Summary
Wnt signaling regulates a variety of developmental processes in animals. Although the β-catenin dependent (canonical) pathway is known to control cell fate, a similar role for noncanonical Wnt signaling has not been established in mammals. Moreover, the intracellular cascades for noncanonical Wnt signaling remain to be elucidated. Here we delineate a pathway in which Wnt3a signals through the Gαq/11 subunits of G proteins to activate phosphatidylinositol signaling and PKCδ in the murine ST2 cells. The Gαq/11-PKCδ signaling is required for Wnt3a-induced osteoblastogenesis in these cells, and PKCδ homozygous mutant mice exhibit a deficit in embryonic bone formation. Furthermore, Wnt7b, expressed by osteogenic cells in vivo, induces osteoblast differentiation in vitro via the PKCδ-mediated pathway; ablation of Wnt7b in skeletal progenitors results in less bone in the mouse embryo. Together these results reveal a novel Wnt-dependent osteogenic mechanism, and provide a potential target pathway for designing therapeutics to promote bone formation.
Introduction
The Wnt family of proteins are conserved from coelenterate to human regulating cell proliferation, fate specification, polarity and migration (Cadigan and Nusse, 1997; Lee et al., 2006). In the canonical Wnt pathway (Huelsken and Birchmeier, 2001; Wodarz and Nusse, 1998), Wnt binding to Frizzled receptors and the low-density lipoprotein receptor-related protein 5 or 6 (LRP5/6) in vertebrates (Mao et al., 2001b; Pinson et al., 2000; Tamai et al., 2000), stabilizes β-catenin and thereby activate transcription of downstream target genes via lymphoid enhancer-binding factor-1 (Lef-1) and T cell factors (Tcf1, 3, 4). The amplitude of signaling is fine tuned in part via negative feedback mechanisms that include the secreted molecule Dickkopf 1 (Dkk1) (Glinka et al., 1998), itself a direct transcriptional target of canonical Wnt signaling (Chamorro et al., 2005; Niida et al., 2004). Dkk1 antagonizes the pathway by interfering with LRP5/6 and Wnt interactions (Bafico et al., 2001; Mao et al., 2001a; Semenov et al., 2001).
Wnts also signal through β-catenin-independent (noncanonical) mechanisms to regulate morphogenesis during vertebrate development (Veeman et al., 2003). Most notably, noncanonical Wnt signaling has been implicated in convergence and extension of the body axis during embryogenesis, in Xenopus (Tada and Smith, 2000; Wallingford et al., 2000), zebrafish (Heisenberg et al., 2000), and mice (Kibar et al., 2001; Wang et al., 2006). In addition, noncanonical Wnt signaling was shown to regulate both polarized extension and planar cell polarity (PCP) in the mouse cochlea (Curtin et al., 2003; Kibar et al., 2001; Montcouquiol et al., 2003; Wang et al., 2005). Thus, noncanonical Wnt signaling directs cell polarity and cell movement in a variety of vertebrate species.
The intracellular cascade responsible for noncanonical Wnt signaling in vertebrates is not well understood. Overexpression of Xenopus Wnt5a, rat Frizzled 2 or a Xenopus Dishevelled construct lacking the DIX domain (XDshΔDIX) in Xenopus or zebrafish embryos stimulated calcium flux and PKC activity by activating phosphatidylinositol signaling sensitive to pertussis toxin (Kuhl et al., 2000; Sheldahl et al., 2003; Slusarski et al., 1997). However, the role of classic PKC is not known, although PKCδ, a novel PKC isoform, was shown to regulate Xenopus convergent extension in response to Frizzled signaling (Kinoshita et al., 2003). Neither is it known whether a similar signaling cascade operates in mammalian cells. In addition, certain Wnts or Frizzled molecules were found to activate the Rho family of small GTPases in HEK293T cells and Xenopus embryos (Habas et al., 2003; Habas et al., 2001). The pathway was implicated in convergent extension in Xenopus embryos (Choi and Han, 2002; Habas et al., 2003; Habas et al., 2001; Penzo-Mendez et al., 2003), but a similar role has not been demonstrated in mammals.
The importance of canonical Wnt signaling in bone is supported by genetic evidence from mammals. In humans, loss- or gain-of function mutations in LRP-5 were linked with the osteoporosis-pseudoglioma syndrome (Gong et al., 2001), or a high bone density syndrome (Boyden et al., 2002; Little et al., 2002), respectively. Mice lacking LRP-5 (LRP-5−/−) (Kato et al., 2002) or Wnt10b (Wnt10b−/−) (Bennett et al., 2005) exhibited a postnatal low bone mass phenotype. Conversely, mice lacking the Wnt antagonist secreted Frizzled related protein 1 (sFRP1−/−) developed more bone postnatally (Bodine et al., 2004). Moreover, genetic deletion of β-catenin from early osteoprogenitors resulted in lack of mature osteoblasts in the mouse embryo (Day et al., 2005; Hill et al., 2005; Hu et al., 2005; Rodda and McMahon, 2006), whereas forced activation of β-catenin greatly enhanced osteogenesis (Rodda and McMahon, 2006). Specifically, β-catenin was shown to be required both prior to Osterix (Osx) expression (Hu et al., 2005), and for the progression of Osx-positive cells to mature osteoblasts (Rodda and McMahon, 2006). Finally, β-catenin signaling in more mature osteoblasts was found to indirectly control bone mass by regulating osteoclast formation through the control of Osteoprotegerin expression (Glass et al., 2005).
A role for noncanonical Wnt signaling in bone has not been described. Here we report a Wnt-Gαq/11-PKCδ noncanonical pathway that operates in mammalian osteoprogenitors to promote osteoblast development. We further demonstrate that Wnt7b likely promotes bone formation in the mouse in part via this mechanism.
Results
Wnt3a induces osteoblastogenesis coupled with PKC activation in ST2 cells
To investigate the molecular mechanism underlying Wnt signaling during osteoblast differentiation, we established a Wnt-responsive osteoblastogenesis system. The murine bone marrow-derived stromal cell line ST2 (Ogawa et al., 1988), upon incubation with a conditioned medium containing Wnt3a (hereafter Wnt3a medium), expressed a markedly higher alkaline phosphatase (AP) activity than cells cultured in a control conditioned medium (hereafter L medium) (Fig. 1A). Real-time PCR revealed that AP mRNA levels steadily increased during the first 3 days of Wnt3a treatment before reaching a plateau (Fig. 1C). Similarly, expression of bone sialoprotein (Bsp) was activated within the first 24 hours, and reached a plateau by 72 hours of Wnt3a treatment (Fig. 1C). On the other hand, osteocalcin (OC), a marker for mature osteoblasts, was induced only after 96 hours of Wnt3a stimulation (Fig. 1C). Interestingly, Wnt3a did not stimulate Runx2 expression, but significantly induced Osterix (Osx) after 96 hours (Fig. 1C). Moreover, in the presence of ascorbic acid and β-glycerophosphate, Wnt3a induced widespread formation of mineralized nodules (Fig. 1B). Finally, purified recombinant Wnt3a dose-dependently induced AP in ST2 cells in a serum-free medium (Fig. 1D). Thus, Wnt3a is sufficient to induce osteoblast differentiation in ST2 cells.
Figure 1. Wnt3a induces osteoblast differentiation and MARCKS phosphorylation via PKCδ in ST2 cells.

(A) AP detection by substrate staining after incubation for 48 hrs in conditioned medium. (B) Detection of bone nodules by alizarin red after incubation for 14 days in mineralization medium.
(C) Real-time PCR assays of mRNA expressed as folds over GAPDH.
(D) AP expression in cells cultured for 48 hrs in serum-free medium with recombinant Wnt3a.
(E) Upregulation of MARCKS (arrows) by Wnt3a.
(F) Western analyses of total MARCKS in whole cell lysates from cells incubated in Wnt3a (W) or L medium.
(G) Western analyses of phospho-MARCKS in cytosolic fractions of cells.
(H) Western analyses of phospho-MARCKS in cytosolic fractions of cells incubated in serum-free medium. Wnt3a used at 50 ng/ml, and rottlerin at 5 μM.
Bar graphs: n=3. Western signals normalized to α-tubulin.
To identify downstream molecules responsible for Wnt-induced osteoblastogenesis, a proteomics approach was taken to compare protein profiles in ST2 cells cultured in Wnt3a versus L medium. Meristoylated alanine-rich C kinase substrate (MARCKS), a prototypic substrate for protein kinase C (PKC) (Blackshear, 1993) was detected at a increased level after 24 hours of Wnt3a stimulation (Fig. 1E, arrows), a result confirmed by Western analyses of total cell lysates (Fig. 1F). As MARCKS is known to redistribute from the plasma membrane to the cytosol following phosphorylation (Arbuzova et al., 2002), the cytosolic fractions of cells were therefore analyzed for the levels of phosphorylated MARCKS, using a phospho-specific antibody. These studies revealed that MARCKS phosphorylation was markedly enhanced after 1 hour of incubation in Wnt3a medium, and that upregulation was sustained for at least 24 hours (Fig. 1G). In fact, when cells were stimulated with purified Wnt3a without serum, phospho-MARCKS was readily detectable at 10 minutes post-stimulation, and steadily rose within the first hour (Fig. 1H). Thus, Wnt3a robustly induces MARCKS phosphorylation in ST2 cells.
