Abstract
The Carney triad is a rare syndrome of unknown aetiology, with synchronous or metachronous appearance of rare neoplasms: gastrointestinal stromal tumours (GISTs), pulmonary chondromas and extra‐adrenal paragangliomas. In most cases, the Carney triad is incomplete. The combination encountered typically, GISTs and pulmonary chondromas, was also seen in our patient, a 22‐year‐old woman. She was diagnosed with the triad after Billroth II gastrectomy for histologically proved gastric GISTs. The diagnosis of pulmonary chondromas was confirmed by transthoracic, computed tomography‐guided needle biopsy. An oesophageal leiomyoma was resected 2 years after the initial diagnosis, on suspicion of paraganglioma. The clinical course of the patient has been uneventful since. The last follow‐up was carried out 6 years after the initial diagnosis. On histological examination, the cells of gastric GIST were partly positive for CD34, whereas CD117 was expressed in all areas in variable intensity and S‐100 protein was negative. The oesophageal tumour was classified as leiomyoma due to strong immunopositivity for smooth muscle actin and desmin, being negative for CD34 and CD117. Two different gastric GIST lesions as well as the oesophageal leiomyoma and normal tissue were analysed for activating mutations in common hot spots of KIT (exon 9 and 11) and platelet‐derived growth factor receptor α (exon 18), but in all probes wild‐type sequences were found. These results are in accordance with the first published analyses of GIST lesions from Carney patients.
In the Carney triad, at least two of the following rare neoplasms are present: multifocal gastrointestinal stromal tumours (GISTs), pulmonary chondromas and extra‐adrenal paragangliomas.1 Clinical symptoms in this syndrome of unknown aetiology are highly variable. Since 1977, some 80 patients were diagnosed with the Carney triad; it was incomplete in most cases.2,3 Surgery has been the sole treatment option for GISTs and paragangliomas. Imatinib mesylate, a tyrosine kinase inhibitor that targets BCR–ABL, KIT and platelet‐derived growth factor receptor α (PDGRFα) has emerged as an effective agent in the treatment of (mostly sporadic) GISTs and is now widely used in advanced disease and has been investigated as an adjuvant treatment.4 Only recently, the first report on molecular assessment of GISTs in the Carney triad that were found to display KIT wild type became available the findings of which were subsequently confirmed by a second investigation extending the mutational analysis on PDGFRα gene.5,6
Case report
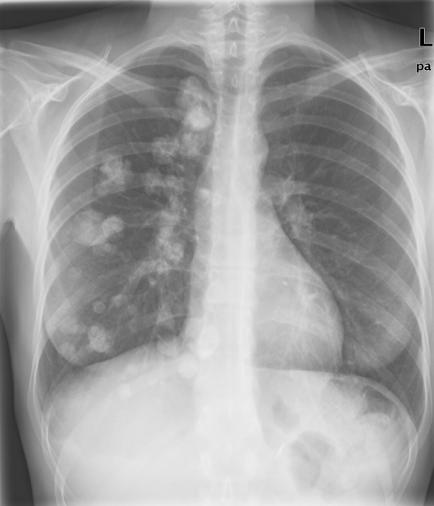
A previously healthy 22‐year‐old woman presented with a short history of epigastric pain. Gastroscopy showed multiple submucosal lesions in the corpus and antrum, which were suspected of being lymphoma. However, biopsy findings were consistent with a gastrointestinal stromal tumour, showing expression of CD34 and CD117 (KIT receptor ) and leading to Billroth II gastrectomy and Roux‐en‐Y anastomosis. Multiple pulmonary nodules had been detected in a preoperative chest x ray (fig 1) and thus the patient subsequently underwent transthoracic needle biopsy. Chondroma was diagnosed on histological examination, which, with gastric GISTs, was suggestive of incomplete Carney triad. An extramural oesophageal mass appeared in endoscopic ultrasound 2 years later, suspected of being paraganglioma. The tumour was resected endoscopically and classified as a leiomyoma of the oesophagus.
