Abstract
Background
RANTES (regulated on activation, normal T cell expressed and secreted) expression is increased in inflammatory bowel disease (IBD). RANTES is produced at higher levels in granulomatous conditions, so increased RANTES expression can be expected in Crohn's disease compared with ulcerative colitis.
Aim
To compare RANTES expression between intestinal biopsy specimens of patients with Crohn's disease and those with ulcerative colitis.
Materials and methods
A prospective study of patients presenting with lower gastrointestinal symptoms at the Bahrain Specialist Hospital from July 2004 to April 2005 was carried out. Endoscopic colonic biopsy specimens were taken from every patient and subjected to (a) routine haematoxylin and eosin staining examination by light microscopy, (b) immunohistochemistry for examination of RANTES protein expression by light microscopy and (c) in situ hybridisation for examination of RANTES mRNA expression by light microscopy. RANTES expression was assessed and quantified.
Results
58 patients were enrolled to the study. Of them, 40 had IBD (21 had Crohn's disease and 19 had ulcerative colitis), 15 were controls with normal colonic biopsy results or non‐inflammatory lesions and 3 had colonic inflammatory lesions other than IBD. RANTES expression in lymphocytes or histiocytes was significantly higher (p = 0.04) in new patients with ulcerative colitis than in those with Crohn's disease analysed by immunohistochemistry (IHC).
Conclusion
RANTES expression in lymphocytes or histiocytes is significantly higher in patients with ulcerative colitis than in those with Crohn's disease. Hence, RANTES IHC can be an effective method for distinguishing between biopsy specimens of patients with ulcerative colitis from those of patients with Crohn's disease, where routine histological features are indeterminate. RANTES IHC may prove to be a useful technique for identifying early or equivocal granulomas.
The term inflammatory bowel disease (IBD) encompasses Crohn's disease and ulcerative colitis. The two diseases usually differ enough to be clearly distinguishable, although there are overlapping features such that in a proportion of IBD cases, a clear distinction is not possible and a diagnosis of “indeterminate” colitis is necessitated. Crohn's disease and ulcerative colitis have different clinical courses and natural histories, and require different management strategies.
The normal intestinal immune system is under a carefully controlled regulatory balance in which pro‐inflammatory and anti‐inflammatory cells and molecules promote a normal host mucosal defence capability without destruction of intestinal tissue.1 The recruitment and activation of leucocytes at sites of intestinal inflammation and injury are the hallmarks of IBD and chemokines are central to this.2 In IBD, the down‐regulatory processes that should turn off inflammatory events seem to be deficient.
Chemokines have a plethora of overlapping functions and are produced by a wide variety of cell types during an inflammatory response. The coordinated expression of chemokines and adhesion molecules is responsible for selective trafficking of leucocyte populations from intravascular to extravascular compartments. Chemokines such as interleukin (IL)8, monocyte chemoattractant protein (MCP)1 and epithelial neotrophil activating peptide 78 have an important role in the immunopathogenesis of IBD.3,4,5,6
Few published data are available on RANTES (regulated on activation, normal T cell expressed and secreted) in connection with IBD.
RANTES expression is increased in IBD (Crohn's disease and ulcerative colitis)2 and RANTES mRNA transcription is markedly increased in the intestinal mucosa from patients with Crohn's disease as compared with normal controls.7 RANTES is produced at a high level in sarcoidosis and tuberculosis—granulomatous disorders in which there is a close association of memory T cells with macrophages.8 To date, there have been few conclusive studies directly comparing the characteristics of RANTES expression in Crohn's disease versus ulcerative colitis.9
We expect that the pattern of RANTES expression in Crohn's disease (granulomatous) is different (granulomatous) from that in ulcerative colitis (non‐granulomatous). If there is a definite difference in the pattern of RANTES expression in Crohn's disease and that in ulcerative colitis, then theoretically it may be possible to make a definitive diagnosis in biopsy specimens, where the histological features alone prove to be inconclusive.
We investigated and compared the intensity of expression and spatial distribution of RANTES in intestinal mucosal biopsy specimens from patients with Crohn's disease, those with ulcerative colitis and controls by using immunohistochemical (antibodies to RANTES) and molecular biological (in situ hybridisation of RANTES mRNA) techniques, and assessed objectively whether there is a marked difference in the RANTES staining characteristics of Crohn's disease as compared with ulcerative colitis.
Materials and methods
This was a mostly prospective study and partly a retrospective study. Patients presenting with lower gastrointestinal symptoms to the Bahrain Specialist Hospital (BSH), Bahrain, from July 2004 to February 2005 were enrolled prospectively. A proportion of these patients were known to have IBD (either Crohn's disease or ulcerative colitis), whereas the rest were new patients presenting to the clinic for the first time.
