Abstract
Aim
To compare cell phenotypes displayed by cholangiocarcinomas and adjacent bile duct lesions in patients from an area endemic in liver‐fluke infestation and those with sporadic cholangiocarcinoma.
Methods
65 fluke‐associated and 47 sporadic cholangiocarcinomas and 6 normal livers were studied. Serial paraffin‐wax sections were stained immunohistochemically with monoclonal antibodies characterising a Brunner or pyloric gland metaplasia cell phenotype (antigens D10 and 1F6), intestinal goblet cells (antigen 17NM), gastric foveolar apomucin (MUC5AC), a gastrointestinal epithelium cytokeratin (CK20) and the p53 protein.
Results
60% of the 112 cholangiocarcinomas expressed antigen D10, 68% MUC5AC, 33% antigen 17NM and 20% CK20; 37% showed overexpression of p53. When present together in a cholangiocarcinoma, cancer cells expressing D10 were distinct from those displaying 17NM or MUC5AC. Many more fluke‐associated cholangiocarcinomas than sporadic cholangiocarcinomas displayed 17NM and p53 expression. Most cases of hyperplastic and dysplastic biliary epithelium expressed D10 strongly. Pyloric gland metaplasia and peribiliary glands displayed D10 and 1F6, with peribiliary gland hyperplasia more evident in the livers with fluke‐associated cholangiocarcinoma; goblet cells in intestinal metaplasia stained for 17NM. No notable association of expression between any two antigens (including p53) was found in the cancers.
Conclusions
Most cases of dysplastic biliary epithelium and cholangiocarcinoma display a Brunner or pyloric gland cell phenotype and a gastric foveolar cell phenotype. The expression of D10 in hyperplastic and dysplastic epithelium and in cholangiocarcinoma is consistent with a dysplasia–carcinoma sequence. Many more fluke‐associated cholangiocarcinomas than sporadic cholangiocarcinoma display an intestinal goblet cell phenotype and overexpress p53, indicating differences in the aetiopathology of the cancers in the two groups of patients.
North East Thailand has the highest incidence of cholangiocarcinoma in the world.1 Infestation with the liver fluke Opisthorchis viverrini is endemic in the region, and the incidence of cholangiocarcinoma is at least 12 times higher than in other parts of Thailand, where fluke infestation is not endemic.2 Studies have confirmed an appreciable association between O viverrini infestation and cholangiocarcinoma,1,3 with at least two thirds of the cancers in northeast Thailand being attributed to O viverrini infestation.4 By contrast, Australia is among the countries with the lowest age‐standardised incidence rates for cholangiocarcinoma.5 Known aetiological factors for sporadic cholangiocarcinoma in Western countries include anabolic steroid treatment and Thorotrast administration, with congenital anomalies of the biliary tree, primary sclerosing cholangitis and inflammatory bowel disease also contributing to the risk.6
Tumours generally retain the phenotype of their tissue of origin, but may also inappropriately express components normally found only in another tissue. Bile duct epithelium does not usually express MUC6 (a mucin secreted by gastric mucous neck cells, pyloric glands, Brunner glands and peribiliary glands), MUC5AC (a major secretory mucin of gastric foveolar epithelium) or CK20 (cytokeratin 20—a cytokeratin specific to gastrointestinal epithelium), but one or both of these mucins and CK20 may be expressed by cholangiocarcinoma.7,8 The p53 tumour suppressor protein is also expressed by some cholangiocarcinomas.9
In this investigation, we used monoclonal antibodies to five gastrointestinal‐specific antigens and to the p53 protein to compare the cell phenotypes displayed by cholangiocarcinomas and adjacent bile duct lesions in patients with fluke‐associated and sporadic cholangiocarcinomas.
Materials and methods
Patients and tissue samples
Archival paraffin‐wax‐embedded tissue from surgical specimens resected at the Khon Kaen University Hospital, Thailand, during the period 1993–96 were used. These included 27 hepatic lobectomies with intrahepatic cholangiocarcinoma and 38 with extrahepatic cholangiocarcinoma. Nineteen of the extrahepatic cancers were hilar, 11 primarily at the common hepatic duct and 8 at the common bile duct. Ages of the 44 male patients ranged from 25 to 80 years (mean 52, median 53 years), and those of the 21 female patients from 42 to 75 years (mean 56, median 54 years).
