Abstract
Aim
To search for patched homologue 1 (PTCH1) mutations in four families with basal cell nevus syndrome (BCNS).
Methods
Mutation analysis of PTCH1 in unrelated Japanese families affected with BCNS was carried out by direct sequencing.
Results
Six novel PTCH1 mutations, 833G→A in exon 6, 1415C→A and 1451G→T in exon 10, 2798delC in exon 17, 2918–2925dupAGTTCCCT in exon 18 and 3956C→A in exon 23, were identified.
Conclusions
Among the six PTCH1 mutations, two frameshift mutations (2798delC and 2918–2925dupAGTTCCCT) and one nonsense mutation (833G→A) are predicted to lead to premature termination of PTCH1 protein translation. Three simultaneous mutations, 1415C→A (A472D) and 1451G→T (G484V) in exon 10, and 3956G→A (R1319H) in exon 23, were found on one allele in only affected members in one family and none of them were found among 90 unrelated healthy Japanese. The three mutations on one chromosome may have resulted from errors in the recombinational repair process and this is the first report on the PTCH1 mutations due to such a mechanism.
Basal cell nevus syndrome (BCNS, OMIM *109400), known as Gorlin syndrome, is inherited in an autosomal dominant mode. This syndrome is characterised by multiple basal cell nevi, odontogenic keratocysts and skeletal anomalies.1 At the minimum, its prevalence is estimated to be 1 in 57 000.2
BCNS is caused by mutations in the patched homologue 1 (PTCH1) gene located at chromosome 9q22.3, the human homologue of the Drosophila segment polarity gene patched (Ptch).3,4PTCH1 encodes a transmembrane receptor protein for the secreted molecule, sonic hedgehog.5,6PTCH1 gene encompasses about 34 kb and consists of at least 23 exons, encodes 1447 amino acid proteins with a 12‐transmembrane domain, two extracellular loops and a putative sterol‐sensing domain.7,8,9 Bailey et al10 reported that missense mutations could abolish PTCH1 function, possibly by blocking protein maturation.
It has been reported that basal cell carcinoma occurs in >90% of patients with BCNS by the age of 40 years.11,12,13 Identification of mutations is very helpful for genetic counselling and clinical service in the BCNS family, because patients with mutations have a high risk for BCNS. At least 101 PTCH1 mutations have been reported so far in patients with BCNS.3,4,5,6,14,15,16,17,18,19,20,21,22,23
Here we report the results of a search for PTCH1 mutations in four unrelated families with BCNS.
Materials and methods
Families
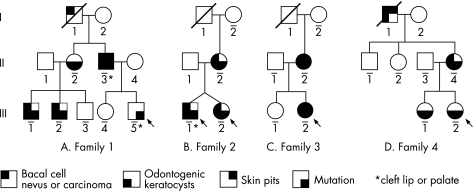
Four unrelated Japanese families, clients of Aichi‐Gakuin University, were diagnosed as having BCNS according to the criteria of Shanley et al.12 The four families had a total of 13 affected and 14 unaffected members (fig 1).
Figure 1 Four families with basal cell nevus syndrome. Arrow indicates proband. People who were analysed are indicated with a horizontal line below their individual symbol.
Family 1
The proband (fig 1A, III‐5) was a boy with cleft lip and palate. His father (II‐3) had a cleft lip, multiple basal cell nevi, odontogenic keratocysts, multiple skin pits on the palm and sole; a paternal elder sister (II‐2) and her two children (III‐1 and III‐2) had multiple basal cell nevi and odontogenic keratocysts, whereas the mother (II‐4) had no malformations. The paternal grandfather (I‐1) had died from skin cancer, according to the father, but the precise diagnosis was not known.
Family 2
The probands (fig 1B, III‐1 and III‐2) were 10‐year‐old twins, a girl and a boy with cleft lip and palate. Both the twins and their mother (II‐2) had typical BCNS, whereas the father (II‐2) was free from malformations on physical examination. The maternal grandparents (I‐1 and I‐2) were phenotypically normal.
Family 3
The proband (fig 1C, III‐2) was an 18‐year‐old girl with BCNS. Her mother (II‐2) also had typical malformations for BCNS, whereas the father (II‐1) was phenotypically normal. An elder sister (III‐1) of the proband and the maternal grandparents (I‐1 and I‐2) were all healthy.