PKCδ mediates Wnt3a-induced osteoblastogenesis, independent of β-catenin
Induction of MARCKS phosphorylation prompted us to examine the role of PKC in Wnt3a-induced osteoblastogenesis. The PKC family of serine and threonine protein kinases consists of at least 11 members, including the classic PKC isoforms (α, β1, β2,γ) activated by diacylglycerol (DAG), phosphatidylserine and Ca++, the novel PKC subfamily (δ, ε, η, θ) by DAG and phosphatidylserine, and the atypical PKC isoforms (λ, ι, ζ) only by phosphatidylserine (Newton, 1997). Ro-31-8220, an inhibitor for all PKC isoforms, significantly impaired AP induction by Wnt3a (Fig. 2A). However, Gö 6976, an inhibitor specific for classic PKC, had no effect even at 10 μM (IC50 2.3 nM for PKCα, 6.7 nM for PKCβ) (Fig. 2A). Similarly, the intracellular Ca++ chelator BAPTA/AM did not inhibit Wnt3a-induced AP expression (Fig. 2A). In addition, a peptide inhibitor specific for atypical PKC (PKCζ pseudosubstrate) also failed to inhibit Wnt3a-induced osteoblastogenesis (data not shown). In contrast, rottlerin, a selective inhibitor for PKCδ and PKCθ, significantly reduced AP induction in a dose-dependent manner (Fig. 2A), but a PKCθ pseudosubstrate had no effect (data not shown). The inhibition of osteoblastogenesis by rottlerin was confirmed by real-time PCR of osteoblast markers (Supplementary data, Fig. S1). Similarly, rottlerin inhibited Wnt3a-induced AP activity in primary cultures of limb primordial cells, isolated from E13.5 mouse embryos and containing osteoprogenitors but no mature osteoblasts (Fig. 2B). Moreover, rottlerin abolished Wnt3a-induced MARCKS phosphorylation in ST2 cells (Fig. 1H). Thus, PKCδ is required for Wnt3a-induced osteoblast differentiation and MARCKS phosphorylation.
Figure 2. PKCδ is required for Wnt3a-induced osteoblastogenesis but not for canonical Wnt signaling in ST2 cells.
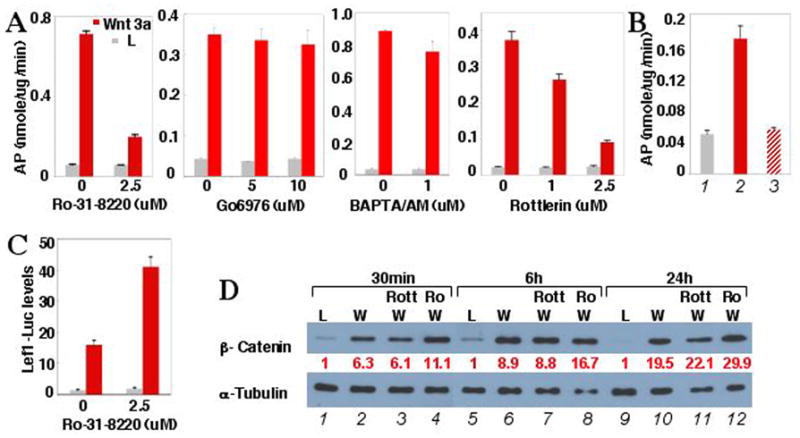
(A) Effects of PKC inhibitors on Wnt3a-induced AP expression in cells incubated for 48 hrs in conditioned medium.
(B) Wnt3a-induced AP expression, and inhibition by rottlerin in primary E13.5 limb primordial cells after 96 hrs of incubation. 1: L medium; 2: Wnt3a medium; 3: Wnt3a medium plus 5 μM rottlerin.
(C) Effect of Ro-31-8220 on Wnt3a-induced expression of Lef1-luciferase reporter.
(D) Western analyses of β-catenin in cytosolic fractions of cells following incubation in Wnt3a (W) or L medium, with or without 5 μM rottlerin (Rott) or Ro-31-8220 (Ro). When inhibitors were used, cells were pretreated with inhibitor for 1 hr in normal growth medium. Bar graphs: n=3. β-catenin level normalized to α-tubulin.
To determine whether inhibition of PKC interfered with canonical Wnt signaling, we examined the potential effect of Ro-31-8220 or rottlerin on Wnt3a-induced transcriptional activation of a Lef1-luciferase reporter, and β-catenin stabilization. Not only did Ro-31-8220 not impair Wnt3a-induced luciferase expression or β-catenin stabilization, it synergized with Wnt3a (Fig. 2, C–D). The fact that Ro-31-8220 also inhibits GSK3β, a known negative regulator of canonical Wnt signaling, may explain this observation. Similarly, rottlerin did not impair Wnt3a-induced β-catenin stabilization (Fig. 2D). Thus, PKCδ mediates Wnt-induced osteoblastogenesis independent of canonical Wnt signaling.
To corroborate the role of PKCδ, we knocked down its expression with siRNA. Western analyses confirmed that PKCδ siRNA reduced the protein level of PKCδ by ~66% (Fig. 3E). Importantly, the knockdown decreased Wnt3a-induced AP induction by approximately 50% (Fig. 3A). Similarly, overexpression of a dominant negative form of PKCδ (PKCδ-ΔC) using a retroviral vector severely impaired Wnt3a-induced AP expression (data not shown). Moreover, when cultured in a mineralization medium, cells expressing PKCδ-ΔC formed significantly fewer bone nodules than control cells expressing GFP (Fig. 3, G and H). These results support the conclusion that PKCδ activity is required in Wnt3a-induced osteoblast differentiation.
Figure 3. PKCδ activation via Gq signaling promotes Wnt3a-induced osteoblastogenesis and requires Dvl in ST2 cells.

(A–D) AP expression in cells incubated for 48 hrs in conditioned medium. In A and C, cells transfected with siRNA were incubated in normal growth medium for 96 hrs before cultured in conditioned medium. In B, cells were first infected with retrovirus before incubated in conditioned medium. n=3.
(E–F) Western analyses for PKCδ (E) and Gαq/Gα11 (F) in whole cell lysates of cells at 96 hrs after siRNA transfection, with GAPDH siRNA as control.
(G–L) Detection of bone nodules by alizarin red. In G–J, cells were infected with retrovirus before incubated in Wnt3a-mineralization medium. U73122 used at 5 μM.
(M–N) Western analyses for β-catenin and phospho-MARCKS in cytosolic fractions. Cells prestarved for 24 hrs were cultured in serum-free medium for 1 hr with or without recombinant Wnt3a. In N, cells were first infected with viruses, and then starved for 24 hrs before Wnt3a stimulation.
Western signals normalized to α-tubulin.
Gq-activated phosphatidylinositol signaling, independent of β-catenin, mediates Wnt3a-induced osteoblastogenesis
We next set out to unravel the signaling cascade leading to PKCδ activation in response to Wnt3a. PKCδ is activated by DAG, which is in turn produced through hydrolysis of phosphatidylinositol 4,5-bisphosphate (PIP2) by phospholipase C (PLC). Since the PLC-βisoenzymes are activated by both the Gq subfamily of α subunits and the βγ subunits of heterotrimeric G proteins (Morris and Malbon, 1999), we examined whether G protein-linked phosphatidylinositol signaling was responsible for Wnt3a-induced PKCδ activation and subsequent osteoblastogenesis. Pertussis toxin, which catalyzes ADP-ribosylation of the Gi family of the α-subunits thus uncoupling them from their activating receptors, is known to inhibit PLC-β activation by the βγ-subunits (Morris and Malbon, 1999). The toxin, however, did not inhibit Wnt3a-induced osteoblast differentiation in ST2 cells (data not shown). We therefore focused subsequent studies on Gq signaling.
To examine the role of Gq signaling in Wnt-induced osteoblastogenesis, we took advantage of a dominant negative reagent GqI, which is a COOH-terminal peptide (a.a. 305–359) of Gαq previously shown to partially inhibit Gq signaling (Akhter et al., 1998). GqI expression significantly reduced Wnt3a-induced AP expression (Fig. 3B), as well as bone nodule formation (Fig. 3, I and J). Thus, Gq signaling likely mediates Wnt3a-induced osteoblastogenesis.
To confirm the role of Gq subfamily of α-subunits, we reduced the levels of Gαq and Gα11, the two widely expressed members, with siRNA. A combination of siRNA oligonucleotides against Gαq or Gα11, reduced their combined protein levels by ~43%, as detected by an antibody recognizing both molecules (Fig. 3F). Importantly, these oligonucleotides reduced Wnt3a-induced AP expression by >50% (Fig. 3C). Similarly, single knockdowns of either Gαq or Gα11 also partially inhibited AP induction (data not shown). Thus, both Gαq and Gα11 are likely to mediate Wnt3a-induced osteoblast differentiation.