All of the patients presenting to the hospital were examined by flexible colonoscopy after obtaining informed consent, and four biopsy specimens were taken, from appropriate mucosal sites, from each patient.
In addition, tissue blocks from known cases of IBD were retrieved from the archives of the pathology department at the Salmaniya Medical Complex, Bahrain. This added a retrospective component to the study.
Two biopsy specimens were obtained from each patient at BSH, placed in formalin fixative and transported to the Arabian Gulf University for storage and further investigation.
At the time of endoscopic examination, NZ entered clinical data and endoscopic findings pertaining to each patient on an appropriately designed proforma. All available relevant clinical data were accurately recorded and filed.
Each of the formalin‐fixed biopsy specimens (two from each patient) was routinely processed and embedded in paraffin wax (each biopsy specimen was placed in a separate tissue block). For each patient, the two tissue blocks containing formalin‐fixed paraffin wax‐embedded tissue were labelled A and B. These were processed as follows:
Block A: A 5‐μm‐thick tissue section was produced at the microtome, placed on a glass slide and stained with haematoxylin and eosin.
Block A: Another 5‐μm‐thick tissue section was produced at the microtome, placed on a glass slide and subjected to immunohistochemical assessment of the RANTES antigen. The section was dewaxed and rehydrated with graded alcohol. It was stained with a monoclonal antibody to RANTES (R&D Systems, Minneapolis, Minnesota, USA; dilution 1:100). Standard avidin–biotin–peroxidase methods with overnight incubation and enzyme antigen retrieval were used. Positive controls comprised lymph node biopsy specimens containing granulomas. Omission of primary antibodies served as negative controls.
Block B: A 5‐μm‐thick tissue section was produced at the microtome, placed on a glass slide and subjected to assessment of RANTES mRNA by in situ hybridisation (ISH). The method included hybridisation with a biotin‐labelled RANTES oligonucleotide probe (Gene Detect, Auckland, New Zealand) and detection by horseradish peroxidase with tyramide signal amplification, and is described well in the literature.10
The tissue blocks obtained from the Salmaniya Medical Complex were processed in a manner identical to that described earlier for the prospectively recruited patients from BSH.
For each participant, the histological slides stained with haematoxylin and eosin were examined by two pathologists (NA, AAS), and a diagnosis was rendered in conjunction with all clinical and endoscopic data available. The participants were classified into six categories on this basis:
Crohn's disease treated (old)
Crohn's disease (new)
Ulcerative colitis treated (old)
Ulcerative colitis (new)
Control and
Others.
RANTES expression was assessed and quantified (IHC and ISH) in tissues from each patient by identifying the cell types that produced positive signals and by counting the number of positive cell signals per high‐power field.
The intensity of staining and spatial distribution of RANTES was compared in specimens from all the six categories of patients. During the examination process, the authors examining the slides (NA, AAS, JA)— were blinded to the diagnostic category of each case.
Data were statistically analysed with SPSS.
Results
In all, 58 patients were enrolled to the study. Of them, 45 were enrolled prospectively and 13 retrospectively. The prospective group presented to the Gastroenterology Clinic at the BSH and biopsy specimens were taken by NZ at the time of endoscopic examination. Tissue blocks from the retrospective group were retrieved from the Salmaniya Medical Complex pathology archives.
A final diagnosis was made for each participant on the basis of clinical data, endoscopic findings and histological features. Table 1 shows the classification of the 58 patients.
Table 1 Classification of the patients (n = 58).
| CD | 16 |
| CD old (treated) | 5 |
| UC new | 15 |
| UC old (treated) | 4 |
| Control | 15 |
| Others | 3 |
CD, Crohn's disease; Control, patients who had either normal colonic biopsy results or non‐inflammatory pathology; IBD, inflammatory bowel disease; others, patients with non‐IBD inflammatory lesions; UC, ulcerative colitis.
Table 2 shows the frequencies of histological findings in relation to diagnostic category. Radiological data were not available in this study.
Table 2 Frequency of histological findings in relation to diagnostic category.
| Diagnostic category | Granuloma | Cryptitis | Crypt abscess | Crypt distortion | GCD | Thickening of muscularis mucosae | Granulation tissue | Lymph aggregate | Oedema | Acute inflammation | Chronic inflammation |
|---|---|---|---|---|---|---|---|---|---|---|---|
| CD new (n = 16) | 4 | 3 | 3 | 10 | 10 | 1 | 3 | 3 | 4 | 10 | 14 |
| CD old (n = 5) | 1 | 5 | 5 | 1 | 1 | 2 | 5 | ||||
| UC new (n = 15) | 8 | 8 | 13 | 13 | 1 | 4 | 11 | ||||
| UC old (n = 4) | 2 | 2 | 2 | 3 | 1 | 4 | |||||
| Others (n = 3) 1 each with fibrinopurulent exudate, eruptive exudate and SRUS | |||||||||||
| Control (n = 15) | 1 | 1 | 4 | 7 | |||||||
| Total (n = 58) | 4 | 13 | 14 | 30 | 31 | 2 | 4 | 4 | 10 | 16 | — |
CD, Crohn's disease; GCD, goblet cell depletion; SRUS, solitary rectal ulcer syndrome; UC, ulcerative colitis.
