Abstract
Background
Vascular tumours such as Kaposi's sarcoma and capillary haemangioma are characterised by abnormal vascularisation and proliferation of endothelial cells or neoplastic cells. Adrenomedullin, a potent vasodilative peptide, and its receptor, calcitonin receptor‐like receptor (CRLR), play an important part in angiogenesis.
Aim
To establish whether this system also plays a part in vascular diseases, showing abnormal proliferation such as vascular tumours.
Methods
CRLR expression was investigated in several specimens of Kaposi's sarcoma and other vascular tumours, using immunohistochemical analysis with a previously described CRLR‐specific polyclonal antibody and reverse transcriptase‐polymerase chain reaction.
Results
Intense and specific CRLR‐immunoreactive staining of neoplastic cells was observed in all specimens, which was of greater intensity than similar staining of adjacent normal endothelium.
Conclusions
CRLR is expressed in vascular tumours and, with adrenomedullin, may have a role in neoplastic vascular growth.
Calcitonin gene‐related peptide and adrenomedullin (ADM) are structurally homologous peptides with potent vasodilative effects.1 Their actions are mediated by a common receptor, the calcitonin receptor‐like receptor (CRLR), which is a G protein‐associated receptor whose function and ligand specificity uniquely depend on the coexpression of the receptor activity‐modifying proteins 1–3 (RAMP1–3). The association of CRLR with RAMP1 produces a calcitonin gene‐related peptide receptor, whereas the interaction of CRLR with RAMP2 or RAMP3 constitutes an adrenomedullin 1 or adrenomedullin 2 receptor.2
ADM, in addition to exerting acute vascular effects, has an important role in angiogenesis and vasculogenesis. This is indicated by pharmacological studies of ADM showing proliferative effects on vascular endothelial cells,3 the potent angiogenity of ADM using the chicken chorioallantoic membrane assay4 and the observation that animals with a loss‐of‐function mutation for ADM display severe morphological abnormalities of the cardiovascular system.5 The CRLR has been detected in cardiovascular structures early in embryogenesis6 and the association of the CRLR or RAMP system in angiogenesis and vasculogenesis has been recently shown.7
These findings raise the question whether CRLR is responsible for the pathophysiology of vascular diseases, in particular those characterised by abnormal angioproliferation. In a first approach to dealing with this question, we scrutinised various vascular tumours for the presence of CRLR‐like immunoreactivity (CRLR‐LI).
Materials and methods
Human tissues
Formalin‐fixed, paraffin‐wax‐embedded tissue samples were examined from the following vascular tumours: Kaposi's sarcoma (seven, skin; two, rectum; and one, tonsil), capillary haemangioma (two, nose), malignant haemangioendothelioma (one, pleura) and glomus tumour (one, ear). Histological diagnosis was made by two separate pathologists using the panel of antibodies routinely used against CD31, CD34 and factor VIII‐related antigen. The study represents a refinement of the diagnostic procedures, which received the informed consent of the patients.
Reverse transcriptase‐polymerase chain reaction
Total RNA was reverse transcribed according to the manufacturer's instructions, using Superscript II reverse transcriptase (InVitrogen, Karlsruhe, Germany). Polymerase chain reaction (PCR) experiments were carried out as described previously.8 The primers used were as follows:
ADM primers: forward 5′‐CTC TGA GTC GTG GGA AGA GG‐3′, reverse 5′‐CCC TGG AAG TTG TTC ATG CT‐3′, GenBank accession number NM_001124
CRLR primers: forward 5′‐CAC TAT GCC TGA TGT GAC GC‐3′, reverse 5′‐CAT CAA TGG TGT GCT GGA AC‐3′, GenBank accession number NM_005795
RAMP1 primers: forward 5′‐ACC TCT TCA TGA CCA CTG CC‐3′, reverse 5′‐GTA GCT CCT GAT GGT CCT GC‐3′, GenBank accession number AJ001014
RAMP2 primers: forward 5′‐GCC ATG ATT AGC AGG CCT TA‐3′, reverse 5′‐GTT GGC AAA GTG GAT CTG GT‐3′, GenBank accession number AJ001015
RAMP3 primers: forward 5′‐CTC ATC CCG CTG ATC GTT AT‐3′, reverse 5′‐AAC TTT CTT CCA GCT TGC CA‐3′, GenBank accession number AJ001016
Glyceraldehyde‐3‐phosphate‐dehydrogenase (GAPDH) primers: forward 5′‐CAT CAC CAT CTT CCA GGA GCG A‐3′, reverse 5′‐GTC TTC TGG GTG GCA GTG ATG G‐3′, GenBank accession number BC023632.