To further test the function of phosphatidylinositol signaling, we examined the effect of U73122, a PLC inhibitor, on Wnt3a-induced osteoblastogenesis. U73122 not only inhibited AP induction by ~50% (Fig. 3D), but also reduced bone nodule formation (Fig. 3, K and L). Thus, PLC activity is important for Wnt-induced osteoblast differentiation.
Next we examined whether inhibition of Gq signaling or PLC activity affected Wnt3a-induced PKCδ activation or β-catenin stabilization. Both U73122 (Fig. 3M) and GqI (Fig. 3N) markedly reduced phospho-MARCKS, without significantly altering β-catenin stabilization. These results indicate that Wnt3a activates a Gαq/11→PLCβ→PKCδ pathway independent of β-catenin signaling to promote osteoblast differentiation.
PKCδ activation by Wnt3a requires Dvl but is insensitive to Dkk1
We next examined whether PKCδ activation involves the Dishevelled (Dvl) family of molecules. Immunostaining revealed that in unstimulated ST2 cells, both PKCδ and Dvl-2 were present diffusely in the cytosol (Fig. 4E–G). However, within 30 minutes of Wnt3a stimulation, both molecules were translocated to the plasma membrane in a punctate pattern, although some signal remained in the perinuclear region (Fig. 4A–B). Remarkably, PKCδ and Dvl-2 co-localized at the plasma membrane (Fig. 4C). Similar results were observed with Dvl-1 and Dvl-3 (Supplementary data, Fig. S2, S3). Thus, Wnt3a signaling translocates PKCδ and Dvl to common domains within the plasma membrane.
Figure 4. PKCδ activation by Wnt3a correlates with Dvl-2 translocation to the plasma membrane and is insensitive to Dkk1 in ST2 cells.

(A–H) Immunostaining of PKCδ and Dvl-2 in cells with (A–D) or without (E–H) Wnt3a stimulation. In D and H, cells were infected with a retrovirus co-expressing Dkk1 and nuclear GFP (Dkk1-GFP).
(I–J) Western analyses in cytosolic fractions. Cells were prestarved in serum-free medium for 24 hrs before stimulated with recombinant Wnt3a for indicated times (I) or for 1 hr (J). In J, cells were first infected with retroviruses expressing either GFP or Dkk1 before starvation. Signal levels normalized to α-tubulin.
(K) Effect of Dkk1 on Lef1-luciferase activation by Wnt3a.
To determine the kinetics of membrane translocation for PKCδ and Dvl-2, we used Western analyses to quantify the levels of these proteins in the cytosol following Wnt3a stimulation. At 10 minutes post-stimulation, both PKCδ and Dvl-2 were markedly reduced in the cytosol (Fig. 4I, lane 2). At 30 minutes, the cytosolic content of PKCδ or Dvl-2 was partially recovered but remained significantly lower than the pre-stimulation level (Fig. 4I, lane 3). Interestingly, at 60 minutes both proteins were present in the cytosol at a higher than pre-stimulation level (Fig. 4I, lane 4). Consistent with activation of PKCδ at the cell membrane, the levels of phospho-MARCKS in the cytosol steadily rose within the first hour of stimulation (Fig. 4I; Fig. 1H). Thus, concurrent with PKCδ activation, Wnt3a signaling acutely and transiently translocates PKCδ and Dvl-2 to the plasma membrane with similar kinetics.
We next examined whether Dvl signaling is required for PKCδ activation. Since the DIX, PDZ and DEP domains were reported to preferentially mediate distinct Wnt pathways, we set out to evaluate whether overexpression of Dvl-2 variants lacking one of the three conserved domains (ΔDIX, ΔPDZ and ΔDEP) (Habas et al., 2001) differentially affects Wnt3a-induced PKCδ activation versus β-catenin stabilization. Cells expressing any of the Dvl-2 variants showed a marked reduction in MARCKS phosphorylation (Fig. 3N). Similarly and unexpectedly, all three Dvl-2 variants also inhibited β-catenin accumulation (Fig. 3N). These results support that Dvl proteins are required for both PKCδ activation and β-catenin stabilization in response to Wnt3a, and that all three conserved domains may participate in both pathways.
To determine whether the Wnt-PKCδ pathway requires LRP5/6 signaling, we examined whether the membrane translocation of PKCδ in response to Wnt3a is sensitive to Dkk1 inhibition. Here, cells infected with a retrovirus co-expressing Dkk1 and nuclear GFP were immunostained for endogenous PKCδ, with or without Wnt3a stimulation. As in control cells (Fig. 4A), Wnt3a induced characteristic translocation of PKCδ from the cytosol (Fig. 4H) to the plasma membrane (Fig. 4D) in cells overexpressing Dkk1. Thus, Dkk1 does not inhibit Wnt3a-induced membrane translocation of PKCδ.
We next examined whether Dkk1 inhibits Wnt3a-induced PKCδ activation. To this end, cytosolic proteins from virally infected cells with or without Wnt3a stimulation were assayed for phospho-MARCKS by Western analyses. As a control for the efficacy of the Dkk1 virus, β-catenin levels were also analyzed. In addition, the virally infected cells were transfected with the Lef1-luciferase reporter and assayed for response to Wnt3a. As expected, in Dkk1-overexpressing cells, Wnt3a failed to either stabilize β-catenin (Fig. 4J), or activate transcription of the reporter (Fig. 4K). On the other hand, Wnt3a induced phosphorylation of MARCKS in these cells, similar to that in the control cells (Fig. 4J). Thus, in contrast to the canonical Wnt pathway, Wnt-PKCδ signaling does not appear to engage the LRP5/6 co-receptors.
Genetic deletion of PKCδ results in a reduction in embryonic bone formation
To determine the role of PKCδ in bone formation in vivo, we analyzed the skeleton of PKCδ knockout mice (PKCδ−/−). The PKCδ −/− animals are viable and fertile but were reported to exhibit hyperactivation of B-cell proliferation and auto-immunity (Miyamoto et al., 2002), as well as deficiency in stress-induced apoptosis of blood vessel smooth muscle cells (Leitges et al., 2001). We reasoned that removal of PKCδ might lead to quantitative defects more evident during early phases of bone formation, and therefore focused our analyses on early embryos. At E15.5, the wild type embryos showed obvious ossification in the maxilla, the mandible, the ribs as well as the limbs (Fig. 5A). In contrast, PKCδ −/− littermates exhibited much less ossification (Fig. 5B). In particular, the maxilla and the mandible of mutant embryos showed only minimal mineralization compared with wild type littermates (Fig. 5, compare C and D). In limbs, bone collars of ossifying skeletal elements were notably shorter in the mutant embryo (Fig. 5E). Accordingly, von Kossa staining on sections of long bones showed that bone collars were shortened in mutants at both E14.5 and E15.5 (Fig. 5F). Indeed, the relative bone collar length normalized to the total skeletal element length was significantly reduced in the mutant (Fig. 5G). Thus, removal of PKCδ results in less bone in the early embryonic skeleton.
Figure 5. Removal of PKCδ results in a deficit in embryonic bone formation.

(A–E) Whole-mount skeletal staining of wild type and PKCδ−/− littermates at E15.5. Bone stained red; cartilage stained blue. Vertical lines in E demarcate ends of bone collar; horizontal red line denote deficit in mutant. R: ribs; Mx: maxilla; Mb: mandible.
(F) von Kossa staining on longitudinal sections of E14.5 humerus and E15.5 radius in wild type versus PKCδ−/− littermates. Vertical lines demarcate ends of bone collar; horizontal red line denote deficit in mutant. Double-headed arrows indicate lengths of bone collar (x) and total radius (y).
(G) Relative bone length (x/y) in radius of E15.5 wild type versus PKCδ−/− embryos. n=4, p<0.001.
(H) In situ hybridization for chondrocyte markers on longitudinal sections of humerus in wild type versus PKCδ−/− E14.5 littermates. Vertical lines denote ends of Colα1(X)-expressing domain in wild type embryo. Double-headed arrows indicate wild-type distance between two major PTHrP-R and Ihh expression domains. Asterisk: PTHrP-R signal in skin; arrow: MMP13 signal in osteoblast-lineage cell.
(I) In situ hybridization for osteoblast markers on longitudinal sections of tibia in wild type versus PKCδ−/− littermates at E15.5. Adjacent sections used for each genotype. Purple vertical lines: leading edge of Colα1(X)-expressing domain; orange vertical lines: leading edge of Osx or Bsp in wild type embryo; green vertical lines: leading edge of Osx or Bsp in PKCδ−/− embryo; red horizontal lines: deficit in PKCδ−/− embryo. Arrows: signal in perichondrium.
(J) Western analyses of cytosolic fractions of limb primordial cells from E14.5 wild type versus PKC −/− littermates.
(K–N) Detection of bone (K–L) and cartilage (M–N) nodules in primary cultures of limb primordial cells from E13.5 wild type (K and M) versus PKCδ−/− (L and N) embryos. Bone nodules stained dark red; cartilage nodules blue. Relative nodule numbers between normal and mutant genotypes were indicated.
(O) AP expression by wild type versus PKCδ−/− BMSC at 72 hrs after confluence.
