Abstract
It was recently found that cooling the skin to temperatures as mild as 25°–30°C can induce nociceptive sensations (burning, stinging or pricking) that are strongly suppressed by dynamic contact between the thermode and skin (contact suppression). Here we investigated whether nociceptive sensations produced by menthol can be similarly suppressed. In the first experiment subjects rated the intensity of cold and burning/stinging/pricking sensations before and after application of 10% l-menthol to the forearm. Ratings were compared at resting skin temperature (≈ 33°C) and at 28°, 24°, or 20°C during static or dynamic contact cooling via a Peltier thermode. At resting skin temperature, menthol produced cold and nociceptive sensations, both of which were suppressed by dynamic contact. When the skin was cooled during static contact, menthol increased nociceptive sensations but not cold sensations; when the skin was cooled during dynamic contact, cold sensations were again unchanged while nociceptive sensations were suppressed. A second experiment tested whether contact suppression of menthol’s cold and nociceptive sensations at resting skin temperature was caused by slight deviations of thermode temperature above skin temperature. The results showed that suppression occurred even when the thermode was slightly cooler (−0.5°C) than the skin. These findings support other evidence that the menthol-sensitive channel, TRPM8, plays a role in cold nociception, and raise new questions about how dynamic tactile stimulation may modify perception of nonpainful cold stimulation.
Keywords: menthol, cold, nociception, touch, inhibition, psychophysics
Introduction
Cooling the skin to temperatures above the cold pain threshold has been assumed to produce only sensations of cold. Recent evidence [1] indicates that sensations of burning, stinging, or pricking can be evoked at temperatures as mild as 25° to 31°C when cooling occurs after a thermode is already in contact with the skin (i.e., static contact cooling). However, these sensations, which were termed innocuous cold nociception (ICN), can be greatly reduced when cooling occurs by touching an already cold thermode to the skin (i.e., dynamic contact cooling). Additional experiments indicated that the reduction in ICN during dynamic contact cooling was most likely caused by tactile stimulation produced as the thermode touched the skin [1;2].
The occurrence of ICN provides evidence that mild cooling stimulates the nociceptive system as well as the cold system. This finding raises questions about the longstanding assumption that painful and nonpainful cold are mediated by afferent fibers that have very different sensitivities to cold. Although examples can be found in the literature of C- or A-δ fibers that respond to noxious cold yet have thresholds above 25°C [3–5], such fibers have not been considered important for perception of nonpainful cold. Consistent with this view, the nonspecific cation channel TRPM8 [6–8], which is sensitive to menthol and has a threshold to cooling ≤28°C, has been designated as a cold receptor. But menthol can induce nociceptive sensations of burning, stinging or pricking as well as cold sensations [9–12], and a recent study demonstrated that topically applied menthol can induce cold hyperalgesia [13]. In addition, there is evidence that some fibers that express TRPM8 project in the nociceptive system [14], and TRPM8 has been reported to be co-expressed in rat dorsal root ganglion (DRG) neurons with the heat, irritant- and pH-sensitive channel, TRPV1 [15;16]. Although other studies have failed to find co-expression of TRPM8 and TRPV1, the weight of the current evidence points to TRPM8 as the receptor most likely to mediate ICN.
Accordingly, the primary objective of the present study was to determine whether nociceptive sensations produced by menthol could be suppressed by dynamic contact. The approach was to measure the intensity of nociceptive sensations produced by topically applied menthol under conditions of static and dynamic contact at both resting skin temperature (RST) and during active cooling. Suppression of menthol’s nociceptive sensations by dynamic contact would imply that menthol stimulates the same class of fibers that mediate ICN, and that these fibers are a type of nociceptor that responds to mild cooling. The results of two experiments supported this hypothesis. After finding in the first experiment that at RST dynamic contact suppressed menthol’s cold sensations as well as its nociceptive sensations, we ran a second experiment to rule out the possibility that suppression of cold might have resulted from systematic miss-adjustments of thermode temperature rather than from dynamic mechanical contact.
Methods
Subjects
Thirty-nine subjects (21 females and 18 males) served in exp. 1, and 25 (14 females and 11 males) served in exp. 2. All were self-reported healthy individuals between the ages of 18 and 45 yrs with no history of nerve injury or neuropathology.