Archival blocks of paraffin wax from 47 surgical specimens were also obtained from The Royal Melbourne Hospital, Victoria, and The Princess Alexandra Hospital, Queensland. These included 13 intrahepatic and 34 extrahepatic cholangiocarcinomas. Of the extrahepatic cancers, 13 were hilar, 9 were associated with the common hepatic duct, 8 with the common bile duct and the location of 4 was not precisely known. Patients included 25 men aged 41–74 years (mean 61, median 64 years) and 22 women aged 30–80 years (mean 58, median 59 years). Six livers obtained from the Organ Donor Registry of the Royal Melbourne Hospital that were unsuitable for organ transplantation, served as normal controls. These tissues were collected as approved by The Royal Melbourne Hospital Clinical Research and Ethics Committee.
Antigens studied
Preparation of the monoclonal antibodies identifying antigens D10, 1F6 and 17NM and the tissue distributions of these three antigens have been reported elsewhere.10,11,12 The epitope recognised on antigen D10 has been highly conserved throughout the evolution of terrestrial vertebrates.13 Antigen 17NM is normally restricted to the mucous vacuole of intestinal goblet cells and the goblet cells of intestinal metaplasia.12,14 Expression of the human gastric mucin MUC5AC,15 CK20,16 and p5317 were also investigated.
Immunohistochemistry
Microwave heating18 was used to enhance staining of antigens 17NM, MUC5AC, CK20 and p53. Sections were stained by a four‐stage procedure as previously described,10 with 3‐3′‐diaminobenzidine as chromogen. Tris buffer containing 10% fetal calf serum was used in place of the primary antibody as a negative control. The monoclonal antibody to MUC5AC (clone 45M1, Novocastra Laboratories, Newcastle upon Tyne, UK) was used at a dilution of 1/50, that to CK20 (clone KS20.8, Dakocytomation Pty. Ltd., Botany, Australia) at a dilution of 1/50 and that to p53 (clone DO‐7, Novocastra Laboratories) at a dilution of 1/100. After immunohistochemical staining, sections were stained with Alcian blue (pH 2.5) for acid mucins,19 and with haematoxylin.
For double labelling, sections were first stained for antigen D10, treated with glycine buffer, pH 2.5, for 2 min, washed in water, heated by microwave and then stained for the second antigen by using the NovaRED substrate (Vector Laboratories, Burlingame, California, USA) as chromogen.
Proportion of cells expressing antigen
The proportion of cancer cells stained was subjectively recorded as −, no cells stained; 1+, occasional cells stained; 2+, numerous cells stained; 3+, most cells stained. For p53, strong staining of most cancer cell nuclei was regarded as positive, and cholangiocarcinoma with only focally positive cells as negative.
Statistical analysis
The significance of differences in the number of cancers staining for antigen in different groups of patients was determined using the χ2 test with Yates' correction.20
Results
Antigen expression in normal bile duct epithelium and peribiliary glands
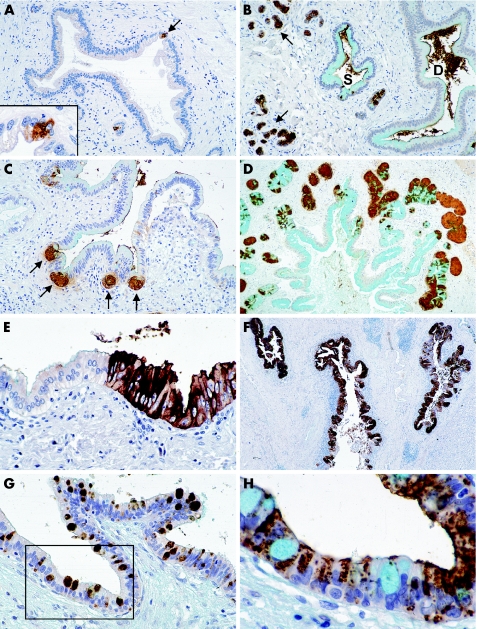
Occasional microscopic foci of D10‐positive epithelial cells were seen in some large bile ducts in four of the control livers (fig 1A), and a limited focal expression of MUC5AC in an occasional large bile duct and saccule of Beale in two (table 1). The acinar cells of peribiliary glands consistently expressed D10 and 1F6 (fig 1B).