As bone collar formation in the embryo is coupled with cartilage development, we examined the status of chondrocyte maturation in PKCδ −/− versus wild type littermates. At E14.5, in mutant embryos, the hypertrophic zone expressing Colα1(X) was shortened; the domains expressing parathyroid hormone related peptide-receptor (PTHrP-R), and Indian hedgehog (Ihh) were less well separated, and the terminal hypertrophic zone expressing matrix metalloproteinase 13 (MMP 13) was reduced (Fig. 5H). Thus loss of PKCδ delays chondrocyte maturation in long bones.
We next examined whether progression of osteoblast differentiation was perturbed in PKCδ −/−embryos. To this end, we took advantage of the fact that osteoblastogenesis in the perichondrium of long bones progresses linearly along the epiphysis to diaphysis axis. In particular, the onset of expression for later osteoblast markers is coupled with chondrocyte maturation, resulting in characteristic positioning of the leading edge in relation to the hypertrophic cartilage. Thus we examined by in situ hybridization the expression of a panel of osteoblast markers in the perichondrium, and evaluated whether their positioning against the Colα1(X)-expressing hypertrophic chondrocytes on adjacent sections was altered in PKCδ −/−versus wild type littermates at E15.5. In the wild type embryo, the early markers AP (Fig. 5I, arrow), Colα1(I) and Runx2 (data not shown) were detected throughout much of the metaphyseal perichondrium. On the other hand, Osx and Bsp were activated in perichondrial cells immediately preceding the hypertrophic region, with their leading edges (orange vertical lines) positioned at a characteristic distance from the first row of Colα1(X)-positive cells (purple vertical line) (Fig. 5I). In the PKCδ −/− embryo, the expression patterns of AP (Fig. 5I), Colα1(I) and Runx2 (data not shown) were similar to those in the wild type littermate. However, the leading edges of Osx and Bsp (green vertical lines) were significantly closer to the boundary of the hypertrophic zone (purple vertical line) (Fig. 5I). Thus, removal of PKCδ appears to delay the onset of Osx expression in the osteoblast lineage, which may in turn impede subsequent differentiation.
To confirm that loss of PKCδ results in intrinsic deficits in osteoblast differentiation, we performed in vitro osteoblastogenesis assays using primary cell cultures. We first assayed for bone nodule formation by E13.5 limb primordial cells. PKC −/− cells produced significantly fewer bone nodules than normal cells (Fig. 5, compare K and L), but instead generated more cartilage nodules (Fig. 5, compare M and N). Secondly, we assayed for AP production by primary bone marrow stromal cells (BMSC) in culture. Here, PKC −/− BMSC showed a significantly lower level than wild type cells (Fig. 5O). These results therefore support the conclusion that PKCδ in skeletal progenitor cells promotes osteoblast differentiation.
Lastly, we examined MARCKS phosphorylation levels in the cytosol of E14.5 limb primordial cells. The level of phospho-MARCKS was markedly lower in PKC −/− cells than in wild type cells (Fig. 5J). Thus, MARCKS is likely an endogenous substrate of PKCδin vivo.
Wnt7b induces osteoblastogenesis via a noncanonical, PKCδ-dependent mechanism
To assess the physiological relevance of Wnt-PKCδ signaling in bone formation, we investigated whether Wnt7b, a ligand expressed by osteogenic cells in vivo and able to induce osteoblast differentiation in vitro (Hu et al., 2005), signals through this pathway. In keeping with the previous finding, overexpression of Wnt7b, either by transient transfection (Fig. 6F), or by viral infection (Fig. 6G), induced AP expression in the multipotent mouse embryonic mesenchymal cell line C3H10T1/2 (Taylor and Jones, 1979). Moreover, Wnt7b overexpression induced formation of bone nodules in both C3H10T1/2 and ST2 cells in mineralization medium (Fig. 6A). Finally, Wnt7b also induced osteoblast differentiation in primary cultures of E13.5 limb primordial cells (Fig. 6H). Interestingly, in the limb cells, Wnt7b induced more robust osteoblastogenesis than a dominant active form of β-catenin (daβcat) (Fig. 6H), even though >90% cells expressed daβcat as judged by co-expression of GFP (data not shown). Thus, Wnt7b activates the osteogenic program in multiple cell systems, and activation may include alternative pathways to that mediated by β-catenin.
Figure 6. Wnt7b induces osteoblastogenesis and PKCδ activity but does not activate canonical Wnt signaling.

(A) Detection of bone nodules by alizarin red in ST2 and C3H10T1/2 cells expressing GFP or Wnt7b.
(B–C) Lef1-luciferase activation by Wnt3a, Wnt7b or daβcat. In B, Wnt7b was virally expressed whereas Wnt3a was present in conditioned medium. In C, cells were co-transfected with the reporter and a daβcat- or Wnt7b- expressing plasmid, or the empty expression vector pCIG. n=3.
(D–E) Western analyses in cytosolic fractions of ST2 (D) or C3H10T1/2 (E) cells virally expressing GFP or Wnt7b, after incubation in serum-free medium for 24 hrs. Western signals normalized to GAPDH.
(F–H) AP expression in C3H10T1/2 (F and G) or primary cultures of E13.5 limb primordial cells (H). Cells either transiently transfected (F) or virally infected (G–H) were cultured in normal growth medium for 48 hrs (F–G) or 72 hrs (H). n=3.
We next examined whether Wnt7b activates the canonical or the PKCδ pathway in the cell cultures. Wnt7b failed to activate Lef1-Luciferase expression in either ST2 or C3H10T1/2 cells, even though Wnt3a and daβcat greatly stimulated expression (Fig. 6, B and C). Accordingly, Wnt7b failed to stabilize β-catenin in C3H10T1/2 cells (Fig. 6E, top panel). On the other hand, Wnt7b induced MARCKS phosphorylation in both ST2 (Fig. 6D) and C3H10T1/2 cells (Fig. 6E). Thus Wnt7b does not stimulate canonical Wnt signaling in either ST2 or C3H10T1/2 cells, but activates PKCδ in both cell types.
We then evaluated the potential role of canonical versus PKCδ signaling in Wnt7b-induced osteoblastogenesis. Co-expression of Dkk1 did not impair AP induction by Wnt7b in either C3H10T1/2 (Fig. 6G) or the primary limb primordial cells (data not shown). However, rottlerin strongly inhibited Wnt7b-induced AP activity in both cell types (Fig. 6G and data not shown). Thus, Wnt7b induces osteoblast differentiation in multiple cell systems via the PKCδ-mediated noncanonical mechanism.
Genetic ablation of Wnt7b results in deficiency in embryonic bone formation
To assess the physiological role of Wnt7b in bone formation, we genetically removed Wnt7b from the skeletal progenitors by using the Cre-loxP technique. An initial report examining E18.5 embryos devoid of Wnt7b failed to show any clear skeletal phenotype (Rodda and McMahon, 2006). Here, we generated Wnt7b mutant mice (Dermo1-Cre; Wnt7bn/c3) carrying a Wnt7b null allele (Wnt7bn) (Parr et al., 2001), a Wnt7b conditional allele (Wnt7bc3, JR, TJC and APM in preparation), and also a Dermo1-Cre allele (Yu et al., 2003). The Wnt7bc3 allele had loxP sites flanking the essential exon 3 and functioned as a null allele upon recombination by Cre (to be reported elsewhere). Wnt7b mutant animals were viable after birth with no obvious phenotype. However, whole-mount skeletal staining revealed that, at E15.5 when wild type embryos showed obvious ossification, Wnt7b mutant littermates exhibited a diminution in ossification, while some mutant embryos also appeared to be slightly smaller (Fig. 7A). Regardless of the overall size, the bone collar of long bones was consistently shorter in mutant littermates (Fig. 7B), as confirmed by quantitation of the relative bone collar length over total length of the element (Fig. 7C). At E18.5, mutant skulls exhibited less alizarin red staining and widened sutures (Fig. 7D). Thus, Wnt7b deficiency results in less bone in mouse embryos.
Figure 7. Removal of Wnt7b in skeletal cells results in defects in bone formation.

(A) Whole-mount skeletal staining of wild type (WT) or Wnt7b mutant (MT1 and MT2) littermates at E15.5. Note smaller size of MT2. Representative bones shown at a higher magnification below corresponding whole skeleton. R: ribs; Mx: Maxilla; Mb: mandible; s: scapula; h: humerus; c: clavicle.
(B) von Kossa staining on longitudinal sections of humerus in wild type (WT) versus Wnt7b mutant (MT) E15.5 littermates. Vertical lines demarcate length of bone collars; horizontal line denotes deficit in mutant embryo.
(C) Relative bone length (bone collar over total length) in humerus of wild type versus Wnt7b mutant E14.5 littermates. n=5; p<0.001.
(D) Skulls of wild type (WT) or Wnt7b mutant (MT) littermates at E18.5. Boxed regions shown at a higher magnification below. White contour demarcates sutures; arrow denotes a nearly fused suture.
(E) Western analyses of cytosolic fractions of limb primordial cells from wild type versus Wnt7b mutant E14.5 littermates.
(F) In situ hybridization for chondrocyte markers on longitudinal sections of humerus from wild type (WT) versus Wnt7b mutant (MT) embryos. Vertical lines denote ends of Colα1(X)-expressing domain in wild type embryo. Double-headed arrows indicate wild-type distance between the two major PTHrP-R, Ihh or Colα1(X) expression domains. Asterisk: PTHrP-R signal in skin.