Of the 21 patients with Crohn's disease, only four showed histological evidence of granuloma formation. Granulomas were either well formed and composed of epithelioid cells or giant cells without central necrosis, or were ill‐defined collections of epithelioid histiocytes. None of the patients with ulcerative colitis had granulomas or giant‐cell reaction to ruptured crypts.
Histological features identified in the IBD cases included the following:
Chronic inflammatory cell infiltrates (plasmacytosis was more commonly seen in patients with ulcerative colitis)
Cryptitis (more common in patients with ulcerative colitis)
Crypt abscesses (more common in patients with ulcerative colitis)
Crypt distortion (equally seen in Crohn's disease and in ulcerative colitis)
Goblet cell depletion (approximately equally seen in Crohn's disease and in ulcerative colitis)
Submucosal muscularisation (Crohn's disease only)
Thickening of muscularis mucosae (Crohn's disease only)
Granulation tissue (more frequent in Crohn's disease)
Lymphoid aggregates with or without germinal centres (almost entirely in Crohn's disease)
Oedema (most often in Crohn's disease).
None of the patients with IBD showed any evidence of epithelial dysplasia on microscopy.
The diagnoses for the three “others” cases were pseudomembranous colitis, solitary rectal ulcer syndrome and microscopic colitis. The 15 “control” showed a narrow range of histological features from “within normal limits” to mild non‐specific inflammation.
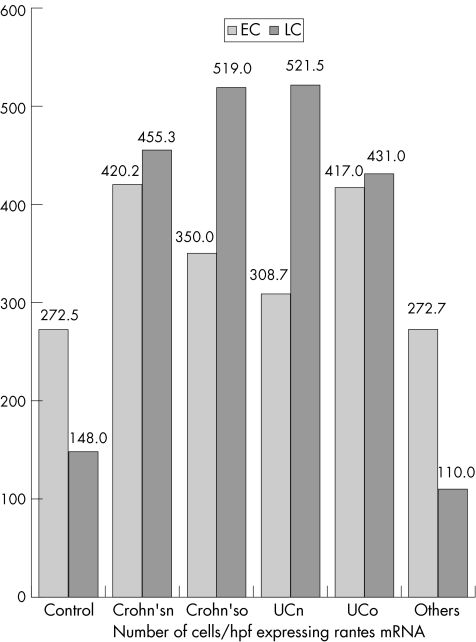
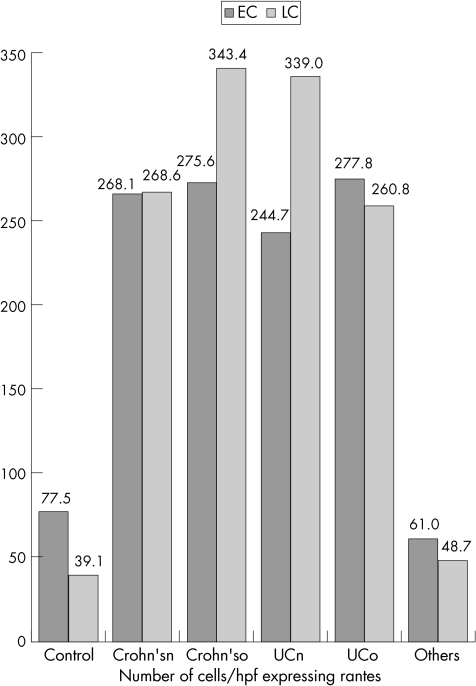
RANTES expression (protein and mRNA) was detected in crypt epithelial cells and inflammatory cells—especially lymphocytes and histiocytes. Table 3 and fig 1 give the data relating to the epithelial and lymphocyte expression of RANTES in the different study groups by using ISH; table 4 and fig 2 give the data relating to IHC. Figures 3–5 show microscopic (haematoxylin and eosin staining) findings from selected patients. Figures 6–10 are photomicrographs of RANTES expression (IHC and ISH) from selected cases.