Figure 1 Immunohistochemically stained paraffin‐wax sections, counterstained with Alcian blue for acid mucins and with haematoxylin, from livers of transplant donors (A, B), and patients with sporadic (C) and fluke‐associated (D–H) cholangiocarcinoma. (A) The typically limited focal expression of antigen D10 (arrow) in a normal intrahepatic bile duct. The inset shows a higher power view of the D10‐positive cells. (B) Strong staining for antigen D10 in the acini of extramural peribiliary glands (arrows) and some cells in a saccule of Beale (S). The duct epithelium of the adjacent bile duct (D) is unstained, but the antigen lies free in the lumen of both saccule and duct. (C) Antigen D10‐positive epithelial cells budding from invaginations (arrows) in the common bile duct. These buds are early stages in the development of peribiliary glands or pyloric gland metaplasia. (D) Peribiliary gland hyperplasia around an intrahepatic bile duct. The acini of the glands stain strongly for antigen D10. (E) Part of a small area of focal biliary epithelial cell hyperplasia staining strongly for antigen D10 and sharply demarcated from the normal epithelium on the left. (F) Large intrahepatic bile ducts with extensive biliary epithelial cell hyperplasia staining strongly for antigen D10. The epithelium of these ducts also show areas of cell atypia not discernable at this magnification. (G) An intrahepatic bile duct showing intraepithelial neoplasia with goblet cell metaplasia staining for antigen 17NM. (H) Serial section to the area outlined in (G) with goblet cells stained by Alcian blue for acid mucins and dysplastic epithelial cells expressing antigen D10 (brown). Original magnification of figures: (F) ×10; (D) ×25; (A–C) ×50; (E, G) ×100; (H) and inset in (A) ×250.
Table 1 Antigen expression in non‐neoplastic biliary epithelium of livers from transplant donors and patients with fluke‐associated or sporadic cholangiocarcinoma.
| D10 | 1F6 | 17NM | MUC5AC | CK20 | p53 | |
|---|---|---|---|---|---|---|
| Donor livers | ||||||
| Duct epithelium | 4/6* | 0/6 | 0/6 | 2/6 | 0/6 | 0/6 |
| Peribiliary glands | 5/5 | 5/5 | 0/5 | 2/5† | 0/5 | 0/5 |
| Livers with CC | ||||||
| Normal duct epithelium | 9/35 (26)* | 0/17 | 0/35 | 2/33 (6)† | 0/34 | 0/34 |
| Hyperplastic duct epithelium | 13/16 (81) | 0/8 | 5/17 (29)‡ | 0/14 | 0/15 | 0/14 |
| Intestinal metaplasia | 2/4 (50) | 0/1 | 4/4 (100) | 3/4 (75) | 0/3 | 0/3 |
| Dysplastic duct epithelium | 6/7 (86) | 0/5 | 1/7 (14)‡ | 4/7 (57) | 1/7 | 0/7 |
| Peribiliary glands | 47/47 (100) | 14/21 (67) | 0/47 | 2/45 (4) | 0/44 | 0/44 |
CC, cholangiocarcinoma.
Values are the number (%) of specimens of liver with tissue staining for antigen/number of specimens tested that contain the tissue. Livers from patients include those with fluke‐associated and sporadic CC combined, except that results for antigen 1F6 expression include only those with sporadic CC. Intact normal bile duct epithelium was found in only 35 of the 112 livers with CC. Serial sections of some lesions were not available to stain for all six antigens studied.
*Antigen D10 was present as occasional microscopic foci of D10‐positive cells in the regional or larger bile ducts (see fig 1A).
†MUC5AC was focally present in an occasional saccule of Beale or large bile duct in these two livers.
‡Only occasional goblet cells staining for 17NM were present in these lesions.
Various stages in the budding of D10‐positive cells and glands from the epithelium of large bile ducts were seen in sections of livers with cholangiocarcinoma (fig 1C). Peribiliary gland hyperplasia, highlighted by D10 expression, was present in both groups of cancers, but was most pronounced in livers with fluke‐associated cholangiocarcinoma (fig 1D).
The antibody to antigen 1F6 gave high background staining of sections of the specimens from Thailand, and no results for 1F6 are given in this group of patients.