(G) In situ hybridization for osteoblast markers on longitudinal sections of humerus in wild type versus Wnt7b mutant (MT) E15.5 littermates. Adjacent sections were used for each genotype. Purple vertical lines: leading edge of Colα1(X)-expressing domain in each genotype; orange vertical lines: leading edge of Osx, PTHrP-R or Bsp in wild type; green vertical lines: leading edge of Osx, PTHrP-R or Bsp in MT embryo; red horizontal lines: deficit in MT embryo. Arrows: signal in perichondrium; asterisks: signal in chondrocytes.
(H) Detection of bone nodules in primary cultures of calvarial cells or bone marrow stromal cells (BMSC) from wild type (WT) or Wnt7b mutant (MT) littermates.
(I) In situ hybridization for Dkk1 and Tcf1 on longitudinal sections of humerus at E15.5. Asterisks: signal in chondrocytes; arrows: signal in perichondrium; PH: prehypertrophic chondrocytes.
(J) Wnt7b signals through a Gαq/11→PLC→PKCδpathway to stimulate progression from Runx2- to Osx-expressing cells during osteoblastogenesis.
We next examined whether chondrocyte maturation was perturbed in Wnt7b mutants. At E14.5, the overall length of the hypertrophic zone expressing Colα1(X) was similar between mutant and wild type littermates (Fig. 7F). However, in mutants the domains expressing PTHrP-R or Ihh were less well separated, and the terminal hypertrophic region expressing MMP13 was clearly reduced. At E15.5, the distance between the two Colα1(X)-expressing domains was significantly reduced in the mutant. Thus, loss of Wnt7b appears to delay subsequent maturation of chondrocytes after the initiation of Colα1(X) expression.
To examine potential intrinsic defects in the osteoblast lineage, we assayed the expression of AP, Bsp, Runx2, Osx and PTHrP-R, in relation to Colα1(X) on adjacent sections of E15.5 long bones. Whereas AP and Runx2 expression in the perichondrium appeared indistinguishable between mutant and wild type littermates, the leading edges for Osx, PTHrP-R and Bsp were consistently closer to the boundary of the hypertrophic zone in the mutant (Fig. 7G). Thus, removal of Wnt7b, similar to that of PKCδ, results in a deficit in Osx activation and subsequent osteoblast differentiation.
To determine whether removal of Wnt7b disrupted canonical Wnt signaling in long bones, we performed in situ hybridization for Dkk1 and Tcf1, two known target genes of the pathway. Both molecules were expressed normally in Wnt7b mutant embryos (Fig. 7I). In contrast, the limb primordial cells from E14.5 mutant embryos contained a significantly lower level of phospho-MARCKS than wild type littermates (Fig. 7E), indicating that Wnt7b stimulates MARCKS phosphorylation in vivo. Thus, the bone defect in Wnt7b mutant embryos is unlikely due to disruption of canonical Wnt signaling, but appears to correlate with impairment in PKCδ activation.
To confirm that Wnt7b directly regulates osteoblast differentiation, we evaluated Wnt7b-deficient cells for their ability to differentiate in vitro. We utilized calvarial cells from neonates, as well as BMSC from adult mice, both containing osteoblast precursors. In both cases, Wnt7b-deficient cells produced significantly fewer bone nodules than wild type cells (Fig. 7H). These results are consistent with the notion that endogenous Wnt7b promotes osteoblast differentiation from progenitors.
In summary, the present study reveals a novel osteogenic pathway in which Wnt molecules, such as Wnt7b, signal through the Gq family of G protein α-subunits to activate PKCδ that in turn promotes the transition from Runx2- to Osx-expressing cells (Fig. 7J).
Discussion
We have delineated a noncanonical Wnt pathway that operates in mammalian osteoblast precursors to promote bone formation. In this pathway, Wnt activates the G protein-linked phosphatidylinositol signaling and subsequently PKCδ, via a mechanism that requires Dvl but is insensitive to Dkk1. Several lines of evidence support that Wnt7b, expressed in osteogenic tissues in vivo, stimulates osteoblast differentiation likely through the PKCδ-mediated pathway. First, genetic ablation of either Wnt7b or PKCδ delayed the onset of Osx expression and reduced embryonic bone; second, Wnt7b- or PKCδ-deficient osteoprogenitors were defective in osteoblastogenesis in vitro; third, deletion of either gene reduced MARCKS phosphorylation in vivo.
G proteins and Wnt signaling
Although the Gαo subunit was recently found to mediate both the Wnt and the planar polarity pathway in Drosophila (Katanaev et al., 2005), the role of G proteins in Wnt signaling in mammals is not clear. In mouse F9 teratocarcinoma cells, overexpression of chimeric receptors between the −2-adrenergic receptor and rat Frizzled-1 or −2, was reported to respectively activate the canonical Wnt pathway (Liu et al., 2001), or decrease intracellular cGMP (Ahumada et al., 2002), both in a pertussis toxin-sensitive manner. More recently, both Gαo and Gαq were found to mediate Wnt3a-induced stabilization of β-catenin in cell cultures (Liu et al., 2005). However, the physiological relevance of these findings is not known. Finally, adenylyl cyclase signaling was implicated in Wnt-induced myogenesis (Chen et al., 2005), but a direct role of G proteins remains to be confirmed.
The present study indicate that the Gq family of α-subunits is required for Wnt-induced PKCδactivation but not β-catenin stabilization in osteoprogenitors, and that Gq signaling is important for Wnt-induced osteoblast differentiation. These conclusions are supported by studies using a dominant negative reagent (GqI) and specific siRNA oligonucleotides against Gαq and Gα11. Of note, mice missing both alleles of Gαq and one copy of Gα11 (Gαq−/−; Gα11+/−) developed to term but exhibited severe bone defects in the craniofacial skeleton (Offermanns et al., 1998). In addition, in Xenopus embryos, Gq but not Gi signaling mediated XWnt8a-induced axis duplication as well as mesoderm ventralization (Wu et al., 2000), although it was not clear whether in that system Gq signaled through β-catenin or an alternative pathway. Finally, it may be of interest to examine whether similar G-protein signaling mediates cardiomyocyte differentiation induced by Wnt11, previously reported to be independent of β-catenin signaling (Koyanagi et al., 2005; Pandur et al., 2002).
Canonical versus noncanonical Wnt signaling
The mechanism for activating canonical versus noncanonical pathways by Wnt molecules is not clear. Wnt3a signaled through both the β-catenin and the PKCδ pathway in ST2 cells whereas Wnt7b selectively activated the later in multiple cell types. The two pathways differed not only in their dependence on the LRP5/6 co-receptor, but also in their requirement for Gq signaling. This could indicate that Wnt3a induces formation of two distinct signaling complexes, or a single complex with dual signaling properties. The specificity of signaling complexes may be dictated by the Frizzled receptor(s), as forced expression of mouse Frizzled 4 in HEK293 cells (Mikels and Nusse, 2006) or human Frizzled 5 in Xenopus embryos (He et al., 1997) was sufficient to transduce canonical signaling by Wnt5a which was otherwise inactive in this regard. Moreover, overexpression of different mouse Frizzled molecules in the Xenopus embryo was found to activate either classic PKC or downstream target genes of β-catenin (Sheldahl et al., 1999). Finally, Wnts may signal through receptors other than Frizzleds, such as the atypical receptor kinase Ryk (Lu et al., 2004; Schmitt et al., 2006), or the orphan receptor tyrosine kinase Ror2 (Mikels and Nusse, 2006). Identification of responsible Frizzled or alternative receptors for different Wnts under physiological conditions will help to elucidate the mechanism for Wnt ligands to generate distinct signals.
Wnt7b, PKC and bone formation
Consistent with the role of Wnt-PKCδ signaling in osteoblastogenesis in vitro, genetic ablation of either Wnt7b or PKCδ resulted in a deficit in bone formation in the mouse embryo. Nonetheless bone formed in both mutants. The modest phenotype could reflect overlapping roles of other Wnts or PKC isoforms important for osteoblastogenesis. Alternatively, Wnt-PKCδ signaling could represent a mechanism that augments other osteogenic signals, but itself is not essential for osteoblast differentiation in vivo. In this scenario, the noncanonical pathway appears to perform a function distinct from that of canonical Wnt signaling, as thus far inferred from genetic studies of β-catenin (Day et al., 2005; Hill et al., 2005; Hu et al., 2005; Rodda and McMahon, 2006).
The mechanism for Wnt7b and PKCδ to regulate the onset of Osx expression is presently unknown. Although an immediate target of Wnt-PKCδ signaling, MARCKS has not been implicated in bone formation. The MARCKS−/− mice had severe defects in neural development and exhibited perinatal lethality (Stumpo et al., 1995), but analyses of the skeletons of these mutant embryos have so far not revealed any obvious defects (data not shown). It is possible that other PKCδ substrates mediate osteoblast differentiation.