Table 3 Data relating to the epithelial and lymphocyte expression of RANTES mRNA by ISH.
| Category | Epithelial | Lymphocyte/histiocyte | ||||
|---|---|---|---|---|---|---|
| Mean | Range | SD | Mean | Range | SD | |
| CD treated, (n = 5) | 350 | 173–513 | 140.4 | 519 | 225–1000 | 294.9 |
| CD new (n = 16) | 420.2 | 96–660 | 189.6 | 455.3 | 180–900 | 217.2 |
| UC treated (n = 4) | 417 | 288–550 | 123.3 | 431.8 | 380–480 | 35.7 |
| UC new (n = 15) | 308.7 | 50–551 | 115.5 | 521.5 | 150–1296 | 281.5 |
| Control (n = 15) | 272.5 | 128–448 | 89.6 | 148 | 2–420 | 105 |
| Others (n = 3) | 272.7 | 180–432 | 113.2 | 110 | 80–150 | 29.4 |
CD, Crohn's disease; ISH, in situ hybridisation; RANTES, regulated on activation, normal T cell expressed and secreted; UC, ulcerative colitis.
Figure 1 Data relating to the epithelial and lymphocyte expression of RANTES (regulated on activation, normal T cell expressed and secreted) mRNA by in situ hybridisation (ISH) group. Crohn'sn, Crohn's disease (new); Crohn'so, Crohn's disease (old); EC, epithelial cells; HPF, high‐power field; LC, lymphocyte; UCn, ulcerative colitis (new); UCo, ulcerative colitis (old).
Table 4 Data relating to the epithelial and lymphocyte expression of RANTES by IHC.
| Category | Epithelial | Lymphocyte/Histocyte | ||||
|---|---|---|---|---|---|---|
| Mean | Range | SD | Mean | Range | SD | |
| CD treated (n = 5) | 275.6 | 234–324 | 30.3 | 343.4 | 264–510 | 86.2 |
| CD new (n = 16) | 268.1 | 148–404 | 62.6 | 268.6 | 193–393 | 63.4 |
| UC treated (n = 4) | 277.8 | 214–341 | 50.3 | 260.8 | 104–442 | 122.3 |
| UC new (n = 15) | 244.7 | 125–354 | 63.6 | 339 | 160–540 | 109.9 |
| Control (n = 15) | 77.5 | 10–451 | 105.7 | 39.1 | 6–112 | 34.7 |
| Others (3) | 61 | 18–101 | 34 | 48.7 | 18–86 | 28.2 |
CD, Crohn's disease; IHC, immunohistochemistry; RANTES, regulated on activation, normal T cell expressed and secreted; UC, ulcerative colitis.
Figure 2 Data relating to the epithelial and lymphocyte expression of RANTES (regulated on activation, normal T cell expressed and secreted) by immunohistochemistry (IHC). Crohn'sn, Crohn's disease (new); Crohn'so, Crohn's disease (old); EC, epithelial cells; HPF, high‐power field; LC, lymphocyte; UCn, ulcerative colitis (new); UCo, ulcerative colitis (old).
Figure 3 Ulcerative colitis (new; case 21). Crypt abscess, goblet cell depletion and severe mixed acute and chronic inflammation. Haematoxylin and eosin staining, ×40.
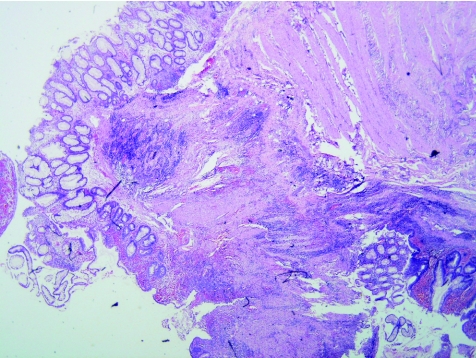
Figure 4 Crohn's disease (new; case 34). Superficial and deep inflammation, muscularisation of the submucosa, moderate crypt distortion. Haematoxylin and eosin staining, ×2.
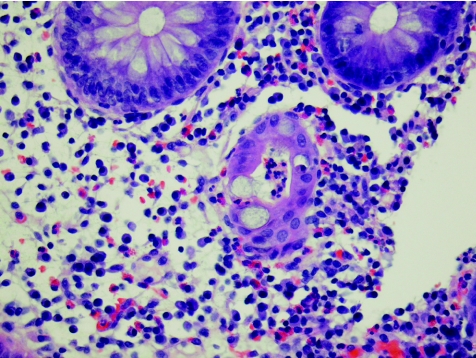
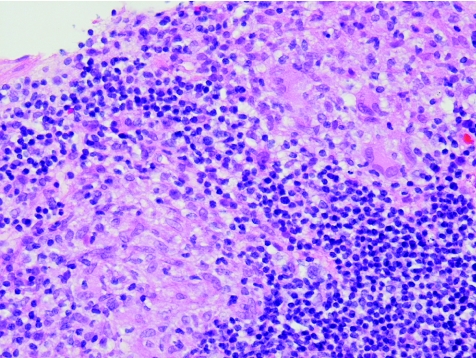
Figure 5 Crohn's disease (new; case 41). Epithelioid cell granulomas. Haematoxylin and eosin staining, ×40.