Antigen expression in hyperplastic, metaplastic and dysplastic biliary epithelium
Focal and extensive areas of epithelial cell hyperplasia were present in the bile ducts of livers with both fluke‐associated and sporadic cholangiocarcinomas (table 1), and generally stained strongly for antigen D10 (fig 1E,F); such epithelium was most extensive in the livers with fluke‐associated cholangiocarcinoma. Pyloric gland metaplasia stained for D10 and 1F6. The occasional goblet cells seen in hyperplastic and dysplastic epithelium and the numerous goblet cells in intestinal metaplasia stained for antigen 17NM (fig 1G,H). Dysplastic epithelium predominantly expressed D10 and MUC5AC (table 1).
Cholangiocarcinoma histology
We found no difference in the frequency of the histological types of cholangiocarcinoma, either between the intrahepatic and extrahepatic cholangiocarcinoma or between the two groups of patients. Of the 112 cholangiocarcinomas studied, 25 were papillary, 8 papillo‐tubular and the remainder comprised 39 well differentiated, 24 moderately differentiated and 16 poorly differentiated tubular adenocarcinomas. Five of the cholangiocarcinomas (three fluke‐associated and two sporadic) were mucinous and five had a cholangiolocellular growth pattern in part of the tumour.
Antigen expression in cholangiocarcinoma
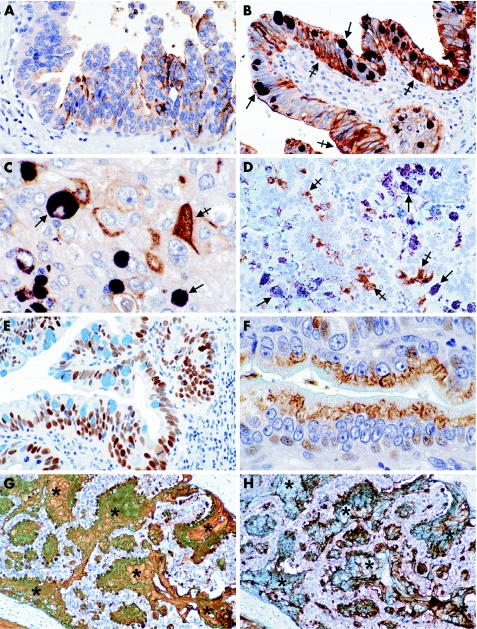
D10, MUC5AC and 17NM were the most commonly expressed antigens (table 2). In five livers with carcinoma in situ, both the carcinoma in situ and invasive cholangiocarcinoma stained for D10 in two cases, but neither the in situ nor the invasive carcinoma stained in the other three (fig 2A–C). Double labelling confirmed that cancer cells staining for D10 were distinct from those expressing 17NM or MUC5AC (fig 2B–D). Cholangiocarcinoma expressing p53 showed most cell nuclei staining for p53 in both cholangiocarcinoma and carcinoma in situ when present (fig 2E). Antigen 1F6 was present either in a supranuclear position (fig 2F), or as granules throughout the cytoplasm of cancer cells. Besides being present in the cytoplasm of cancer cells, antigen D10, 17NM and MUC5AC were also seen extruded into glandular lumina (69% of cholangiocarcinoma) or lying free in the stroma (38% of cholangiocarcinoma; fig 2G,H).
Table 2 Antigen expression in fluke‐associated and sporadic cholangiocarcinoma.
| n* | D10 | 1F6 | 17NM | MUC5AC | CK20 | p53 | |
|---|---|---|---|---|---|---|---|
| Fluke‐associated | |||||||
| Intrahepatic | 27 | 17 (63) | 11 (41) | 19 (70) | 4 (15) | 12 (44) | |
| Extrahepatic | 38 | 26 (68) | 18 (47)† | 26 (68) | 10 (26) | 18 (47) | |
| Total | 65 | 43 (66) | 29 (45)‡ | 45 (69) | 14 (21) | 30 (46)§ | |
| Sporadic | |||||||
| Intrahepatic | 13 | 5 (38) | 1 | 1 (8) | 6 (46) | 2 (15) | 2 (15) |
| Extrahepatic | 34 | 19 (56) | 3 | 7 (21)† | 25 (73) | 6 (18) | 9 (26) |
| Total | 47 | 24 (51) | 4 | 8 (17)‡ | 31 (66) | 8 (17) | 11 (23)§ |
Values are the n (%) of cancers expressing antigen in each subject group.
*Total number of cancers examined in each group.
†χ2 significant difference (0.050>p>0.025) in the numbers of positive cancers in these two subject groups.