Stimuli
Thermal stimuli were delivered via a 16-channel thermode composed of independently controllable, 8mm x 8mm Peltier thermoelectric modules arranged in a 4 x 4 matrix with 2-mm separations between neighboring modules. The 16 modules were bonded with thermally conductive epoxy to a water-circulated heat sink [2;17]. Skin-thermode interface temperature was monitored by a 40-ga type-T thermocouple recessed into the face plate of each module. Baseline temperature, target temperature (28°, 24°, 20°C), rate of temperature change (5°/sec), and dwell time (5 sec) were controlled with LabView software. The thermode was fixed to a floor mounted positioning device that stood next to a modified dental chair in which the subject sat with his or her right forearm resting on a 4-in thick foam pad. A lockable ball joint on the positioning device enabled manual placement of the thermode flush against the surface of the subject’s forearm.
The chemical stimulus was 10% l-menthol (Pfalz & Bauer, Waterbury, CT) dissolved in 95% ethanol and applied for 15 min to the volar surface of the forearm on a saturated filter paper square that was equal in size (16 cm2) to the 16-channel thermode. The filter paper was occluded by a wide strip of parafilm draped across the forearm and weighted at both ends to keep the filter paper flat against the forearm. After the parafilm and filter paper were removed, the forearm was wrapped in a single layer of cellophane (Saran Wrap™, S.C. Johnson) to prevent evaporative cooling from residual menthol and ethanol, and to avoid transference of residual menthol to the thermode during thermal testing. Skin temperature was monitored via a digital thermometer connected to a 40-ga thermocouple wire positioned underneath the cellophane at a point a 2–3 cm from the distal edge of the menthol-treated skin.
General Procedure
Both experiments included a practice session for new subjects who had not participated in thermal perception experiments in this laboratory. The practice consisted of two brief (10–15 min) exercises designed to train individuals to use the Labeled Magnitude Scale (LMS;[18;19]) to rate the intensity of thermal sensations. The LMS is a “category-ratio” scale [20] in which labeled intensity descriptors (e.g., “weak”, “strong”) are spaced according to their empirically-derived semantic magnitudes. The scale is bounded at the bottom by “no sensation” and at the top by “strongest imaginable sensation of any kind”, and subjects enter their ratings by using a computer mouse to move an arrow to the appropriate location on the scale. Subjects were first asked to imagine 16 commonly experienced thermal sensations (e.g., washing hands in cold tap water; walking barefoot on hot pavement) and rate their intensity. This exercise served both to familiarize subjects with the scale and to encourage use of the broadest possible perceptual context. Subjects then received a series of 11 practice thermal stimuli (ranging from 18° to 42°C) presented on two rows of the thermode array as it rested on the right forearm. Warm and cold stimuli were presented alternately across trials on separate pairs of rows of the thermode with an inter-trial interval of 30 sec. Subjects had three tasks per trial: to rate thermal sensation intensity (cool-cold, warm-hot), nociceptive sensation intensity (burning, stinging or pricking), and to indicate the specific sensations they had perceived by clicking on one or more descriptors displayed on the computer screen: nothing, cool, cold, warm, hot, burning, stinging/pricking, aching, and painful. The instructions were to choose as many words as necessary to describe each sensation fully, and to click on “nothing” if no sensation was felt.
Experiment 1: Static vs dynamic contact with and without cooling
This experiment investigated the effect of dynamic mechanical contact on sensations produced by menthol at RST and during cooling to 28°, 24° and 20°C. Two conditions of thermal stimulation, Static Contact and Dynamic Contact, were tested before and after menthol application in separate sessions (see Fig. 1). The inter-stimulus interval (ISI) and placement of the thermode were the same whether or not menthol was present, and the forearm was wrapped with cellophane in both cases. In the Static Condition the thermode was set to RST (measured beneath the plastic wrap) and placed in static contact with the arm for 3 min to allow any suppressive effects of the initial contact to subside before data collection began. Temperature stimulation proceeded in a descending series beginning with 28°, and a 3-min interstimulus interval (ISI) was inserted to minimize thermal interactions across trials. Stimulus duration was 5 sec. After the final stimulus (20°C), the thermode was lifted from the skin and the cellophane was removed from the arm to enable menthol to be applied. After 15-min of menthol application, the filter paper and its occlusive covering were removed and the arm was rewrapped with cellophane. Before returning the thermode to the skin, subjects made initial ratings of thermal and nociceptive sensations produced by the menthol alone. The thermode was then set to the measured RST and placed on the skin for a 3-min adaptation period, after which a second set of intensity ratings was made to assess menthol sensations during static contact with the thermode at RST. Thermal testing followed immediately, with the three test temperatures presented in the same sequence and with the same timing as before menthol application.