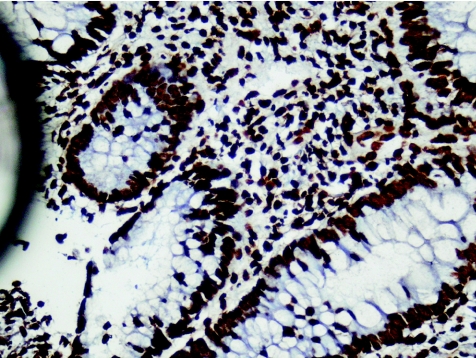
Figure 6 Ulcerative colitis (new; case 8). Diffuse strong nuclear positivity in both epithelial and lymphocyte nuclei. RANTES (regulated on activation, normal T cell expressed and secreted) in situ hybridisation, ×40.
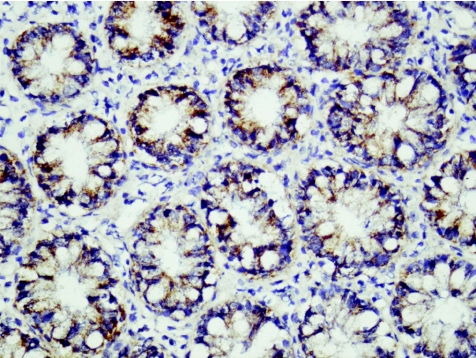
Figure 7 Ulcerative colitis (new; case 20). Strong positive staining for RANTES (regulated on activation, normal T cell expressed and secreted) protein in plasma cells and lymphocytes. RANTES immunohistochemistry, ×40.
Figure 8 Crohn's disease (new; case 33). Strong epithelial cytoplasmic staining and relatively little staining of inflammatory cells of RANTES (regulated on activation, normal T cell expressed and secreted). In situ hybridisation, ×40.
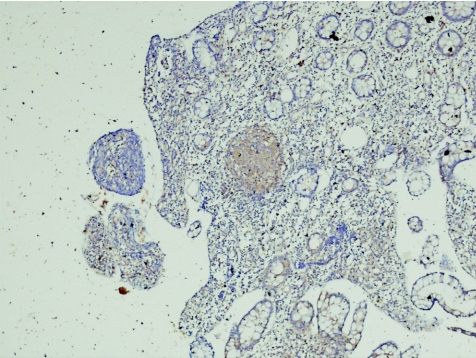
Figure 9 Crohn's disease (new; case 56). The granuloma from fig.35 represented at higher power. RANTES (regulated on activation, normal T cell expressed and secreted) immunohistochemistry, ×10.
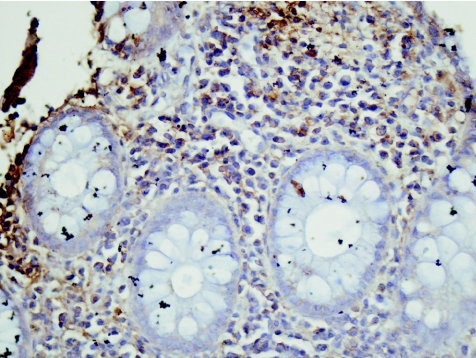
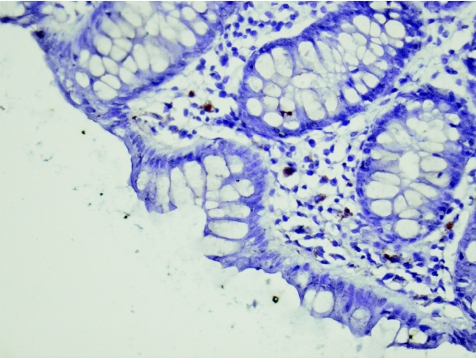
Figure 10 Control (case 3). Negative for RANTES (regulated on activation, normal T cell expressed and secreted ) mRNA. Brown colour is lipofushcin–melanosis coli. In situ hybridisation, ×40.
Epithelial RANTES expression in cases of Crohn's disease (new) was significantly higher than in controls (p<0.05 using ISH, p<0.01 using IHC).
Epithelial RANTES expression in ulcerative colitis (new) was significantly higher than in controls (IHC, p<0.05).
Epithelial RANTES expression in patients with ulcerative colitis (new) and Crohn's disease (new) was not significantly different (p = 0.1 using ISH; p = 0.31 using IHC).
RANTES expression in lymphocytes or histiocytes in patients with Crohn's disease (new) was significantly higher than in controls (p<0.05 using ISH; p<0.01 using IHC).
RANTES expression in lymphocytes or histiocytes in patients with ulcerative colitis (new) was significantly higher than in controls (p = 0.0001 using ISH; p<0.05 using IHC).
RANTES expression in lymphocytes or histiocytes in patients with ulcerative colitis (new) was significantly higher than in those with Crohn's disease (new, IHC, p = 0.04).