‡χ2 significant difference (0.005>p) in the numbers of positive cancers in these two subject groups.
§χ2 significant difference (0.025>p>0.010) in the numbers of positive cancers in these two subject groups.
Figure 2 Immunohistochemically stained paraffin‐wax sections, counterstained with Alcian blue for acid mucins and with haematoxylin, from livers of patients with fluke‐associated (A–C, E, G, H) and sporadic (D, F) cholangiocarcinomas. (A) Expression of antigen D10 (brown colour) by a micropapillary adenocarcinoma in a large intrahepatic bile duct. (B) Intraepithelial neoplasia double labelled for antigens 17NM (deep purple) and D10 (brown). Goblet‐cell mucin vacuoles express antigen 17NM (arrows) and most other tumour cells antigen D10 (crossed arrows). (C) Invasive adenocarcinoma deeper in the same section as in (B), with some cancer cells staining deep purple for antigen 17NM (arrows) and others brown for antigen D10 (crossed arrow). (D) Cholangiocarcinoma of the common hepatic duct showing some cancer cells staining brown for antigen D10 (crossed arrows), and others (on both sides) staining deep purple for MUC5AC (arrows). (E) Most cancer cell nuclei staining for p53 protein in a serial section to that in (B). (F) Papillary cholangiocarcinoma of hepatic duct showing supranuclear staining of cancer cells for antigen 1F6 (brown). (G) Intrahepatic mucinous cholangiocarcinoma with antigen 17NM (brown) in both the cytoplasm of cancer cells and large pools of secreted mucus (asterisks). Alcian blue staining of the mucus has merged with the brown‐staining 17NM in some areas. This tumour did not express antigen D10. (H) Serial section to that in (G) showing predominantly intracellular mucin stained for MUC5AC (brown). Secreted strands of brown‐staining MUC5AC can also be seen in the lakes of Alcian blue‐positive mucin (asterisks). Original magnification of figures: (G, H) ×50; (A, B, D, E) ×100; (C, F) ×250.
Of the 67 cholangiocarcinomas that expressed antigen D10 (table 2), 31% showed 1+ tumour cells staining for D10, 41% 2+ cells staining and 28% 3+ cells staining. For the other antigens expressed by cholangiocarcinoma, the corresponding figures were 51%, 30% and 19%, respectively, for 17NM, 13%, 62% and 25%, respectively, for MUC5AC, and 45%, 50% and 5%, respectively, for CK20.
In both groups of patients, the extrahepatic cholangiocarcinoma tended to express the various antigens studied more often than the intrahepatic cholangiocarcinoma (table 2), but in no case was the difference statistically significant. Much greater proportions of fluke‐associated cholangiocarcinoma expressed antigen 17NM and p53 than in the sporadic cholangiocarcinoma (table 2). We found no statistically significant association of expression of any two of the antigens investigated in individual tumours.
Discussion
In contrast with the general absence of the five antigens studied in bile ducts of normal livers, the epithelial cell hyperplasia and dysplasia present in the large ducts of livers bearing cholangiocarcinoma strongly expressed antigen D10. This de novo expression suggests that it is these tissues, with possibly a contribution from the D10‐positive, hyperplastic peribiliary glands21 that give rise to D10‐positive cholangiocarcinoma.
Brunner glands and pyloric gland metaplasia in chronic gastritis and inflammatory disease of the small bowel characteristically express D10 and 1F6.10,11 Brunner glands and pyloric gland metaplasia have similar cytology and secretory profiles of neutral mucins and biologically active substances (such as trefoil peptides, epidermal growth factor, etc) that have a role in the maintenance and repair of gastrointestinal mucosa.22 The presence of D10 and 1F6 in pyloric gland metaplasia and peribiliary glands suggests that these glands have a similar role in the biliary tree.
The only differences found between the two groups of cholangiocarcinoma were the markedly greater proportions of fluke‐associated cholangiocarcinoma that expressed 17NM and stained for p53 than in the sporadic cholangiocarcinoma. As there was no statistically significant association in expression of 17NM and p53 in individual cancers, the increases are probably independent events. A greater prevalence of intestinal metaplasia in livers with fluke infestation can account for the higher frequency of expression of 17NM observed in the fluke‐associated cholangiocarcinoma, if such metaplasia contributes to an increased risk of transformation.23
The more common overexpression of p53 found in the fluke‐associated cancers was consistent with a reported difference between cholangiocarcinoma in European and Asian patients.9 Marked differences in p53 expression have, however, also been reported between US patients from an area with a high incidence of cholangiocarcinoma and those in the general population.24 It is not clear therefore to what extent fluke infestation or other environmental factors may be responsible for the difference in p53 expression between the two groups of patients.