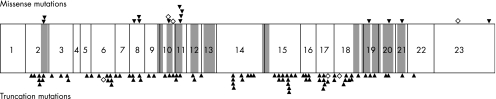
Figure 2 All reported patched homologue 1 (PTCH1) mutations causing basal cell nevus syndrome or basal cell carcinoma.3,4,5,6,14,15,16,17,18,19,20,21,22,23 Numbers in boxes indicate exons. Missense and nonsense mutations are depicted above and below the gene, respectively. Shaded regions, closed triangles and open diamonds indicate transmembrane domains, previously reported mutations and mutations observed in the present families, respectively.
Family 4
The proband (fig 1D, III‐2) was an 8‐year‐old girl with odontogenic keratocysts. Her mother (I‐4) had malformations typical for BCNS, an elder sister (III‐1) had odontogenic keratocysts and the maternal grandfather (I‐1) had BCNS. The father (II‐3) was phenotypically normal.
Mutation searches
Mutation analysis of PTCH1 (NM_000264) by direct sequencing was carried out in the four families. After written informed consent was obtained from the participants of these families, DNA was extracted from their peripheral blood cells by standard methods or from their fingernails by using ISOHAIR (Nippon Gene, Tokyo, Japan). For fingernail DNA, clipped fingernails were cut into small pieces with scissors and DNA was extracted according to the supplied manual of ISOHAIR. However, as DNA from some fingernail samples showed low quality and was a small amount, the method of extracting fingernail DNA was improved. We adopted a frozen‐sample crusher SK‐100 (Tokken, Kashiwa, Chiba, Japan) to crush the fingernail as finely as possible and extract DNA with ISOHAIR. After the DNA was extracted with ISOHAIR, it was dissolved in extraction buffer (10 mM TRIS–hydrochloric acid, pH 7.5; 100 mM EDTA, pH 8.0; 0.5% sodium dodecyl sulphate), treated with 50 μg/ml proteinase K at 55°C for 3 h, extracted with phenol or chloroform, and collected with ethanol and sodium acetate. All exons and exon–intron boundaries of PTCH1 were amplified by polymerase chain reaction (PCR) using our primer pairs designed from its genomic sequence. Amplification of exon 1 was carried out using two sets of pairs because exon 1 is too large as a single fragment for PCR. PCR was carried out in a mixture (10 μl) containing 5 ng genomic DNA, 1 μM each primer, 200 μM deoxynucleotide triphosphates, 0.3 unit TaKaRa ExTaq HS version (Takara, Kyoto, Japan) and 1× PCR buffer supplied by Takara. PCR conditions were as follows: initial incubation at 94°C for 2 min, followed by 35 cycles of denaturation at 94°C for 30 s, annealing at 60°C for 30 s, elongation at 72°C for 30 s and final elongation at 72°C for 7 min. PCR products were treated with ExoSAP‐IT (Amersham Biosciences, Piscataway, New Jersey, USA) following the instruction manual supplied by the company, and sequenced directly using BigDye Sequencing Kit V.3.1 (Applied Biosystems, Foster City, California, USA). Sequenced samples were purified using SephadexG50 (Amersham Biosciences) and run on an automated sequencer Model 3100 (Applied Biosystems). Sequence electropherograms were aligned and analysed using the Auto Assembler software V.2.1 (Applied Biosystems). We carried out sequencing in both directions and repeated it two times to confirm mutations independently.
Results
All affected members in family 1 had a 1‐base deletion at nt 2798 (2798delC) in exon 17. This deletion is predicted to lead to frameshift with 28 amino acids after codon 933, create a premature stop codon at nt 961 (934fsX961) and cause the loss of 487 C‐terminal amino acids. Similarly, in the proband of family 2 and his mother, an 8‐nucleotide duplication at nt 2925−2926 (2918−2925dupAGTTCCCT) in exon 18 was found. The duplication leads to frameshift with substitution of 15 amino acids after codon 976, introduces a premature stop codon at nt 991 (977fsX991) and causes the loss of 457 C‐terminal amino acids. In the proband of family 3 and her mother, a nonsense mutation at nt 833 (833G→A) in exon 6 was found. The mutation leads to premature termination codon (W278X) and loss of the 1170 C‐terminal amino acids. None of the mutations were observed among the 90 unrelated healthy Japanese.
