Abstract
A case of pancreatic polypeptide cell hyperplasia in a 76‐year‐old man who presented with subacute bowel pseudo‐obstruction is reported. A computed tomography scan incidentally showed a pancreatic head lesion that was resected by pancreaticoduodenectomy. Histological examination showed expansion of the endocrine pancreas with increased numbers of pancreatic polypeptide cells in irregularly enlarged islets, ragged endocrine cell clusters, ductulo‐insular complexes and microadenomas. The clinicopathological features of this rare and poorly understood condition are discussed.
Pancreatic polypeptide is produced by pancreatic polypeptide cells, which represent about 10% of the islets of Langerhans.1 It is released after ingestion of food and has inhibitory postprandial effects on gastric emptying, intestinal motility, exocrine pancreatic secretion, hepatic glucose production and gallbladder contraction.2 It is also associated with body weight regulation by influencing food intake and energy metabolism.3
Little is known about pancreatic polypeptide and disease. Published reports of pancreatic polypeptide cell hyperplasia (PPCH) will be reviewed and compared with the present case.
Case report
A 76‐year‐old man presented with epigastric pain, constipation and signs of subacute bowel obstruction. His medical history was unremarkable; in particular there was no personal or family history suggestive of multiple endocrine neoplasia type 1 (MEN I) syndrome. Although abdominal imaging failed to show any cause of obstruction, a computed tomography scan showed a heterogeneous lesion in the pancreatic head, a dilated biliary tree and pancreatic duct, and an atrophic pancreas. Whipple's resection was uneventful. Slicing of the pancreatic head (4×2.5×1.5 cm) showed firm, lobulated parenchyma without evidence of a circumscribed lesion. The common bile duct was slightly dilated. The duodenum and ampulla were normal.
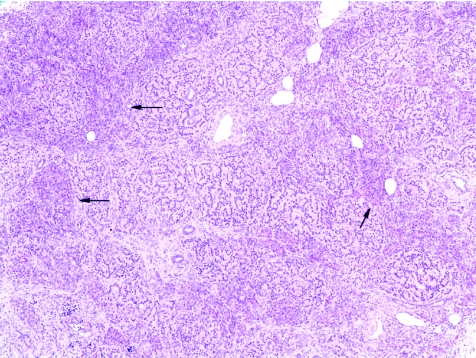
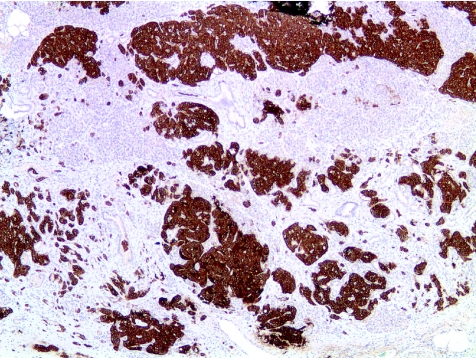
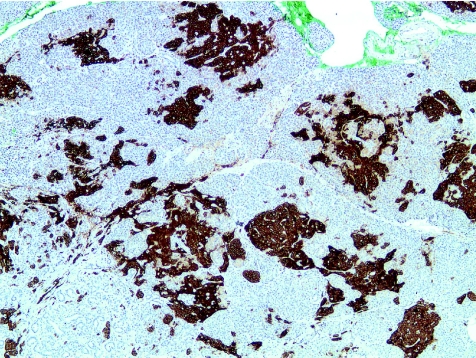
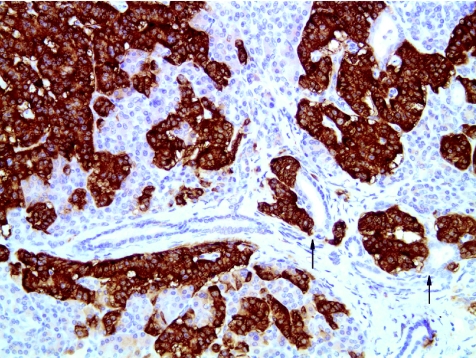
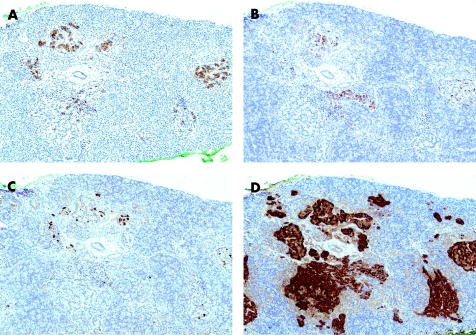
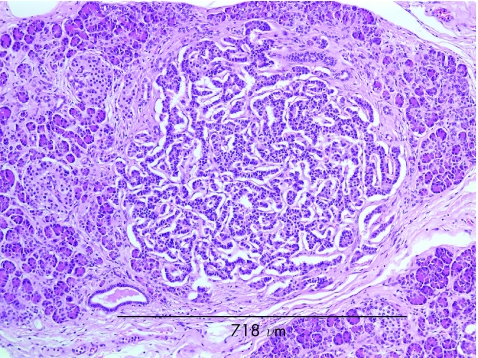
Histological examination showed diffuse islet cell hyperplasia and ductulo‐insular complex (DIC) formation (ie, endocrine cells budding from duct epithelium). The islets were increased in number and size, with irregular outlines, replacing parts of the otherwise normal acinar lobules. They showed an abnormal trabecular and pseudoglandular architecture (fig 1). Synaptophysin immunostaining confirmed these findings (fig 2). Whereas most islets, irregular endocrine cell clusters and DICs harboured α, β, δ and pancreatic polypeptide cells, the quantitative and spatial relationship