Our observations confirmed the frequent expression of the MUC5AC in dysplasia of the bile duct epithelium and cholangiocarcinomas reported by others.7,25 The MUC6 mucin is expressed by most peripheral and hilar cholangiocarcinomas, focally by non‐dysplastic and non‐neoplastic biliary epithelium, and by most examples of dysplastic biliary epithelium.7,25 These investigators also found that MUC5AC and MUC6 were expressed more often by hilar than by peripheral cholangiocarcinoma, but the differences were not statistically significant. These observations on expressions of MUC5AC and MUC6 in the biliary epithelium and in cholangiocarcinomas are in keeping with our findings on the three gastrointestinal mucins studied here.
Intrahepatic cholangiocarcinomas are reported to retain the cytokeratin profile of normal bile duct epithelium (ie, CK7, 8, 18 and 19), but with 23% of the cancers also expressing CK20,26 a proportion similar to that found in both patient groups in the current study. Although CK20 indicates a metaplastic change towards a gastrointestinal phenotype, there was no significant association between expression of CK20 and antigen 17NM in individual cholangiocarcinomas.
This investigation has shown that most cholangiocarcinomas display a Brunner or pyloric gland cell phenotype and a gastric foveolar cell phenotype, with intestinal goblet cell and gastrointestinal‐specific cytokeratin phenotypes less commonly expressed. Fluke‐associated cholangiocarcinomas more commonly display the intestinal goblet cell phenotype and overexpression of p53 than sporadic cholangiocarcinomas, suggesting some differences in aetiopathology. The novel expression of antigen D10 in hyperplastic and dysplastic biliary epithelium, and its presence in populations of cancer cells distinct from those expressing MUC5AC or 17NM indicates a likely origin of the cholangiocarcinoma from dysplastic epithelium.
Acknowledgements
This work was supported partly by a grant from the National Health and Medical Research Council of Australia (grant number 950857).
Footnotes
Competing interests: None.
References
- 1.Haswell‐Elkins M R, Satarug S, Elkins D B.Opisthorchis viverrini infection in North East Thailand and its relationship to cholangiocarcinoma. J Gastroenterol Hepatol 19927538–548. [DOI] [PubMed] [Google Scholar]
- 2.Srivatanakul P, Sontipong S, Chotiwan P.et al Liver cancer in Thailand: temporal and geographic variations. J Gastroenterol Hepatol 19883413–420. [Google Scholar]
- 3.Itoh M, Pairojkul C, Thamawit W.et al Association of antibodies to Opisthorchis viverrini with hepatobiliary disease in North Eastern Thailand. Am J Trop Med Hyg 199451424–429. [PubMed] [Google Scholar]
- 4.Parkin D M, Srivatanakul P, Khalt M.et al Liver cancer in Thailand. I. A case‐control study of cholangiocarcinoma. Int J Cancer 199148323–328. [DOI] [PubMed] [Google Scholar]
- 5.Parkin D M, Ohshima H, Srivatanakul P.et al Cholangiocarcinoma: epidemiology, mechanisms of carcinogenesis and prevention. Cancer Epidemiol Biomarkers Prev 19932537–544. [PubMed] [Google Scholar]
- 6.Anthony P. Tumours and tumour‐like lesions of the liver and biliary tract. In: MacSween RNM, Burt AD, Portman BC, Ishak KG, Scheuer PJ, Anthony PP, eds. Pathology of the liver. Edinburgh: Churchill Livingstone, 2002743–747.