When IHC was used, there seemed to be an increase in both epithelial and lymphocyte RANTES expression in patients with Crohn's disease (old) compared with that in patients with Crohn's disease (new). However, as the number of patients with Crohn's disease (old) in the study was low, this difference was not found to be significant.
We found no significant difference in the RANTES expression in epithelial cells or lymphoctes or histiocytes in patients with ulcerative colitis (new) when compared with those having ulcerative colitis (old).
The study showed sharp positive staining of granulomas with RANTES antibody (IHC).
Discussion
IBD comprises two distinct clinicopathological entities: Crohn's disease and ulcerative colitis. Crohn's disease and ulcerative colitis have different clinical courses and natural histories,11,12,13 and, in most instances, on the basis of haematoxylin and eosin staining of the biopsy specimen, one can be readily favoured over the other.14,15,16,17,18,19 The two conditions do, however, share several histological features, and so in a proportion of cases a clear distinction is not possible (indeterminate colitis).
We aimed to investigate RANTES expression in IBD and to compare the expression of RANTES in Crohn's disease with that in ulcerative colitis and to evaluate whether there were marked differences that may help in distinguishing the two conditions histologically.
The normal intestinal immune system is under a carefully controlled regulatory balance in which pro‐inflammatory and anti‐inflammatory cells and molecules promote a normal host mucosal defence capability without the destruction of intestinal tissue.1 Once this regulatory balance is disturbed, activation of leucocytes can lead to production and release of increased amounts of destructive inflammatory molecules. Disruption of any of several specific immune defence mechanisms may lead to the development of chronic intestinal inflammation. Through constant luminal exposure to potent stimulatory bacterial products, the state of activation of the intestinal immune system and mucosal inflammatory pathways can be chronically increased.
The recruitment and activation of leucocytes at sites of intestinal inflammation and injury are the hallmarks of IBD, and chemokines are central to this.2 Normally, the inhibition of active and destructive immunological and inflammatory events should occur after the resolution of a bacterial or viral infection that has been appropriately defended against and controlled by the mucosal immune system. In IBD, the down‐regulatory processes that should turn off inflammatory events seem to be deficient.20
Chemokines have a plethora of overlapping functions and are produced by a wide variety of cell types during an inflammatory response. The coordinated expression of chemokines and adhesion molecules is responsible for the selective trafficking of leucocyte populations from intravascular to extravascular compartments. Chemokines such as IL8, MCP1 and extractable nuclear antigens 78 have an important role in the immunopathogenesis of IBD.
Few published data are available on RANTES in connection with IBD, most of the work having been carried out on other chemokines, especially IL8 and MCP.
Mazzucchelli et al21 showed transcription of RANTES mRNA to be increased in the intestinal mucosa of both patients with Crohn's disease and those with ulcerative colitis. Berrebi and coworkers7 showed that there was increased expression of the rantes gene in intestinal and lymph node specimens from patients with Crohn's disease, compared with its expression in specimens from controls. Furthermore, considerably raised levels of RANTES expression were identified in granulomas of patients with Crohn's disease as compared with mucosa of normal controls.
RANTES22 is a CC chemokine and shows chemotactic activity in vitro mainly on human CD4 memory T lymphocytes, monocytes and eosinophils, but has no marked chemotactic property on CD4 naive T lymphocytes, CD8 cytotoxic T lymphocytes, B lymphocytes or neutrophils.22 In situ accumulation of CD4 memory T lymphocytes and monocytes leads to the formation of granuloma, which is the histological hallmark of Crohn's disease in the intestine and often in mesenteric lymph nodes.8 RANTES production seems to be increased in other granulomatous disorders, including sarcoidosis and tuberculosis.8
Therefore, we expect RANTES expression to be higher in Crohn's disease (granulomatous disorder) than in ulcerative colitis (non‐granulomatous disorder).
Oki et al9 recently investigated the expression of RANTES and its receptor CCR5 in intestinal biopsy specimens from patients with Crohn's disease and those with ulcerative colitis. Using immunohistochemistry, they found expression of these proteins in small mononuclear cells, in particular Th1 cells. No marked difference was found in RANTES expression in Crohn's disease and that in ulcerative colitis.
Our findings showed that RANTES expression was markedly increased in IBD as compared with that in controls. When comparing Crohn's disease and ulcerative colitis, RANTES expression in epithelial cells was comparable in both groups. RANTES expression by lymphocytes was, however, considerably greater in ulcerative colitis (new) than in Crohn's disease (new). This was an unexpected finding, bearing in mind that RANTES expression is strongly associated with granulomatous disorders. Although we do not know the exact reason for this reversal, we can give some plausible explanations.