Fig. 1.
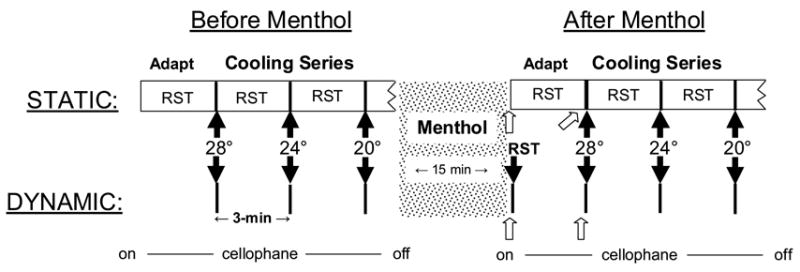
Shown is a diagram of the procedure used in experiment 1. Rectangular boxes in the Static Condition indicate periods when the thermode was in contact with the skin (RST = resting skin temperature). Thick vertical lines in both conditions indicate bouts of 5-sec thermal stimulation at the specified temperatures. Solid black arrows indicate intensity ratings made in response to dynamic contact and/or thermal stimulation; open arrows indicate baseline intensity ratings at RST prior to dynamic contact or thermal stimulation. Tsk indicates measurements of RST.
The procedure in the Dynamic Contact condition (Fig. 1) differed from the Static Contact condition in the following ways: (1) during temperature stimulation the thermode was set to the test temperature before it was touched to the skin for 5 sec; (2) there was no 3-min adaptation period with the thermode in contact with the skin, either before or after menthol; (3) after subjects made initial (baseline) ratings of thermal and nociceptive sensations produced by menthol alone, the thermode was set to RST and touched to the skin to assess the effect of dynamic contact (with thermal stimulation) on menthol sensations. Three minutes later the descending temperature series began, with the stimuli separated by 3-min ISIs.
Experiment 2: Effects of dynamic contact at small temperature offsets
In this experiment subjects rated thermal sensations produced by menthol during dynamic contact when thermode temperature deviated −1.0° to +1.0°C from the measured skin temperature. New subjects served in the same practice session used in exp. 1, and the menthol stimulus and procedure for application were also the same as before. To quantify the intensity as well as the frequency of reports of both warm and cold sensations, subjects made three intensity ratings per trial: cold, warmth and burning/stinging/pricking. The thermal stimulation procedure differed from exp. 1 in three ways: (1) Only dynamic contact stimulation was used, as the hypothesis under test was whether slight warming of the thermode was responsible for suppressing menthol cold and nociceptive sensations below levels reported at RST prior to contact; (2) thermode temperatures were +1.0, +0.5, 0, −0.5, and −1.0°C offsets from the measured RST; (3) ratings of baseline menthol sensations and thermal testing began 5 min rather than 3 min after removing the menthol-saturated filter paper and wrapping the forearm with cellophane. The longer delay was inserted to ensure that any suppression of menthol’s sensations by mechanical stimulation as the arm was wrapped with cellophane would have diminished by the time testing began.
The intensity rating procedure also differed from exp. 1 in that subjects continued to make ratings at 20-sec intervals after initial dynamic contact for a total of 3 min, with the thermode remaining on the arm throughout this period. The thermode was then lifted from the skin for 3 min, allowing a total of 6 min between measurements of the effect of dynamic contact. Only two temperature offsets plus thermal neutrality (RST) were presented in each session, which meant that two sessions were required to complete testing. Half of the subjects were tested with ascending offsets first (i.e., from −1.0°, −0.5, 0°) and half with descending offsets first (i.e., from +1.0°, +0.5, 0°). Replicate ratings were obtained for the same subjects in two additional sessions with the opposite offset series. In addition, a control session was run in which ratings were obtained at all offsets in the absence of menthol. The primary purpose of the this session was to determine whether the thermode was perceived to be slightly warm when set to the measured skin temperature. Such a result would be indicative of a slight deviation in measured skin temperature from actual skin temperature.