Frizzled-G protein signaling as a target for bone anabolic therapeutics
The present study identified a novel mechanism for Wnt signals to stimulate bone formation. Importantly, the new pathway can be uncoupled from canonical Wnt signaling, and is mediated through specific G proteins likely coupled with the Frizzled receptors. Thus, reagents that selectively activate G protein signaling by the relevant receptors may provide specific bone anabolic effects.
Experimental Procedures
Plasmids and oligonucleotides
Full-length Wnt7b cDNA was purchased from ATCC. The Lef1-luciferase reporter (Mao et al., 2001b) and the cDNAs for the dominant active form of β-catenin (Tetsu and McCormick, 1999), GqI (Akhter et al., 1998), Dvl-2 derivatives (Habas et al., 2001), were as previously described. The full-length cDNA for PKCδ was cloned by PCR from a mouse E15 cDNA pool Marathon-Ready (BD Biosciences Clontech), and the cDNA for the truncated form (PKCδ-ΔC, a.a. 1–338) was generated by a second PCR from the full-length cDNA. The siRNA oligonucleotides for PKCδ were purchased from Dharmacon, and those for Gαq and Gα11 were from Ambion.
Antibodies, proteins and chemicals
The polyclonal antibodies against PKCδ, MARCKS, the monoclonal antibodies against β-tubulin, Dvl-1, Dvl-2 and Dvl-3 and the HRP-conjugated secondary antibodies were purchased from Santa Cruz Biotechnology. The monoclonal antibodies against glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and β-catenin were from Chemicon, and BD Biosciences Pharmigen, respectively. The polyclonal antibody against phospho-MARCKS was from Cell Signaling. The polyclonal antibody against Gαq and Gα11 was from Calbiochem. The Alexa 488 or 555-conjugated secondary antibodies were from Molecular Probes. Purified recombinant Wnt3a was from R&D systems. All inhibitors for PKC and PLC were from Calbiochem.
Cell cultures
C3H10T1/2 cells, Wnt3a-expressing and control L cells were obtained from ATCC and maintained in BME with 10% bovine serum (Atlas) as per instructions. ST2 cells (Dr. Steve Teitelbaum, Washington University) were maintained in α-MEM (Sigma). Unless otherwise indicated, ST2 cells were seeded at 1.5x104 cells/cm2 overnight before experiments. Wnt3a and L conditioned medium were used at 1:2 dilution in normal growth medium. Serum-free medium Ultraculture was purchased from Cambrex.
Primary cultures of limb primordial cells were dissociated from the stylopod and zeugopod region of the forelimb of E13.5 mouse embryos; primary cultures of the calvarial cells were isolated from 2-day-old newborn mice. Cells were seeded at 1x105 cells/cm2 in α-MEM with 10% bovine serum, and used without passage.
Primary cultures of the bone marrow stromal cells (BMSC) were isolated from the femur and the tibia of 5-week-old mice, and plated in α-MEM with 15% bovine serum, with medium changed at day 3 and day 6. Cells were passed once at day 7–8 and reseeded at 1x105 cells/cm2 for AP or mineralization assays.
Infections and transfections
Viruses expressing GFP or Dkk1 were as previously described (Hu et al., 2005). Viruses expressing Wnt7b, PKCδ, PKCδ-ΔC, GqI or the Dvl-2 derivatives were generated in the same manner, and all co-expressed nuclear GFP via an internal ribosome entry site (IRES). A proper dilution of each virus stock in appropriate growth medium was chosen to achieve >90% infection as per GFP detection. For infections, cells were incubated at about 50% confluency in virus-containing medium for 24 hrs.
Transient transfections were performed using Lipofectamine (Invitrogen), and in some cases after cells were infected with viruses. Luciferase and AP assays were performed at 48 hrs after transfection.
Transfections of siRNA oligonucleotides were performed using siPORTTM Amine (Ambion). Four distinct siRNA duplexes each for Gαq and Gα11 were individually tested; two in each case were found to be effective and used in experiments reported here. For PKCδ, a pool of four siRNA duplexes was used.
Osteoblast differentiation assays
AP expression was detected as previously described (Katagiri et al., 1994). When inhibitors were used, cells were pretreated with the inhibitor in normal growth medium for 1 hr. For mineralization assays, confluent cells were incubated in the presence of 50 μg/ml ascorbic acid and 50 mM β-glycerophosphate for 14–21 days. Real-time PCR for osteoblast markers was performed as previously described (Hu et al., 2005).
Proteomics, Western analyses and immunocytochemistry
For proteomics, ST2 cells were cultured in Wnt3a or L medium for 24 hrs before total cell lysates were harvested using a lysis buffer containing a protease inhibitor cocktail (Roche) and phosphatase inhibitor cocktail 1 and 2 (Sigma). Proteomic analyses were performed at the Siteman Cancer Center Proteomics Core Facility (Washington University).
Western analyses were performed using ECL Plus Western Blotting Detection System (Amersham Biosciences). The intensity of protein bands was quantified using ImageJ (http://rsb.info.nih.gov/ij/). For analyses of mutant embryos, E14.5 limbs were skinned, homogenized and extracted for cytosolic proteins in the presence of protease and phosphatase inhibitors.
Immunocytochemistry was performed on chamber slides (Nalge Nunc International). Cells seeded at 0.75x104/cm2 were cultured overnight in regular medium, and then either directly switched to serum-free medium for 24 hrs, or first infected with retroviruses before changing to serum-free medium. Cells were then stimulated in fresh serum-free medium for 30 minutes with recombinant Wnt3a protein at 50 ng/ml, and finally immunostained and examined by confocal microscopy.
Mouse strains and analyses
The PKCδ+/− (Miyamoto et al., 2002), the Dermo1-Cre (Yu et al., 2003) and the Wnt7b+/− (Parr et al., 2001) mouse strains were previously described. Whole-mount skeletal staining, mouse embryo tissue processing, von Kossa staining and in situ hybridization were performed as described (Hilton et al., 2005). To quantitate the relative bone length, multiple sections representing different sectioning planes were stained by von Kossa, and ratios of bone collar length over total length of the element were calculated.
Supplementary Material
Acknowledgments
We are indebted to Drs. Xi He, Walter Koch, Christopher Niehrs, Frank McCormick, Steve Teitelbaum for providing reagents. We thank Drs. Deborah Stumpo and Perry Blackshear (NIEHS) for sharing MARCKS−/− mouse embryos. The work was supported in part by NIH grants R01 DK065789 (FL) and P01 DK056246 (APM). KJ was supported by the Korea Science and Engineering Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahumada A, Slusarski DC, Liu X, Moon RT, Malbon CC, Wang HY. Signaling of rat Frizzled-2 through phosphodiesterase and cyclic GMP. Science. 2002;298:2006–2010. doi: 10.1126/science.1073776. [DOI] [PubMed] [Google Scholar]
- Akhter SA, Luttrell LM, Rockman HA, Iaccarino G, Lefkowitz RJ, Koch WJ. Targeting the receptor-Gq interface to inhibit in vivo pressure overload myocardial hypertrophy. Science. 1998;280:574–577. doi: 10.1126/science.280.5363.574. [DOI] [PubMed] [Google Scholar]
- Arbuzova A, Schmitz AA, Vergeres G. Cross-talk unfolded: MARCKS proteins. Biochem J. 2002;362:1–12. doi: 10.1042/0264-6021:3620001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bafico A, Liu G, Yaniv A, Gazit A, Aaronson SA. Novel mechanism of Wnt signalling inhibition mediated by Dickkopf-1 interaction with LRP6/Arrow. Nat Cell Biol. 2001;3:683–686. doi: 10.1038/35083081. [DOI] [PubMed] [Google Scholar]
- Bennett CN, Longo KA, Wright WS, Suva LJ, Lane TF, Hankenson KD, MacDougald OA. Regulation of osteoblastogenesis and bone mass by Wnt10b. Proc Natl Acad Sci U S A. 2005;102:3324–3329. doi: 10.1073/pnas.0408742102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackshear PJ. The MARCKS family of cellular protein kinase C substrates. J Biol Chem. 1993;268:1501–1504. [PubMed] [Google Scholar]