The microenvironment of the intestine in patients with IBD (ulcerative colitis and Crohn's disease) is an intricate system, characterised by large numbers and different types of inflammatory cells expressing and responding to multiple chemical mediators, notably chemokines. A complex interplay therefore exists between cells and different chemokines. Several factors may affect the level of RANTES expression in patients with IBD and these factors may vary between Crohn's disease and ulcerative colitis. Quite possibly, there may be a specific cell–chemokine or chemokine–chemokine interaction in ulcerative colitis resulting in high RANTES expression that is not evident in Crohn's disease. It may therefore be constructive to study the differential expression of more than one chemokine—for example, IL8—and RANTES in patients with Crohn's disease and ulcerative colitis to see if there is a difference.
It should be borne in mind that the exact aetiology of the IBDs is unknown. The pathogenesis of both Crohn's disease and ulcerative colitis is thought to include multiple factors, some of which have been substantiated whereas others are purely theoretical. Genetic factors, microbes, diet and aberrant immune responses have all been implicated. With such a myriad of proposed pathogenetic factors, it is quite feasible that in ulcerative colitis, a pathogenetic mechanism (yet to be defined) leads to increased RANTES levels—above and beyond those seen in Crohn's disease.
Genetics has a role in IBD. Crohn's disease is known to have a stronger genetic component than ulcerative colitis. One gene, NOD2/CARD15, is known to be important in the pathogenesis of Crohn's disease.
Abnormal (mutant) NOD2 protein (in Crohn's disease) interacts in a defective manner with bacterial surface glycoprotein, which ultimately leads to an aberrant immune response in which the bacterium persists and can invoke inflammatory responses that ultimately lead to the morphological expression of Crohn's disease.23 We propose that this aberrant immune response may include several chemokines being expressed, some at increased levels and others at reduced levels. In Crohn's disease, on the basis of this design, RANTES may be one of the chemokines reduced in expression despite the (granulomatous) tendency to increase RANTES expression.
However, taken at face value, this apparent difference in RANTES expression may provide a means of making a definitive diagnosis (ulcerative colitis or Crohn's disease) when the H&E appearances are equivocal and indeterminate. We would urge further investigations in this direction to see if our findings are corroborated, because as far as we are aware, this is the first study that has disclosed real differences in RANTES expression in ulcerative colitis and in Crohn's disease. A useful direction of research would be to identify which subsets of lymphocytes (eg, T, T4, T8, Th1 Th2) are responsible for RANTES expression in the two types of IBD and to assess whether there is a salient difference in subset RANTES expression between Crohn's disease and ulcerative colitis, which can be exploited in terms of diagnostic pathology to refine IBD biopsy diagnoses.
Our study also showed a trend of increasing RANTES expression with chronicity of disease in the case of Crohn's disease. This supports previous work carried out in rats that showed the important role of RANTES in the progression from acute to chronic colitis.24
All the granulomas, which were identified in four biopsy specimens of patients with Crohn's disease, stained strongly for RANTES antibody—a finding similar to that by Oki et al,9 who suggested that, based on this positive staining for RANTES and the fact that epithelioid histiocytes of the granuloma are antigen‐presenting cells, the Crohn's disease granuloma may be one of the crucial sites of Th1‐shifted immune responses in Crohn's disease. Our results support this view.
The staining of granulomas in the biopsy specimens was remarkable. Furthermore, ill‐defined granulomas were also prominently stained. There are instances in diagnostic pathology when the pathologist is doubtful on the basis of morphology stained with haematoxylin and eosin whether a structure truly is or is not a granuloma. We recommend the use of the RANTES antibody as an immunohistochemical marker for granulomas in case of uncertainty.
Conclusions
RANTES expression was markedly increased in colonic biopsy specimens from patients with IBD (both Crohn's disease and ulcerative colitis) compared with those from patients without inflammatory colonic lesions. The levels of epithelial RANTES expression in patients with ulcerative colitis and Crohn's disease were found to be comparable, but our data suggest that RANTES expression in lymphocytes or histiocytes is definitely higher in patients with ulcerative colitis than in those with Crohn's disease. This would imply that there is potential for the use of RANTES IHC when attempting to make a distinction between ulcerative colitis and Crohn's disease in biopsy specimens, where routine histological features are indeterminate. Follow‐up studies to corroborate these findings are recommended. RANTES IHC may prove to be a useful technique for identifying early or equivocal granulomas.
Acknowledgements
We thank the Research Committee of the Arabian Gulf University for awarding a research grant that partly funded the study.