Number of cycles: 38; cycling parameter: 30 s at 94°C or 45 s at 58°C or 1 min at 72°C.
Negative controls were included either by omitting reverse transcriptase from cDNA synthesis or by omitting cDNA from the PCR amplifications.
Immunohistochemical analysis
Immunohistochemical analysis was carried out on paraffin wax‐embedded 1–2‐μm sections mounted on Superfrost or Plus slides. The primary antibodies used were (1) affinity‐purified polyclonal anti‐human CRLR antibody (diluted 1:100)9; and (2) mouse monoclonal anti‐human CD34 antibody (Immunotech, Germany; 1:1000); staining procedures were carried out as described previously.10
Results
Kaposi's sarcoma is a tumour characterised by aberrant proliferation of vascular structures with predominance of spindle‐shaped cells. Most of these cells are endothelial in origin, but some of them are thought to arise also from fibroblasts and macrophages.
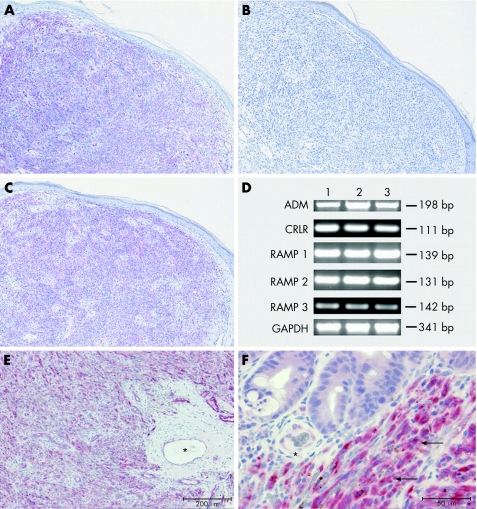
The expression of CRLR‐LI was evaluated in 10 biopsy specimens of patients with Kaposi's sarcoma, including seven from skin (fig 1A), one from tonsil (fig 1E) and two from rectum (fig 1F). All specimens showed a similar staining pattern: CRLR‐LI was most intense over the fascicular pattern of spindle‐shaped cells. Comparatively faint staining was observed over the flattened endothelial cell linings of interspersed blood vessels in the adjacent non‐tumour tissue. CRLR immunostaining was absent after preabsorption of the antiserum with the synthetic peptide CRLR antigen (fig 1B). Reverse transcriptase‐PCR analysis of the RNA extracts from three skin specimens of people with Kaposi's sarcoma showed the presence of the transcript for CRLR in these tissues and showed that, in addition, the genes for RAMP1–3 and ADM are expressed (fig 1C).
Figure 1 Biopsy results of Kaposi's sarcoma from (A) skin, (E) tonsil and (F) rectum specimens, showing intense calcitonin receptor‐like receptor‐like immunoreactivity (CRLR‐LI) over the fascicular pattern of spindle cells (arrows); comparatively faint CRLR‐LI staining over the flattened endothelial cells of interspersed blood vessels (asterisks). Scale bars (A,E) 200 μm and (F) 50 μm, respectively. (C) Immunohistochemistry for CD34 showing reactivity in spindle‐shaped tumour cells. Scale bar 200 μm. (B) Absence of CRLR‐LI after preabsorption of the antiserum with the synthetic peptide CRLR antigen. Scale bar 200 μm. (D) Reverse transcriptase‐PCR products for adrenomedullin (ADM), CRLR and receptor activity‐modifying protein (RAMP1–3) mRNAs of total RNA from Kaposi's sarcoma of the skin of three patients (1–3); glyceraldehyde‐3‐phosphate‐dehydrogenase (GAPDH) served as positive control.