of these cell populations was disrupted in some of the enlarged islets and cell clusters (so‐called islet cell dysplasia). Immunostaining for pancreatic polypeptide showed that between 10% and >50% of all endocrine cells, both in the enlarged islets and DICs, produced pancreatic polypeptide (figs 3, 4), a finding that is markedly higher than even that in the pancreatic polypeptide cell‐rich ventral portion of the normal pancreas (<10%). The normal spatial distribution of islet cells was lost as pancreatic polypeptide cells occupied both a central and a peripheral position in the enlarged islets (fig 5). Mitotic figures were not identified, and Ki‐67 immunostaining showed few positive nuclei (<1%) in the endocrine compartment.
Figure 1 Expansion of the endocrine compartment is characterised by irregular and coalescent endocrine cell clusters with trabecular growth pattern. Arrows indicate residual pancreatic acini (haematoxylin and eosin ×50).
Figure 2 Immunostaining for synaptophysin confirms the expansion of the endocrine compartment (×50).
Figure 3 Immunostaining for pancreatic polypeptide (PP) shows that a large proportion of the expanded endocrine cell population expresses PP (×50).
Figure 4 Ductulo‐insular complex, that is endocrine cell clusters budding from small ducts (arrows), are present (immunostaining for synaptophysin ×200).
Figure 5 Immunostaining shows loss of the normal spatial and quantitative relationship of islet cells producing insulin (A), glucagon (B), somatostatin (C) and pancreatic polypeptide (D); (×100).
A few of the endocrine cell clusters were distinct from the others by their larger size (<0.8 mm), smooth contour, sharp delineation from adjoining parenchyma and monohormonal secretion of pancreatic polypeptide (fig 6), findings that meet the diagnostic criteria of endocrine microadenomas. The endocrine cell population comprised in a focus of ectopic pancreatic tissue in the ampulla and surrounding duodenal wall, showed changes similar to those in the pancreatic head.
Figure 6 A relatively large (0.7 mm) compact and well‐delineated endocrine cell cluster, which showed monohormonal pancreatic polypeptide expression on immunostaining (haematoxylin and eosin ×50).
These findings were reported as PPCH, with the possible presence of pancreatic polypeptide cell microadenomas. Unfortunately, preoperative serum levels of pancreatic polypeptide were not measured.
Discussion
Proliferative changes associated with pancreatic polypeptide cells include three conditions:
tumours exclusively or predominantly composed of pancreatic polypeptide cells (“PPomas”),
pancreatic endocrine tumours (PETs) in which pancreatic polypeptide cells are part of a mixed tumour cell population (mixed PETs) and
PPCH.