Abbreviations
BSH - Bahrain Specialist Hospital
IBD - inflammatory bowel disease
IHC - immunohistochemistry
ISH - in situ hybridisation
MCP - monocyte chemoattractant protein
RANTES - regulated on activation, normal T cell expressed and secreted
References
- 1.Sansonetti P J. War and peace at mucosal surfaces. Nature 20044953–964. [DOI] [PubMed] [Google Scholar]
- 2.McCormack G, Moriarty D, O'Donoghue D.et al Tissue cytokine and chemokine expression in inflammatory bowel disease. Inflamm Res 200150491–495. [DOI] [PubMed] [Google Scholar]
- 3.Grimm M, Elsbury S, Pavli P.et al Interleukin 8: cells of origin in inflammatory bowel disease. Gut 19963990–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reinecker H, Loh E, Ringler D.et al Monocyte‐chemoattractant protein 1 gene expression in intestinal epithelial cells and inflammatory bowel disease mucosa. Gastroenterology 199510840–50. [DOI] [PubMed] [Google Scholar]
- 5.Z'Graggen K, Walz A, Mazzuchelli L.et al The C‐X‐C chemokine ENA‐78 is preferentially expressed in intestinal epithelium in inflammatory bowel disease. Gastroenterology 1997113808–816. [DOI] [PubMed] [Google Scholar]
- 6.Daig R, Andus T, Ashcenbrenner E.et al Increased interleukin 8 expression in the colon mucosa of patients with inflammatory bowel disease. Gut 199638216–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berrebi D, Banerjee A, Paris R.et al In situ Rantes and interferon‐gamma gene expression in pediatric small bowel Crohn's disease. J Pediatr Gastroenterol Nutr 199725371–376. [DOI] [PubMed] [Google Scholar]
- 8.Devergne O, Marfaing‐Koka A, Schall T.et al Production of the Rantes chemokine in delayed‐type hypersensitivity reactions: involvement of macrophages and endothelial cells. J Exp Med 19941791689–1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oki M, Ohtani H, Kinouchi Y.et al Accumulation of CCR5+ T cells around RANTES+ granulomas in Crohn's disease: a pivotal site of Th1‐shifted immune response? Lab Invest 200585137–145. [DOI] [PubMed] [Google Scholar]
- 10.Kenny‐Moynihan M, Unger E. Immunohistochemical and in situ hybridization techniques. In: O'Leary TJ, Becker RL, Frisman DM, eds. Advanced diagnostic methods in pathology: principles, practice, and protocols. Philadelphia: Elsevier Science Health Science Division, 200391–118.
- 11.Farmer R G, Hawk W A, Turnbull R B., Jr Clinical patterns in Crohn's disease. A statistical study of 1615 cases. Gastroenterology 197568627–635. [PubMed] [Google Scholar]
- 12.Farmer R G, Easley K A, Rankin G B. Clinical patterns, natural history and progression of ulcerative colitis. A long‐term follow up of 1116 patients. Dig Dis Sci 1993381137–1146. [DOI] [PubMed] [Google Scholar]
- 13.Cuschieri A, Grace P, Darzi A.et al Disorders of the colon and rectum. In: Cuschieri A, Grace P, Darzi A, et al.Clinical surgery. 2nd edn. Massachusetts, USA: Blackwell Publishing, 2003407–416.
- 14.Polodsky D K. Inflammatory bowel disease. N Engl J Med 2002347417–429. [DOI] [PubMed] [Google Scholar]
- 15.Fielding J F, Toye D K M, Benton D C.et al Crohn's disease of the stomach and duodenum. Gut 1970111001–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosai J. Gastrointestinal tract, anal region. In: Rosai and Ackerman's surgical pathology. Vol 1. 9th edn. Edinburgh: Mosby, 2004858
- 17.Oberhuber G, Stangl P C, Vogelsang H.et al Significant association of strictures and internal fistula formation in Crohn's disease. Virchows Arch 2000437293–297. [DOI] [PubMed] [Google Scholar]
- 18.Shepherd N A. Granulomas in the diagnosis of intestinal Crohn's disease: a myth exploded? Histopathology 200241166–168. [DOI] [PubMed] [Google Scholar]
- 19.Chambers T, Morson B. Large bowel biopsy in the differential diagnosis of inflammatory bowel disease. Invest Cell Pathol 19803159–173. [PubMed] [Google Scholar]
- 20.Tamboli C P, Neut C, Desreumaux P.et al Dysbiosis in inflammatory bowel disease. Gut 2004531–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mazzucchelli L, Hauser C, Z'Graggen K.et al Differential in‐situ expression of the genes encoding the chemokines MCP‐1 and RANTES in human inflammatory bowel disease. J Pathol 1996178201–206. [DOI] [PubMed] [Google Scholar]
- 22.Schall T J, Jongstra J, Dyer B J.et al A human T cell‐specific molecule is a member of a new gene family. J Immunol 19881411018–1025. [PubMed] [Google Scholar]
- 23.Ogura Y, Lala S, Xin W.et al Expression of NOD2 in Paneth cells: a possible link to Crohn's ileitis. Gut 2003521591–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ajuebor M N, Hogaboam C M, Kunkel S L.et al The chemokine RANTES is a crucial mediator of the progression from acute to chronic colitis in the rat. J Immunol 2001166552–558. [DOI] [PubMed] [Google Scholar]