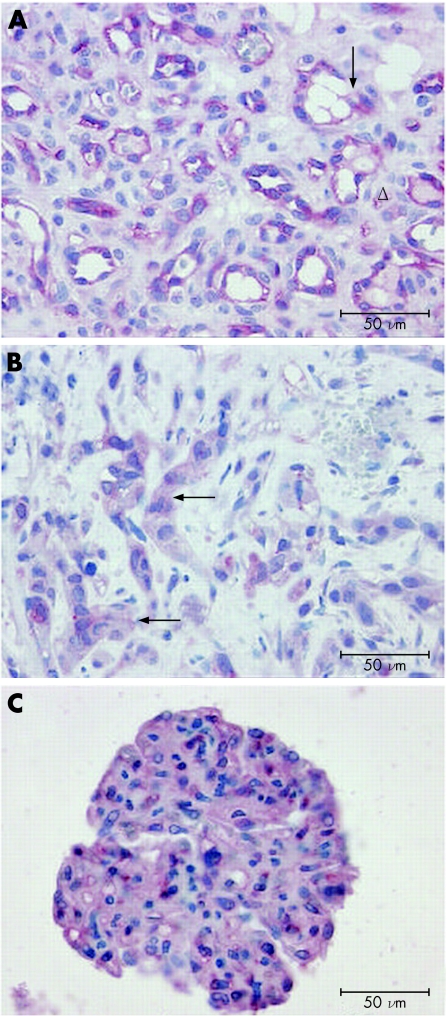
Capillary haemangioma is a tumour of small‐calibre blood vessels whose walls are composed of undifferentiated endothelial cells and characteristically lack elastic fibres or smooth muscles. Immunohistochemical analysis of two specimens of capillary haemangioma showed intense staining of the densely packed cells lining the lumina of the capillary‐like vessels, with no CRLR‐LI over stroma cells (fig 2A). The staining was confined to the cytoplasm, reflecting the distribution of the micro‐organelles associated with the receptor synthesis, processing and intracellular trafficking. Distinct staining of cell membranes was obscured by cytoplasmic immunostaining and was therefore difficult to discern.
Figure 2 (A) Capillary haemangioma with intense calcitonin receptor‐like receptor‐like immunoreactivity (CRLR‐LI) of capillary‐like vessels (arrows). Stroma cells do not show any staining (triangles). (B) Malignant haemangioendothelioma with CRLR‐LI mainly confined to epitheloid cells (arrows). (C) Glomus tumour with intense CRLR‐LI of glomus cells. Scale bar 50 μm.
Malignant haemangioendothelioma shows a poorly circumscribed proliferation of venous or capillary vessels with variable histological features. In the specimen investigated here, CRLR‐LI was mainly confined to streaks or nests of epitheloid cells with vacuolated cytoplasm and immunoreactivity to endothelial markers (fig 2B). Their intense immunoreactivity was in sharp contrast with the weaker signal shown by the vascular endothelium (not shown).
Glomus tumour is a distinct neoplasm that arises from modified smooth‐muscle cells of the normal glomus body, which is a specialised form of arteriovenous anastomosis associated with temperature regulation. Histologically, it consists of circumscribed aggregates of epitheloid cells that express several smooth‐muscle cell proteins, confirming their myogenic origin. The glomus tumour examined here was a well‐circumscribed lesion in the skin, consisting of solid aggregates of round glomus cells, and showed intense CRLR‐LI throughout (fig 2C).
Discussion
Our data provide novel evidence that vascular neoplastic cells express the CRLR protein, which is typically found on vascular endothelial cells. The weaker staining in normal blood vessels than that in vascular tumours suggests that the level of CRLR synthesis in the neoplastic cells is up regulated. We can presently speculate only on the mechanisms underlying this up regulation. Up regulation of CRLR may be driven by hypoxia, which is an angiogenic stimulus that probably prevails in vascular tumours with their high oxygen demand and abnormal blood supply. Interestingly, the promoter region of the human CRLR gene contains a consensus hypoxia‐response element, and transcription of the gene has been found to be up regulated in microvascular endothelial cells under hypoxia.11
The observation that neoplastic cells of vascular origin are still able to synthesise CRLR and at higher rates than normal differentiated endothelial cells suggests that activation of this receptor is requisite for tumour growth. Consistent with this notion are observations that ADM, a ligand of CRLR, inhibits endothelial apoptosis, stimulates endothelial cell migration and proliferation in a CRLR‐dependent manner, and promotes angiogenesis.7,12,13
The presence of the ADM transcript in Kaposi's sarcoma, as described here, argues strongly for an autocrine or paracrine action of the peptide, mediated by CRLR. This is reminiscent of another angiogenic system detected in Kaposi's sarcoma: vascular endothelial‐derived growth factor (VEGF) and its receptors. VEGF, like ADM, is constitutively produced by vascular endothelial cells, is up regulated by hypoxia and acts as an autocrine mitogenic factor in a Kaposi's sarcoma‐derived cell line.14 Recently, a constitutively active G protein‐coupled receptor encoded by the Kaposi's sarcoma‐associated herpes virus has been shown to enhance the expression of VEGF by stimulating the activity of the transcription factor hypoxia‐inducible factor 1α, which activates transcription via a hypoxia‐response element in the VEGF promoter.15 A similar mechanism of gene up regulation may be relevant for the ADM or CRLR system.