Figure 1 Chest x ray showing multiple calcified pulmonary nodules in the right lung consistent with chondromas.
The clinical course was uneventful for the next 4 years. Compared with the first chest x ray, only a slight increase in size and number of pulmonary chondromas was documented in 6 years.
The gastric tumour tissue both from biopsy and gastrectomy as well as oesophageal tissue were embedded, cut and stained. Immunostaining was carried out using the avidin–biotin–peroxidase complex method, with antibodies against CD34, CD117, desmin, S100 protein, chromogranin A, PGP 9.5, smooth muscle actin (Dako, Hamburg, Germany) and Ki‐67 (Dianova, Hamburg, Germany). The tumour was also investigated by nested‐polymerase chain reaction and direct sequencing for mutations in the KIT and the PDGFRα genes as published previously.7
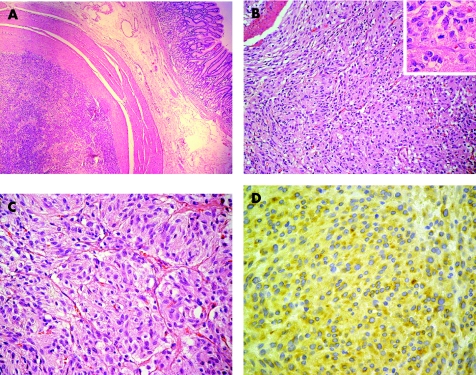
The main surgical specimen showed multiple solid tumours, each being surrounded by a pseudocapsule (fig 2A). Histological examination showed nodular infiltrations by a tumour composed of relatively uniform cells arranged in short fascicles (fig 2B), but also growing in nested paraganglioma‐like pattern (fig 2C). An average of one mitotic figure per 50 high‐power fields was seen. The tumour cells were only partially positive for CD34, whereas CD117 was expressed in all different nodules in variable intensity (fig 2D). We found no reactivity for smooth muscle actin, desmin, chromogranin A, PGP9.5 or S100 proteins. MiB1 (anti‐Ki‐67) stained 5% of the tumour cell nuclei.
Figure 2 Gastrointestinal stromal tumour (GIST). (A) Tumour mass with surrounding fibrous capsule is seen in the gastric wall, leaving the mucosa uninvolved. (B, C) The lesion shows at least partially nested paraganglioma‐like appearance. Only very few mitoses are found (inset). Haematoxylin and eosin. (D) GIST. Tumour cells showing diffuse cytoplasmic positivity with the antibody against CD117. Anti‐CD117 (1595), avidin–biotin–peroxidase complex method.
Histologically, the oesophageal tumour, 7 mm in diameter, was composed of interlacing bundles of smooth muscle cells intermingled with collagen. The cytoplasm showed strong immunopositivity for smooth muscle actin and desmin, but was negative for S100, PGP9.5, CD34 and CD117.
Two different GIST lesions as well as the oesophageal leiomyoma and normal tissue were analysed for activating mutations in common hot spots of KIT (exon 9 and 11) and PDGFRα (exon 18), but wild‐type sequences were found in all probes.
Discussion
We present a new case of incomplete Carney triad in a young woman, with the appearance of metachronous leiomyoma of the oesophagus, thus encountering an additional infrequent neoplasm in the syndrome. A similar constellation was recognised only recently.6
In patients with the triad, symptoms such as gastrointestinal bleeding or epigastric pain are often seen. In general, the Carney triad shows an indolent course.3 However, metastatic or recurrent GIST as well as extra‐adrenal paragangliomas may require treatment. Metastasis occurs infrequently in GIST tumours <5 cm, but occurs in 60% of lesions of at least 10 cm.8 Histologically and immunohistologically, the respective index case uncovered the typical features of the histogenic category of GIST, showing differentiation towards (or being derived from, respectively) interstitial cells of Cajal.9,10 However, histological studies of GIST showed a partially paraganglioma‐like growth pattern, thus suggesting a histogenetic link between these neoplasms, according to a recent paper.11 As no detectable immunophenotypical characteristics of paraganglioma were found in the index case, no evidence of an immediate histogenetic relationship was found between those two entities. Not long ago, activating mutations of the tyrosine kinase KIT (CD117) were discovered, contributing to the understanding of GIST pathophysiology.12 In the GIST of our case, however, no such activating mutations in common hot spots of KIT or PDGFRα were detected, which is in accordance with the first reports on the respective analysis.5,6 Results in studies investigating the tyrosine kinase inhibitor imatinib (Glivec) in advanced GIST were encouraging, although the two patients with the Carney triad who received imatinib mesylate did not respond.6,7 This may directly reflect the absence of both KIT and PDGF receptor mutations.
