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litvinov S. V., Bakker H. A., Gourevitch M. M., Velders M. P., Warnaar S. O. Evidence for a role of the epithelial glycoprotein 40 (Ep-CAM) in epithelial cell-cell adhesion. Cell Adhes Commun. 1994 Oct;2(5):417–428. doi: 10.3109/15419069409004452. [DOI] [PubMed] [Google Scholar]
- Litvinov S. V., Velders M. P., Bakker H. A., Fleuren G. J., Warnaar S. O. Ep-CAM: a human epithelial antigen is a homophilic cell-cell adhesion molecule. J Cell Biol. 1994 Apr;125(2):437–446. doi: 10.1083/jcb.125.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair B. S., Pillai R. Oncogenesis of squamous carcinoma of the uterine cervix. Int J Gynecol Pathol. 1992;11(1):47–57. doi: 10.1097/00004347-199201000-00008. [DOI] [PubMed] [Google Scholar]
- Poumay Y., Boucher F., Leclercq-Smekens M., Degen A., Leloup R. Basal cell adhesion to a culture substratum controls the polarized spatial organization of human epidermal keratinocytes into proliferating basal and terminally differentiating suprabasal populations. Epithelial Cell Biol. 1993 Jan;2(1):7–16. [PubMed] [Google Scholar]
- Quak J. J., Van Dongen G., Brakkee J. G., Hayashida D. J., Balm A. J., Snow G. B., Meijer C. J. Production of a monoclonal antibody (K 931) to a squamous cell carcinoma associated antigen identified as the 17-1A antigen. Hybridoma. 1990 Aug;9(4):377–387. doi: 10.1089/hyb.1990.9.377. [DOI] [PubMed] [Google Scholar]
- Smedts F., Ramaekers F. C., Vooijs P. G. The dynamics of keratin expression in malignant transformation of cervical epithelium: a review. Obstet Gynecol. 1993 Sep;82(3):465–465. [PubMed] [Google Scholar]
- Smedts F., Ramaekers F., Troyanovsky S., Pruszczynski M., Link M., Lane B., Leigh I., Schijf C., Vooijs P. Keratin expression in cervical cancer. Am J Pathol. 1992 Aug;141(2):497–511. [PMC free article] [PubMed] [Google Scholar]
- Smedts F., Ramaekers F., Troyanovsky S., Pruszczynski M., Robben H., Lane B., Leigh I., Plantema F., Vooijs P. Basal-cell keratins in cervical reserve cells and a comparison to their expression in cervical intraepithelial neoplasia. Am J Pathol. 1992 Mar;140(3):601–612. [PMC free article] [PubMed] [Google Scholar]
- Tellechea O., Reis J. P., Domingues J. C., Baptista A. P. Monoclonal antibody Ber EP4 distinguishes basal-cell carcinoma from squamous-cell carcinoma of the skin. Am J Dermatopathol. 1993 Oct;15(5):452–455. doi: 10.1097/00000372-199310000-00007. [DOI] [PubMed] [Google Scholar]
- Tsubura A., Senzaki H., Sasaki M., Hilgers J., Morii S. Immunohistochemical demonstration of breast-derived and/or carcinoma-associated glycoproteins in normal skin appendages and their tumors. J Cutan Pathol. 1992 Feb;19(1):73–79. doi: 10.1111/j.1600-0560.1992.tb01562.x. [DOI] [PubMed] [Google Scholar]
- Tsutsumi K., Sun Q., Yasumoto S., Kikuchi K., Ohta Y., Pater A., Pater M. M. In vitro and in vivo analysis of cellular origin of cervical squamous metaplasia. Am J Pathol. 1993 Oct;143(4):1150–1158. [PMC free article] [PubMed] [Google Scholar]
- Velders M. P., Litvinov S. V., Warnaar S. O., Gorter A., Fleuren G. J., Zurawski V. R., Jr, Coney L. R. New chimeric anti-pancarcinoma monoclonal antibody with superior cytotoxicity-mediating potency. Cancer Res. 1994 Apr 1;54(7):1753–1759. [PubMed] [Google Scholar]
- Watt F. M. Involucrin and other markers of keratinocyte terminal differentiation. J Invest Dermatol. 1983 Jul;81(1 Suppl):100s–103s. doi: 10.1111/1523-1747.ep12540786. [DOI] [PubMed] [Google Scholar]
- Wheelock M. J., Jensen P. J. Regulation of keratinocyte intercellular junction organization and epidermal morphogenesis by E-cadherin. J Cell Biol. 1992 Apr;117(2):415–425. doi: 10.1083/jcb.117.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodworth C. D., Bowden P. E., Doniger J., Pirisi L., Barnes W., Lancaster W. D., DiPaolo J. A. Characterization of normal human exocervical epithelial cells immortalized in vitro by papillomavirus types 16 and 18 DNA. Cancer Res. 1988 Aug 15;48(16):4620–4628. [PubMed] [Google Scholar]
- Woodworth C. D., Waggoner S., Barnes W., Stoler M. H., DiPaolo J. A. Human cervical and foreskin epithelial cells immortalized by human papillomavirus DNAs exhibit dysplastic differentiation in vivo. Cancer Res. 1990 Jun 15;50(12):3709–3715. [PubMed] [Google Scholar]