- Bodine PV, Zhao W, Kharode YP, Bex FJ, Lambert AJ, Goad MB, Gaur T, Stein GS, Lian JB, Komm BS. The Wnt antagonist secreted frizzled-related protein-1 is a negative regulator of trabecular bone formation in adult mice. Mol Endocrinol. 2004;18:1222–1237. doi: 10.1210/me.2003-0498. [DOI] [PubMed] [Google Scholar]
- Boyden LM, Mao J, Belsky J, Mitzner L, Farhi A, Mitnick MA, Wu D, Insogna K, Lifton RP. High bone density due to a mutation in LDL-receptor-related protein 5. N Engl J Med. 2002;346:1513–1521. doi: 10.1056/NEJMoa013444. [DOI] [PubMed] [Google Scholar]
- Cadigan KM, Nusse R. Wnt signaling: a common theme in animal development. Genes Dev. 1997;11:3286–3305. doi: 10.1101/gad.11.24.3286. [DOI] [PubMed] [Google Scholar]
- Chamorro MN, Schwartz DR, Vonica A, Brivanlou AH, Cho KR, Varmus HE. FGF-20 and DKK1 are transcriptional targets of beta-catenin and FGF-20 is implicated in cancer and development. Embo J. 2005;24:73–84. doi: 10.1038/sj.emboj.7600460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen AE, Ginty DD, Fan CM. Protein kinase A signalling via CREB controls myogenesis induced by Wnt proteins. Nature. 2005;433:317–322. doi: 10.1038/nature03126. [DOI] [PubMed] [Google Scholar]
- Choi SC, Han JK. Xenopus Cdc42 regulates convergent extension movements during gastrulation through Wnt/Ca2+ signaling pathway. Dev Biol. 2002;244:342–357. doi: 10.1006/dbio.2002.0602. [DOI] [PubMed] [Google Scholar]
- Curtin JA, Quint E, Tsipouri V, Arkell RM, Cattanach B, Copp AJ, Henderson DJ, Spurr N, Stanier P, Fisher EM, et al. Mutation of Celsr1 disrupts planar polarity of inner ear hair cells and causes severe neural tube defects in the mouse. Curr Biol. 2003;13:1129–1133. doi: 10.1016/s0960-9822(03)00374-9. [DOI] [PubMed] [Google Scholar]
- Day TF, Guo X, Garrett-Beal L, Yang Y. Wnt/beta-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis. Dev Cell. 2005;8:739–750. doi: 10.1016/j.devcel.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Glass DA, 2nd, Bialek P, Ahn JD, Starbuck M, Patel MS, Clevers H, Taketo MM, Long F, McMahon AP, Lang RA, Karsenty G. Canonical Wnt signaling in differentiated osteoblasts controls osteoclast differentiation. Dev Cell. 2005;8:751–764. doi: 10.1016/j.devcel.2005.02.017. [DOI] [PubMed] [Google Scholar]
- Glinka A, Wu W, Delius H, Monaghan AP, Blumenstock C, Niehrs C. Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature. 1998;391:357–362. doi: 10.1038/34848. [DOI] [PubMed] [Google Scholar]
- Gong Y, Slee RB, Fukai N, Rawadi G, Roman-Roman S, Reginato AM, Wang H, Cundy T, Glorieux FH, Lev D, et al. LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell. 2001;107:513–523. doi: 10.1016/s0092-8674(01)00571-2. [DOI] [PubMed] [Google Scholar]
- Habas R, Dawid IB, He X. Coactivation of Rac and Rho by Wnt/Frizzled signaling is required for vertebrate gastrulation. Genes Dev. 2003;17:295–309. doi: 10.1101/gad.1022203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habas R, Kato Y, He X. Wnt/Frizzled activation of Rho regulates vertebrate gastrulation and requires a novel Formin homology protein Daam1. Cell. 2001;107:843–854. doi: 10.1016/s0092-8674(01)00614-6. [DOI] [PubMed] [Google Scholar]
- He X, Saint-Jeannet JP, Wang Y, Nathans J, Dawid I, Varmus H. A member of the Frizzled protein family mediating axis induction by Wnt-5A. Science. 1997;275:1652–1654. doi: 10.1126/science.275.5306.1652. [DOI] [PubMed] [Google Scholar]
- Heisenberg CP, Tada M, Rauch GJ, Saude L, Concha ML, Geisler R, Stemple DL, Smith JC, Wilson SW. Silberblick/Wnt11 mediates convergent extension movements during zebrafish gastrulation. Nature. 2000;405:76–81. doi: 10.1038/35011068. [DOI] [PubMed] [Google Scholar]
- Hill TP, Spater D, Taketo MM, Birchmeier W, Hartmann C. Canonical Wnt/beta-catenin signaling prevents osteoblasts from differentiating into chondrocytes. Dev Cell. 2005;8:727–738. doi: 10.1016/j.devcel.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Hilton MJ, Tu X, Cook J, Hu H, Long F. Ihh controls cartilage development by antagonizing Gli3, but requires additional effectors to regulate osteoblast and vascular development. Development. 2005;132:4339–4351. doi: 10.1242/dev.02025. [DOI] [PubMed] [Google Scholar]
- Hu H, Hilton MJ, Tu X, Yu K, Ornitz DM, Long F. Sequential roles of Hedgehog and Wnt signaling in osteoblast development. Development. 2005;132:49–60. doi: 10.1242/dev.01564. [DOI] [PubMed] [Google Scholar]
- Huelsken J, Birchmeier W. New aspects of Wnt signaling pathways in higher vertebrates. Curr Opin Genet Dev. 2001;11:547–553. doi: 10.1016/s0959-437x(00)00231-8. [DOI] [PubMed] [Google Scholar]
- Katagiri T, Yamaguchi A, Komaki M, Abe E, Takahashi N, Ikeda T, Rosen V, Wozney JM, Fujisawa-Sehara A, Suda T. Bone morphogenetic protein-2 converts the differentiation pathway of C2C12 myoblasts into the osteoblast lineage. J Cell Biol. 1994;127:1755–1766. doi: 10.1083/jcb.127.6.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katanaev VL, Ponzielli R, Semeriva M, Tomlinson A. Trimeric G protein-dependent frizzled signaling in Drosophila. Cell. 2005;120:111–122. doi: 10.1016/j.cell.2004.11.014. [DOI] [PubMed] [Google Scholar]
- Kato M, Patel MS, Levasseur R, Lobov I, Chang BH, Glass DA, 2nd, Hartmann C, Li L, Hwang TH, Brayton CF, et al. Cbfa1-independent decrease in osteoblast proliferation, osteopenia, and persistent embryonic eye vascularization in mice deficient in Lrp5, a Wnt coreceptor. J Cell Biol. 2002;157:303–314. doi: 10.1083/jcb.200201089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kibar Z, Vogan KJ, Groulx N, Justice MJ, Underhill DA, Gros P. Ltap, a mammalian homolog of Drosophila Strabismus/Van Gogh, is altered in the mouse neural tube mutant Loop-tail. Nat Genet. 2001;28:251–255. doi: 10.1038/90081. [DOI] [PubMed] [Google Scholar]
- Kinoshita N, Iioka H, Miyakoshi A, Ueno N. PKC delta is essential for Dishevelled function in a noncanonical Wnt pathway that regulates Xenopus convergent extension movements. Genes Dev. 2003;17:1663–1676. doi: 10.1101/gad.1101303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyanagi M, Haendeler J, Badorff C, Brandes RP, Hoffmann J, Pandur P, Zeiher AM, Kuhl M, Dimmeler S. Non-canonical Wnt signaling enhances differentiation of human circulating progenitor cells to cardiomyogenic cells. J Biol Chem. 2005;280:16838–16842. doi: 10.1074/jbc.M500323200. [DOI] [PubMed] [Google Scholar]
- Kuhl M, Sheldahl LC, Malbon CC, Moon RT. Ca(2+)/calmodulin-dependent protein kinase II is stimulated by Wnt and Frizzled homologs and promotes ventral cell fates in Xenopus. J Biol Chem. 2000;275:12701–12711. doi: 10.1074/jbc.275.17.12701. [DOI] [PubMed] [Google Scholar]
- Lee PN, Pang K, Matus DQ, Martindale MQ. A WNT of things to come: evolution of Wnt signaling and polarity in cnidarians. Semin Cell Dev Biol. 2006;17:157–167. doi: 10.1016/j.semcdb.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Leitges M, Mayr M, Braun U, Mayr U, Li C, Pfister G, Ghaffari-Tabrizi N, Baier G, Hu Y, Xu Q. Exacerbated vein graft arteriosclerosis in protein kinase Cdelta-null mice. J Clin Invest. 2001;108:1505–1512. doi: 10.1172/JCI12902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little RD, Carulli JP, Del Mastro RG, Dupuis J, Osborne M, Folz C, Manning SP, Swain PM, Zhao SC, Eustace B, et al. A mutation in the LDL receptor-related protein 5 gene results in the autosomal dominant high-bone-mass trait. Am J Hum Genet. 2002;70:11–19. doi: 10.1086/338450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, DeCostanzo AJ, Liu X, Wang H, Hallagan S, Moon RT, Malbon CC. G protein signaling from activated rat frizzled-1 to the beta-catenin-Lef-Tcf pathway. Science. 2001;292:1718–1722. doi: 10.1126/science.1060100. [DOI] [PubMed] [Google Scholar]
- Liu X, Rubin JS, Kimmel AR. Rapid, Wnt-induced changes in GSK3beta associations that regulate beta-catenin stabilization are mediated by Galpha proteins. Curr Biol. 2005;15:1989–1997. doi: 10.1016/j.cub.2005.10.050. [DOI] [PubMed] [Google Scholar]