Abbreviations
GIST - gastrointestinal stromal tumour
PDGFRα - platelet‐derived growth factor receptor α
Footnotes
Competing interests: None declared.
Patient consent was obtained for this study.
References
- 1.Wales P W, Drab S A, Kim P C. An unusual case of complete Carney's triad in a 14‐year‐old boy.J Pediatr Surg 2002371228–1231. [DOI] [PubMed] [Google Scholar]
- 2.Carney J A, Sheps S G, Go V L.et al The triad of gastric leiomyosarcoma, functioning extra‐adrenal paraganglioma and pulmonary chondroma.N Engl J Med 19772961517–1518. [DOI] [PubMed] [Google Scholar]
- 3.Carney J A. Gastric stromal sarcoma, pulmonary chondroma, and extra‐adrenal paraganglioma (Carney triad): natural history, adrenocortical component, and possible familial occurrence. Mayo Clin Proc 199974543–552. [DOI] [PubMed] [Google Scholar]
- 4.Demetri G D, von Mehren M, Blanke C D.et al Efficacy and safety of imatinib mesylate in advcanced gastrointestinal stromal tumors. N Engl J Med 2002347472–480. [DOI] [PubMed] [Google Scholar]
- 5.Spatz A, Bressac‐de‐Paillerets B, Raymond E. Soft tissue sarcomas. Case 3. Gastrointestinal stromal tumor and Carney's triad. J Clin Oncol 2004222029–2031. [DOI] [PubMed] [Google Scholar]
- 6.Diment J, Tamborini E, Casali P.et al Carney triad: case report and molecular analysis of gastric tumor. Hum Pathol 200536112–116. [DOI] [PubMed] [Google Scholar]
- 7.Wardelmann E, Losen I, Hans V.et al Deletion of Trp‐557 and Lys‐558 in the juxtamembrane domain of the c‐kit protooncogene is associated with metastatic behaviour of gastrointestinal stromal tumors. Int J Cancer 2003106887–895. [DOI] [PubMed] [Google Scholar]
- 8.Shiu M H, Farr G H, Papachristou D N.et al Myosarcomas of the stomach: natural history, prognostic factors and management. Cancer 198249177–187. [DOI] [PubMed] [Google Scholar]
- 9.Fletcher C D, Berman J J, Corless C.et al Diagnosis of gastrointestinal stromal tumors: a consensus approach. Hum Pathol 200233459–465. [DOI] [PubMed] [Google Scholar]
- 10.Kindblom L ‐ G, Remotti H E, Aldenborg F.et al Gastrointestinal pacemaker cell tumor (GIPACT): gastrointestinal stromal tumors show phenotypic characteristics of the interstitial cells of Cajal. Am J Pathol 1998521259–1269. [PMC free article] [PubMed] [Google Scholar]
- 11.Horenstein M G, Hitchcock T A, Tucker J A. Dual CD117 expression in gastrointestinal stromal tumor (GIST) and paraganglioma of Carney triad: a case report. Int J Surg Pathol 20051387–92. [DOI] [PubMed] [Google Scholar]
- 12.Miettinen M, Majidi M, Lasota J. Pathology and diagnostic criteria of gastrointestinal stromal tumors (GISTs): a review. Eur J Cancer 200238(Suppl)39–51. [DOI] [PubMed] [Google Scholar]