In all the affected members (the proband, her elder sister and mother) in family 4, three simultaneous nucleotide substitutions leading to amino acid replacements were found—that is, 1415C→A (A472D) and 1451G→T (G484V) in exon 10 and 3956G→A (R1319H) in exon 23. Neither mutation was observed in the father or in unaffected family members, as well as among the 90 unrelated healthy Japanese.
Discussion
The mutation search showed six novel PTCH1 mutations in the four families (table 1).
Table 1 Six mutations in patched homologue 1 (PTCH1) found in four families with basal cell nevus syndrome.
| Exon | Mutation | Nucleotide change | Amino acid change | Family no |
|---|---|---|---|---|
| 6 | Nonsense | 833G→A | W278X | 3 |
| 10 | Missense | 1415C→A | A472D | 4 |
| 10 | Missense | 1451G→T | G484V | 4 |
| 17 | Deletion | 2798delC | 934fsX961 | 1 |
| 18 | Duplication | 2918–2925dup(AGTTCCCT) | 977fsX991 | 2 |
| 23 | Missense | 3956G→A | R1319H | 4 |
Mutations observed in family 4 merit comment. These mutations could be errors in the recombination repair process rather than three independent single‐nucleotide substitutions, because they are all located on the same allele. Two (A472D and G484V) of the three are located around the third transmembrane domains. The other (R1319H) is in the last intercellular C‐terminal domain of PTCH1; the C‐terminal domain was recently identified as an important regulatory region essential for proper signalling of sonic hedgehog or PTCH1.24 On the other hand, according to the Prediction of Transmembrane Regions and Orientation programme (http://www.ch.embnet.org/), it is predicted that A472D changes the length of the transmembrane region of PTCH1, but G484V never alters the transmembrane topology dramatically. These findings favour 1415C→A or 3956G→A as the causative mutation for BCNS in the family.
Most (around 90%) reported PTCH1 mutations in BCNS were nonsense, splice site, insertion, deletion or duplication mutations leading to frameshift, and each of them was predicted to generate a truncated protein, although there were a few missense mutations. These mutations were located in most exons and spread all over the PTCH1 gene, indicating the absence of a mutational hotspot (fig 2). Three nucleotide changes observed in families 1–3 result in protein truncation corresponding to the previous findings. Although three missense mutations were simultaneously observed on one chromosome in family 4, it remains uncertain which mutation actually affects protein function and is pathogenic. Nevertheless, the identification of mutations is very helpful for genetic counselling and clinical service in the family, because patients with mutations have a high risk for BCNS.
Acknowledgements
We thank the families for their contribution to this study, as well as Drs T Kawai, N Natsume, H Furukawa and J Machida for referring the patients. We also thank W Yoshida, K Miyazaki, Y Noguchi and N Yanai for their technical assistance. This study was supported partly by Grants‐in‐Aid for Scientific Research on Priority Area “Medical Genome Science” for K‐iY; Category S (no 13854024) for NN; and by Grant for “AGU High‐Tech Research Center Project” for private universities: Matching Fund Subsidy, 2003−2007, from the Ministry of Education, Culture, Sports, Science and Technology.
Abbreviations
BCNS - basal cell nevus syndrome
PCR - polymerase chain reaction
PTCH1 - patched homologue 1
Footnotes
Competing interests: None declared.