- Lu W, Yamamoto V, Ortega B, Baltimore D. Mammalian Ryk is a Wnt coreceptor required for stimulation of neurite outgrowth. Cell. 2004;119:97–108. doi: 10.1016/j.cell.2004.09.019. [DOI] [PubMed] [Google Scholar]
- Mao B, Wu W, Li Y, Hoppe D, Stannek P, Glinka A, Niehrs C. LDL-receptor-related protein 6 is a receptor for Dickkopf proteins. Nature. 2001a;411:321–325. doi: 10.1038/35077108. [DOI] [PubMed] [Google Scholar]
- Mao J, Wang J, Liu B, Pan W, Farr GH, 3rd, Flynn C, Yuan H, Takada S, Kimelman D, Li L, Wu D. Low-density lipoprotein receptor-related protein-5 binds to Axin and regulates the canonical Wnt signaling pathway. Mol Cell. 2001b;7:801–809. doi: 10.1016/s1097-2765(01)00224-6. [DOI] [PubMed] [Google Scholar]
- Mikels AJ, Nusse R. Purified Wnt5a protein activates or inhibits beta-catenin-TCF signaling depending on receptor context. PLoS Biol. 2006;4:e115. doi: 10.1371/journal.pbio.0040115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto A, Nakayama K, Imaki H, Hirose S, Jiang Y, Abe M, Tsukiyama T, Nagahama H, Ohno S, Hatakeyama S, Nakayama KI. Increased proliferation of B cells and auto-immunity in mice lacking protein kinase Cdelta. Nature. 2002;416:865–869. doi: 10.1038/416865a. [DOI] [PubMed] [Google Scholar]
- Montcouquiol M, Rachel RA, Lanford PJ, Copeland NG, Jenkins NA, Kelley MW. Identification of Vangl2 and Scrb1 as planar polarity genes in mammals. Nature. 2003;423:173–177. doi: 10.1038/nature01618. [DOI] [PubMed] [Google Scholar]
- Morris AJ, Malbon CC. Physiological regulation of G protein-linked signaling. Physiol Rev. 1999;79:1373–1430. doi: 10.1152/physrev.1999.79.4.1373. [DOI] [PubMed] [Google Scholar]
- Newton AC. Regulation of protein kinase C. Curr Opin Cell Biol. 1997;9:161–167. doi: 10.1016/s0955-0674(97)80058-0. [DOI] [PubMed] [Google Scholar]
- Niida A, Hiroko T, Kasai M, Furukawa Y, Nakamura Y, Suzuki Y, Sugano S, Akiyama T. DKK1, a negative regulator of Wnt signaling, is a target of the beta-catenin/TCF pathway. Oncogene. 2004;23:8520–8526. doi: 10.1038/sj.onc.1207892. [DOI] [PubMed] [Google Scholar]
- Offermanns S, Zhao LP, Gohla A, Sarosi I, Simon MI, Wilkie TM. Embryonic cardiomyocyte hypoplasia and craniofacial defects in G alpha q/G alpha 11-mutant mice. Embo J. 1998;17:4304–4312. doi: 10.1093/emboj/17.15.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa M, Nishikawa S, Ikuta K, Yamamura F, Naito M, Takahashi K. B cell ontogeny in murine embryo studied by a culture system with the monolayer of a stromal cell clone, ST2: B cell progenitor develops first in the embryonal body rather than in the yolk sac. Embo J. 1988;7:1337–1343. doi: 10.1002/j.1460-2075.1988.tb02949.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandur P, Lasche M, Eisenberg LM, Kuhl M. Wnt-11 activation of a non-canonical Wnt signalling pathway is required for cardiogenesis. Nature. 2002;418:636–641. doi: 10.1038/nature00921. [DOI] [PubMed] [Google Scholar]
- Parr BA, Cornish VA, Cybulsky MI, McMahon AP. Wnt7b regulates placental development in mice. Dev Biol. 2001;237:324–332. doi: 10.1006/dbio.2001.0373. [DOI] [PubMed] [Google Scholar]
- Penzo-Mendez A, Umbhauer M, Djiane A, Boucaut JC, Riou JF. Activation of Gbetagamma signaling downstream of Wnt-11/Xfz7 regulates Cdc42 activity during Xenopus gastrulation. Dev Biol. 2003;257:302–314. doi: 10.1016/s0012-1606(03)00067-8. [DOI] [PubMed] [Google Scholar]
- Pinson KI, Brennan J, Monkley S, Avery BJ, Skarnes WC. An LDL-receptor-related protein mediates Wnt signalling in mice. Nature. 2000;407:535–538. doi: 10.1038/35035124. [DOI] [PubMed] [Google Scholar]
- Rodda SJ, McMahon AP. Distinct roles for Hedgehog and canonical Wnt signaling in specification, differentiation and maintenance of osteoblast progenitors. Development. 2006;133:3231–3244. doi: 10.1242/dev.02480. [DOI] [PubMed] [Google Scholar]
- Schmitt AM, Shi J, Wolf AM, Lu CC, King LA, Zou Y. Wnt-Ryk signalling mediates medial-lateral retinotectal topographic mapping. Nature. 2006;439:31–37. doi: 10.1038/nature04334. [DOI] [PubMed] [Google Scholar]
- Semenov MV, Tamai K, Brott BK, Kuhl M, Sokol S, He X. Head inducer Dickkopf-1 is a ligand for Wnt coreceptor LRP6. Curr Biol. 2001;11:951–961. doi: 10.1016/s0960-9822(01)00290-1. [DOI] [PubMed] [Google Scholar]
- Sheldahl LC, Park M, Malbon CC, Moon RT. Protein kinase C is differentially stimulated by Wnt and Frizzled homologs in a G-protein-dependent manner. Curr Biol. 1999;9:695–698. doi: 10.1016/s0960-9822(99)80310-8. [DOI] [PubMed] [Google Scholar]
- Sheldahl LC, Slusarski DC, Pandur P, Miller JR, Kuhl M, Moon RT. Dishevelled activates Ca2+ flux, PKC, and CamKII in vertebrate embryos. J Cell Biol. 2003;161:769–777. doi: 10.1083/jcb.200211094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slusarski DC, Corces VG, Moon RT. Interaction of Wnt and a Frizzled homologue triggers G-protein-linked phosphatidylinositol signalling. Nature. 1997;390:410–413. doi: 10.1038/37138. [DOI] [PubMed] [Google Scholar]
- Stumpo DJ, Bock CB, Tuttle JS, Blackshear PJ. MARCKS deficiency in mice leads to abnormal brain development and perinatal death. Proc Natl Acad Sci U S A. 1995;92:944–948. doi: 10.1073/pnas.92.4.944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada M, Smith JC. Xwnt11 is a target of Xenopus Brachyury: regulation of gastrulation movements via Dishevelled, but not through the canonical Wnt pathway. Development. 2000;127:2227–2238. doi: 10.1242/dev.127.10.2227. [DOI] [PubMed] [Google Scholar]
- Tamai K, Semenov M, Kato Y, Spokony R, Liu C, Katsuyama Y, Hess F, Saint-Jeannet JP, He X. LDL-receptor-related proteins in Wnt signal transduction. Nature. 2000;407:530–535. doi: 10.1038/35035117. [DOI] [PubMed] [Google Scholar]
- Taylor SM, Jones PA. Multiple new phenotypes induced in 10T1/2 and 3T3 cells treated with 5-azacytidine. Cell. 1979;17:771–779. doi: 10.1016/0092-8674(79)90317-9. [DOI] [PubMed] [Google Scholar]
- Tetsu O, McCormick F. Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature. 1999;398:422–426. doi: 10.1038/18884. [DOI] [PubMed] [Google Scholar]
- Veeman MT, Axelrod JD, Moon RT. A second canon. Functions and mechanisms of beta-catenin-independent Wnt signaling. Dev Cell. 2003;5:367–377. doi: 10.1016/s1534-5807(03)00266-1. [DOI] [PubMed] [Google Scholar]
- Wallingford JB, Rowning BA, Vogeli KM, Rothbacher U, Fraser SE, Harland RM. Dishevelled controls cell polarity during Xenopus gastrulation. Nature. 2000;405:81–85. doi: 10.1038/35011077. [DOI] [PubMed] [Google Scholar]
- Wang J, Hamblet NS, Mark S, Dickinson ME, Brinkman BC, Segil N, Fraser SE, Chen P, Wallingford JB, Wynshaw-Boris A. Dishevelled genes mediate a conserved mammalian PCP pathway to regulate convergent extension during neurulation. Development. 2006;133:1767–1778. doi: 10.1242/dev.02347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Mark S, Zhang X, Qian D, Yoo SJ, Radde-Gallwitz K, Zhang Y, Lin X, Collazo A, Wynshaw-Boris A, Chen P. Regulation of polarized extension and planar cell polarity in the cochlea by the vertebrate PCP pathway. Nat Genet. 2005;37:980–985. doi: 10.1038/ng1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wodarz A, Nusse R. Mechanisms of Wnt signaling in development. Annu Rev Cell Dev Biol. 1998;14:59–88. doi: 10.1146/annurev.cellbio.14.1.59. [DOI] [PubMed] [Google Scholar]
- Wu C, Zeng Q, Blumer KJ, Muslin AJ. RGS proteins inhibit Xwnt-8 signaling in Xenopus embryonic development. Development. 2000;127:2773–2784. doi: 10.1242/dev.127.13.2773. [DOI] [PubMed] [Google Scholar]
- Yu K, Xu J, Liu Z, Sosic D, Shao J, Olson EN, Towler DA, Ornitz DM. Conditional inactivation of FGF receptor 2 reveals an essential role for FGF signaling in the regulation of osteoblast function and bone growth. Development. 2003;130:3063–3074. doi: 10.1242/dev.00491. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