- 7.Sasaki M, Nakanuma Y, Ho S B.et al Cholangiocarcinoma arising in cirrhosis and combined hepatocellular‐cholangiocellular carcinomas share apomucin profiles. Am J Clin Pathol 1998109302–308. [DOI] [PubMed] [Google Scholar]
- 8.Rullier A, Le Bail B, Fawaz R.et al Cytokeratin 7 and 20 expression in cholangiocarcinomas varies along the biliary tract but still differs from that in colorectal carcinoma metastasis. Am J Surg Pathol 200024870–876. [DOI] [PubMed] [Google Scholar]
- 9.Tullo A, D'Erchia A M, Honda K.et al New p53 mutations in hilar cholangiocarcinoma. Eur J Clin Invest 200030798–803. [DOI] [PubMed] [Google Scholar]
- 10.Hughes N R, Bhathal P S, Francis D M A. Phenotypic identity of gastric mucous neck cells and mucous cells of cardiac, pyloric, and Brunner's glands. J Clin Pathol 19944753–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhathal P S, Hughes N R, Goodman Z D. The so‐called bile duct adenoma is a peribiliary gland hamartoma. Am J Surg Pathol 199620858–864. [DOI] [PubMed] [Google Scholar]
- 12.Hughes N R, Walls R S, Newland R C.et al Antigen expression in normal and neoplastic colonic mucosa: three tissue‐specific antigens using monoclonal antibodies to isolated colonic glands. Cancer Res 1986462164–2171. [PubMed] [Google Scholar]
- 13.Royce S G, Hughes N R, Binos S.et al Vertebrate phylogeny of antigen D10: identification of a conserved foregut cell lineage. Histochem Cell Biol 2000114125–135. [DOI] [PubMed] [Google Scholar]
- 14.Hughes N R, Bhathal P S. Gastric mucous neck cell and intestinal goblet cell phenotypes in gastric adenocarcinoma. J Clin Pathol 199750741–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bara J, Chastre E, Mahiou J.et al Gastric M1 mucin, an early oncofetal marker of colon carcinogenesis, is encoded by the MUC5AC gene. Int J Cancer 199875767–773. [DOI] [PubMed] [Google Scholar]
- 16.Moll R, Lowe A, Laufer J.et al Cytokeratin 20 in human carcinomas. A new histodiagnostic marker detected by monoclonal antibodies. Am J Pathol 1992140427–447. [PMC free article] [PubMed] [Google Scholar]
- 17.Vojtesek B, Bartek J, Midgley C A.et al An immunochemical analysis of the human nuclear phosphoprotein p53. J Immunol Methods 1992151237–244. [DOI] [PubMed] [Google Scholar]
- 18.Cattoretti G, Pileri S, Parravicini C.et al Antigen unmasking on formalin‐fixed, paraffin‐embedded tissue sections. J Pathol 199317183–98. [DOI] [PubMed] [Google Scholar]
- 19.Cook H C. Carbohydrates. In: Bancroft JD, Stevens A, eds. The theory and practice of histological techniques. Edinburgh: Churchill Livingstone, 1982180–216.
- 20.Hill A B.A short textbook of medical statistics. Sydney: Hodder and Stoughton, 1977154–155.
- 21.Nakanuma Y, Sasaki M, Terada T.et al Intrahepatic peribiliary glands of humans. II. Pathological spectrum. J Gastroenterol Hepatol 1994980–86. [DOI] [PubMed] [Google Scholar]
- 22.Ahnen J A, Poulsom R, Stamp G W H.et al The ulceration‐associated cell lineage (UACL) reiterates the Brunner's gland differentiation programme but acquires the proliferative organization of the gastric gland. J Pathol 1994173317–326. [DOI] [PubMed] [Google Scholar]
- 23.Kozuka S, Kurashina M, Tsubone M.et al Significance of intestinal metaplasia for the evolution of cancer in the biliary tree. Cancer 1984542277–2285. [DOI] [PubMed] [Google Scholar]
- 24.Sturm P D J, Baas I O, Clement M J.et al Alterations of the p53 tumour‐suppressor gene and K‐ras oncogene in perihilar cholangiocarcinoma from a high‐incidence area. Int JCancer199878695–698. [DOI] [PubMed] [Google Scholar]
- 25.Sasaki M, Nakanuma Y, Kim Y S. Characterization of apomucin expression in intrahepatic cholangiocarcinoma and their precursor lesions: an immunohistochemical study. Hepatology 1996241074–1078. [DOI] [PubMed] [Google Scholar]
- 26.Shimonishi T, Miyazaki K, Nakanuma Y. Cytokeratin profile relates to histological subtypes and intrahepatic location of intrahepatic cholangiocarcinoma and primary sites of metastatic adenocarcinoma of liver. Histopathology 20003755–63. [DOI] [PubMed] [Google Scholar]