References
- 1.Gorlin R J. Nevoid basal‐cell carcinoma syndrome. Medicine 19876698–113. [DOI] [PubMed] [Google Scholar]
- 2.Farndon P A, Del Mastro R G, Evans D G R.et al Location of gene for Gorlin syndrome. Lancet 1992339581–582. [DOI] [PubMed] [Google Scholar]
- 3.Hahn H, Wicking C, Zaphiropoulos P G.et al Mutations of the human homolog of Drosophila patched in the nevoid basal cell carcinoma syndrome. Cell 199685841–851. [DOI] [PubMed] [Google Scholar]
- 4.Johnson R L, Rothman A L, Xie J.et al Human homolog of patched, a candidate gene for the basal cell nevus syndrome. Science 19962721668–1671. [DOI] [PubMed] [Google Scholar]
- 5.Unden A B, Holmberg E, Lundh‐Rozell B.et al Mutations in the human homologue of Drosophila patched (PTCH) in basal cell carcinomas and the Gorlin syndrome: different in vivo mechanisms of PTCH inactivation. Cancer Res 1996564562–4565. [PubMed] [Google Scholar]
- 6.Tate G, Li M, Suzuki T.et al A new germline mutation of the PTCH gene in a Japanese patient with nevoid basal cell carcinoma syndrome associated with meningioma. Jpn J Clin Oncol 20033347–50. [DOI] [PubMed] [Google Scholar]
- 7.Stone D M, Hynes M, Armanini M.et al The tumour‐suppressor gene patched encodes a candidate receptor for Sonic hedgehog. Nature 1996384129–134. [DOI] [PubMed] [Google Scholar]
- 8.Marigo V, Davey R A, Zuo Y.et al Biochemical evidence that patched is the Hedgehog receptor. Nature 1996384176–179. [DOI] [PubMed] [Google Scholar]
- 9.Wicking C, Shanley S, Smyth I.et al Most germ‐line mutations in the nevoid basal cell carcinoma syndrome lead to a premature termination of the PATCHED protein, and no genotype‐phenotype correlations are evident. Am J Hum Genet 19976021–26. [PMC free article] [PubMed] [Google Scholar]
- 10.Bailey E C, Zhou L, Johnson R L. Several human PATCHED1 mutations block protein maturation. Cancer Res 2003631636–1638. [PubMed] [Google Scholar]
- 11.Evans D G R, Ladusans E J, Rimmer S.et al Complications of the naevoid basal cell carcinoma syndrome: results of a population based study. J Med Genet 199330460–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shanley S, Ratcliffe J, Hockey A.et al Nevoid basal cell carcinoma syndrome: review of 118 affected individuals. Am J Med Genet 199450282–290. [DOI] [PubMed] [Google Scholar]
- 13.Kimonis V E, Goldstein A M, Pastakia B.et al Clinical manifestations in 105 persons with nevoid basal cell carcinoma syndrome. Am J Med Genet 199769299–308. [PubMed] [Google Scholar]
- 14.Chidambaram A, Goldstein A M, Gailani M R.et al Mutations in the human homologue of the Drosophila patched gene in Caucasian and African‐American nevoid basal cell carcinoma syndrome patients. Cancer Res 1996564599–4601. [PubMed] [Google Scholar]
- 15.Wicking C, Gillies S, Smyth I.et al De novo mutations of the patched gene in nevoid basal cell carcinoma syndrome help to define the clinical phenotype. Am J Med Genet 199773304–307. [PubMed] [Google Scholar]
- 16.Lench N J, Telford E A, High A S.et al Characterisation of human patched germ line mutations in naevoid basal cell carcinoma syndrome. Hum Genet 1997100497–502. [DOI] [PubMed] [Google Scholar]
- 17.Minami M, Urano Y, Ishigami T.et al Germline mutations of the PTCH gene in Japanese patients with nevoid basal cell carcinoma syndrome. J Dermatol Sci 20012721–26. [DOI] [PubMed] [Google Scholar]
- 18.Barreto D C, Bale A E, De Marco L.et al Immunolocalization of PTCH protein in odontogenic cysts and tumors. J Dent Res 200281757–760. [DOI] [PubMed] [Google Scholar]
- 19.Lam C W, Leung C Y, Lee K C.et al Novel mutations in the PATCHED gene in basal cell nevus syndrome. Mol Genet Metab 20027657–61. [DOI] [PubMed] [Google Scholar]
- 20.Fujii K, Kohno Y, Sugita K.et al Mutations in the human homologue of Drosophila patched in Japanese nevoid basal cell carcinoma syndrome patients. Hum Mutat 200321451–452. [DOI] [PubMed] [Google Scholar]
- 21.Fujii K, Miyashita T, Omata T.et al Gorlin syndrome with ulcerative colitis in a Japanese girl. Am J Med Genet 200312165–68. [DOI] [PubMed] [Google Scholar]
- 22.Pastorino L, Cusano R, Nasti S.et al Molecular characterization of Italian nevoid basal cell carcinoma syndrome patients. Hum Mutat 200525322–323. [DOI] [PubMed] [Google Scholar]
- 23.Tanioka M, Takahashi K, Kawabata T.et al Germline mutations of the PTCH gene in Japanese patients with nevoid basal cell carcinoma syndrome. Arch Dermatol Res 2005296303–308. [DOI] [PubMed] [Google Scholar]
- 24.Johnson R L, Milenkovic L, Scott M P. In vivo functions of the patched protein: requirement of the C‐terminus for target gene inactivation but not hedgehog sequestration. Mol Cell 20006467–478. [DOI] [PubMed] [Google Scholar]