Acknowledgements
We thank H Mittag (Department of Dermatology, Marburg) for helpful discussion. This work was supported partly by the Medizin‐Stiftung, Marburg.
Abbreviations
ADM - adrenomedullin
CRLR - calcitonin receptor‐like receptor
CRLR‐LI - calcitonin receptor‐like receptor‐like immunoreactivity
PCR - polymerase chain reaction
RAMP - receptor activity‐modifying protein
VEGF - vascular endothelial‐derived growth factor
Footnotes
Competing interests: None declared.
References
- 1.Brain S D, Grant A D. Vascular actions of calcitonin gene‐related peptide and adrenomedullin. Physiol Rev 200484903–934. [DOI] [PubMed] [Google Scholar]
- 2.Poyner D R, Sexton P M, Marshall I.et al International Union of Pharmacology. XXXII. The mammalian calcitonin gene‐related peptides, adrenomedullin, amylin, and calcitonin receptors. Pharmacol Rev 200254233–246. [DOI] [PubMed] [Google Scholar]
- 3.Miyashita K, Itoh H, Sawada N.et al Adrenomedullin promotes proliferation and migration of cultured endothelial cells. Hypertens Res 200326S93–S98. [DOI] [PubMed] [Google Scholar]
- 4.Zhao Y, Hague S, Manek S.et al PCR display identifies tamoxifen induction of the novel angiogenic factor adrenomedullin by a non‐estrogenic mechanism in the human endometrium. Oncogene 199816409–415. [DOI] [PubMed] [Google Scholar]
- 5.Caron K M, Smithies O. Extreme hydrops fetalis and cardiovascular abnormalities in mice lacking a functional adrenomedullin gene. Proc Natl Acad Sci USA 200198615–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dvorakova M, Haberberger R V, Hagner S.et al Expression and distribution of the calcitonin receptor‐like receptor in the developing rat heart. Anat Embryol 2003207307–315. [DOI] [PubMed] [Google Scholar]
- 7.Fernandez‐Sauze S, Delfino C, Mabrouk K.et al Effects of adrenomedullin on endothelial cells in the multistep process of angiogenesis: involvement of CRLR/RAMP2 and CRLR/RAMP3 receptors. Int J Cancer 2004108797–804. [DOI] [PubMed] [Google Scholar]
- 8.Hagner S, Knauer J, Haberberger R.et al Calcitonin receptor‐like receptor is expressed on gastrointestinal immune cells. Digestion 200266197–203. [DOI] [PubMed] [Google Scholar]
- 9.Hagner S, Haberberger R, Kummer W.et al Immunohistochemical detection of calcitonin gene‐related peptide receptor (CGRP)‐1 in the endothelium of human coronary artery and bronchial vessels. Neuropeptides 2001351–7. [DOI] [PubMed] [Google Scholar]
- 10.Hagner S, Stahl U, Knoblauch B.et al Calcitonin receptor‐like receptor: identification and distribution in human peripheral tissues. Cell Tissue Res 200231041–50. [DOI] [PubMed] [Google Scholar]
- 11.Nikitenko L L, Smith D M, Bicknell R.et al Transcriptional regulation of the CRLR gene in human microvascular endothelial cells by hypoxia. FASEB J 2003171499–1501. [DOI] [PubMed] [Google Scholar]
- 12.Shichiri M, Hirata Y. Regulation of cell growth and apoptosis by adrenomedullin. Hypertens Res 200326S9–14. [DOI] [PubMed] [Google Scholar]
- 13.Kim W, Moon S O, Sung M J.et al Angiogenic role of adrenomedullin through activation of Akt, mitogen‐activated protein kinase, and focal adhesion kinase in endothelial cells. FASEB J 2003171937–1939. [DOI] [PubMed] [Google Scholar]
- 14.Masood R, Cai J, Zheng T.et al Vascular endothelial growth factor vascular permeability factor is an autocrine growth factor for AIDS‐Kaposi sarcoma. Proc Natl Acad Sci USA 199794979–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang L, Wakisaka N, Tomlinson C C.et al The Kaposi's sarcoma‐associated herpesvirus (KSHV/HHV‐8) K1 protein induces expression of angiogenic and invasion factors. Cancer Res 2004642774–2781. [DOI] [PubMed] [Google Scholar]