The condition described as PPCH is surrounded by confusion due to inconsistencies in histological criteria and terminology. This may be related to the heterogeneous distribution of pancreatic polypeptide cells throughout the pancreas and the ensuing sampling problems. Pancreatic polypeptide cells are mostly confined to the pancreatic region of ventral embryological origin,1 whereas islets of the tail, body and other parts of the pancreatic head contain only a small number of these cells. Within the islets, pancreatic polypeptide cells often take up a peripheral position, mixed with α and δ cells.
In this case, pancreatic histology was characterised by marked expansion of the endocrine compartment in the form of irregularly enlarged islets or ragged cell clusters and foci of DICs. The normal spatial and quantitative relationship between the four islet cell populations was disrupted (so‐called islet cell dysplasia) and characterised by a marked increase in pancreatic polypeptide cells. Interestingly, similar pancreatic polypeptide cell changes were present in a small focus of ectopic pancreatic tissue in the periampullary duodenal wall, suggesting that the process may be diffuse, affecting the entire pancreas.
The clinical presentation of pancreatic polypeptide hypersecretion is usually silent. In this case it could be speculated that intestinal (pseudo‐) obstruction was effected by the inhibitory action of pancreatic polypeptide on the upper gastrointestinal tract. Of the seven published cases of PPCH (table 1),4,5,6,7 three were associated with and assumed to be the cause of watery diarrhoea syndrome (Verner–Morrison syndrome).4,5,6 This assumption was not supported by other observations, as highly increased pancreatic polypeptide levels were found in the plasma without concomitant diarrhoea or achlorhydria. PPCH has also been reported in three patients with sporadic pancreatic or duodenal gastrinoma.7 In one of these patients, PPCH was seen 15 years after normalisation of gastrin levels by removal of a pancreatic gastrinoma, a finding arguing against a trophic role for hypergastrinaemia in the development of PPCH. Initial studies reported diffuse pancreatic polypeptide cell changes in the context of MEN I syndrome; however, subsequent large MEN I series identified microadenomatosis (ie, multiple well‐circumscribed monohormonal tumours) as the most distinctive feature, while PPCH did not belong to the spectrum of lesions.8 Hence, the association of PPCH seems to be limited to sporadic gastrinoma (and possibly other sporadic PETs), whereby the relationship between the two lesions remains unclear.
Table 1 Clinicopathological details of case reports of PPCH in the literature.
| Reference | Age | Sex | Clinical details | Preoperative PP serum | Imaging | Specimen | Histology of pancreas |
|---|---|---|---|---|---|---|---|
| Tomita et al4 | 62 | F | Gastric cancer | Mildly increased | Normal pancreas | Distal subtotal pancreatectomy | DICs and islets with increased numbers of PP cells |
| 70 | F | Watery diarrhoea | N/A | CT: 4×5 cm mass in HOP | Whipple's | Large and irregular islets and DICs with predominantly PP cells | |
| Farley et al (case 2)5 | 66 | M | Diarrhoea | Strongly increased | Intra‐operative USS: 2 cm lesion in HOP | Whipple's | Large and irregular islets and DICs with predominantly PP cells |
| Martella et al7 | 70 | F | ZE syndrome, gastrinoma in HOP resected at age 56 | N/A | N/A | Autopsy | Increased number of PP cells arranged in irregular clusters and adenomatous foci in lobules and associated with ducts |
| 50 | F | ZE syndrome, duodenal gastrinoma; granulosa cell tumour; metastasising breast cancer | N/A | N/A | Autopsy | Increased number of PP cells arranged in irregular clusters in lobules and associated with ducts | |
| 54 | F | Diarrhoea, ZE syndrome, metastasising duodenal gastrinoma | Increased in 1/4 samples over 4 consecutive years | N/A | Whipple's | Increased number of PP cells arranged in irregular clusters and adenomatous foci in lobules and associated with ducts | |
| Pasieka et al6 | 37 | F | Watery diarrhoea | N/A | Normal pancreas | Distal pancreatectomy | Increase in both number and size of islets; increased PP cells in islets and along ducts |
CT, computed tomography; DIC, ductulo‐insular complex; HOP, head of pancreas; N/A, not assessed; PP, pancreatic polypeptide; PPCH, pancreatic polypeptide cell hyperplasia; USS, ultrasonography; ZE, Zollinger–Ellison.
The morphological changes associated with the pancreatic polypeptide cell population in our case are remarkably similar to those of β cells in persistent hyperinsulinaemic hypoglycaemia, a rare condition in neonates and also in adults.9 Interestingly, similar histological changes affecting the α cells have recently been described,10 indicating that functional and morphological abnormalities may affect all three cell types. With the genetic defects underlying persistent hyperinsulinaemic hypoglycaemia being identified,11 abnormalities of genes controlling pancreatic polypeptide release could in a parallel way lead to similar histological changes. Although diffuse hyperplasia characterises the adaptive process of the affected β, α or pancreatic polypeptide cell population, the presence of a few microadenomas in this case suggests that neoplastic transformation may occur if the underlying cause is longstanding and unabated. As the prognosis of PPCH is unknown, long‐term follow‐up is recommended.
Abbreviations
DIC - ductulo‐insular complex
MEN I - multiple endocrine neoplasia type 1
PET - pancreatic endocrine tumour
PPCH - pancreatic polypeptide cell hyperplasia
Footnotes
Competing interests: None.
References
- 1.Bommer G, Friedl U, Heitz P U.et al Pancreatic PP cell distribution and hyperplasia: immunocytochemical morphology in the normal human pancreas, in chronic pancreatitis and pancreatic carcinoma. Virchows Arch A 1980387319–331. [DOI] [PubMed] [Google Scholar]
- 2.Miller L J. Gastrointestinal hormones and receptors. In: Yamada T, Alpens DH, Laine L, Owyang G, Powel DW, eds. Textbook of gastroenterology. New York: Lippincott, 199935–66.
- 3.Asakawa A, Inui A, Yuzuriha H.et al Characterization of the effects of pancreatic polypeptide in the regulation of energy balance. Gastroenterology 20031241325–1336. [DOI] [PubMed] [Google Scholar]
- 4.Tomita T, Kimmel J R, Friesen S R.et al Pancreatic polypeptide cell hyperplasia with and without watery diarrhea syndrome. J Surg Oncol 19801411–20. [DOI] [PubMed] [Google Scholar]
- 5.Farley D R, van Heerden J A, Myers J L. Adult pancreatic nesidioblastosis. Unusual presentations of a rare entity. Arch Surg 1994129329–332. [DOI] [PubMed] [Google Scholar]
- 6.Pasieka J, Hershfield N. Pancreatic polypeptide hyperplasia causing watery diarrhea syndrome—a case report. Can J Surg 19994255–59. [PMC free article] [PubMed] [Google Scholar]
- 7.Martella E M, Ferraro G, Azzoni C.et al Pancreatic‐polypeptide cell hyperplasia associated with pancreatic or duodenal gastrinomas. Hum Pathol 199728149–153. [DOI] [PubMed] [Google Scholar]
- 8.Klöppel G, Willemer S, Stamm B.et al Pancreatic lesions and hormonal profile of pancreatic tumors in multiple endocrine neoplasia type I. Cancer 1986571824–1832. [DOI] [PubMed] [Google Scholar]
- 9.Klöppel G, Reinecke‐Lüthge A, Koschorek F. Focal and diffuse beta cell changes in persistent hyperinsulinemic hypoglycemia of infancy. Endocr Pathol 199910299–304. [DOI] [PubMed] [Google Scholar]
- 10.Martignoni M E, Kated H, Stiegler M.et al Nesidioblastosis with glucagon‐reactive islet cell hyperplasia—a case report. Pancreas 200326402–407. [DOI] [PubMed] [Google Scholar]
- 11.Meissner T, Beinbrech B, Mayatepek E. Congenital hyperinsulinism: molecular basis of a heterogeneous disease. Hum Mutat 199913351–361. [DOI] [PubMed] [Google Scholar]