Results
Experiment 1: Menthol sensations and the effect of dynamic contact
Fig. 2 shows that at RST and in the absence of any contact by the thermode, 10% menthol induced thermal and nociceptive sensations on the forearm that were rated between barely detectable and weak in strength. Thermal sensations tended to be rated as more intense than nociceptive sensations, but only marginally so [t-test for non-independent means, t(38)=1.99, p=0.053]. Surprisingly, under conditions of static contact cooling, menthol failed to enhance cold but strongly enhanced nociceptive sensations. At each test temperature, intensity ratings of burning/stinging/pricking were 3 to 4 times higher after menthol treatment than before. A repeated-measures ANOVA with condition (before vs. after menthol), temperature, and sensation quality as factors confirmed there was a significant main effect of condition [F(1,38)=24.0; p<0.0001] that was qualified by a significant condition x sensation quality interaction [F(1,38)=29.2, p<0.0001]. Also of interest was a significant interaction between temperature and sensation quality [F(2,76)=7.8, p<0.005], which reflected the absence of an increase in cold sensation across temperature compared to a monotonic increase in ratings of nociceptive sensation. The relatively flat psychophysical functions for cold sensation were surprising given the evidence that perceived cold increases significantly between 28° and 20°C [21–24]. The use of only three relatively closely-spaced temperatures may have led to this result, as a similar result was found in a recent study that employed only three temperatures between 28° and 18°C [2]. Alternatively, in this temperature range cold sensation per se may not increase as rapidly as nociceptive sensations, which typically have not been rated separately from cold sensations.
Fig. 2.
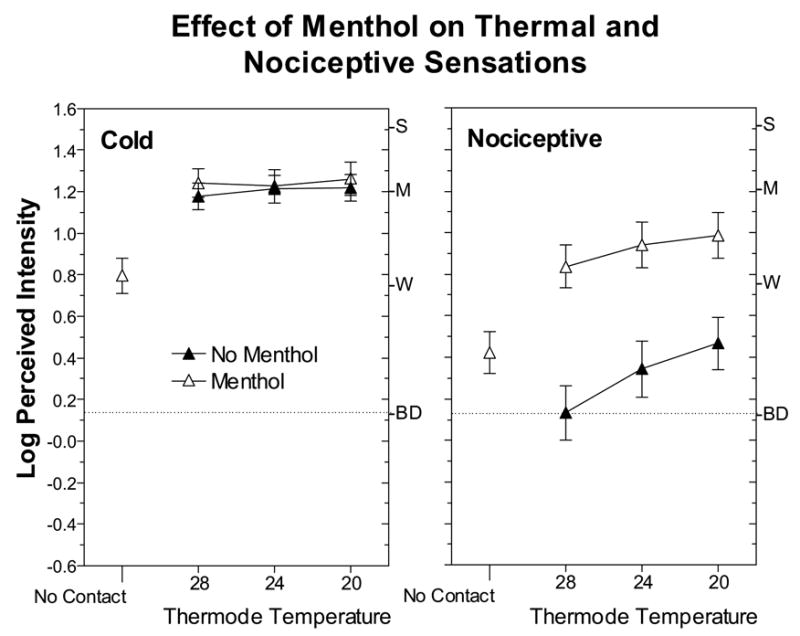
Log-mean perceived intensity ratings of thermal and nociceptive sensations are shown separately as a function of thermode temperature before (empty symbols)and after (filled symbols) application of 10% menthol to the forearm. Intensity ratings labeled “No Contact” were obtained at RST after menthol had been applied to the skin and just prior to thermal testing in the Dynamic Contact condition. Error bars indicate ± SEMs.
Fig. 3 compares ratings of thermal and nociceptive sensations under conditions of static and dynamic contact after the skin had been treated with menthol. At RST, dynamic contact significantly reduced both types of sensations [main effect of condition; F(1,38)=14.8, p<0.0005]. In fact, suppression was greater for cold sensations than for nociceptive sensations [condition x trial x sensation quality; F(1,38)=10.0, p<0.005], although the initially higher intensity of menthol-induced cold may have contributed to this difference. In contrast, when the skin was actively cooled, dynamic contact reduced only nociceptive sensations [condition x sensation quality; F(1,38)=4.4, p<0.05]. Similarly, Fig. 4 shows that before menthol treatment, dynamic contact caused more pronounced and consistent suppression of nociceptive sensations than cold sensations. An ANOVA revealed a significant condition x sensation quality (thermal vs. nociceptive) interaction [F(1,38)=8.22, p<0.01], and post hoc Tukey HSD tests confirmed that suppression of cold was significant only for the 28°C stimulus (p<0.05).
Fig. 3.
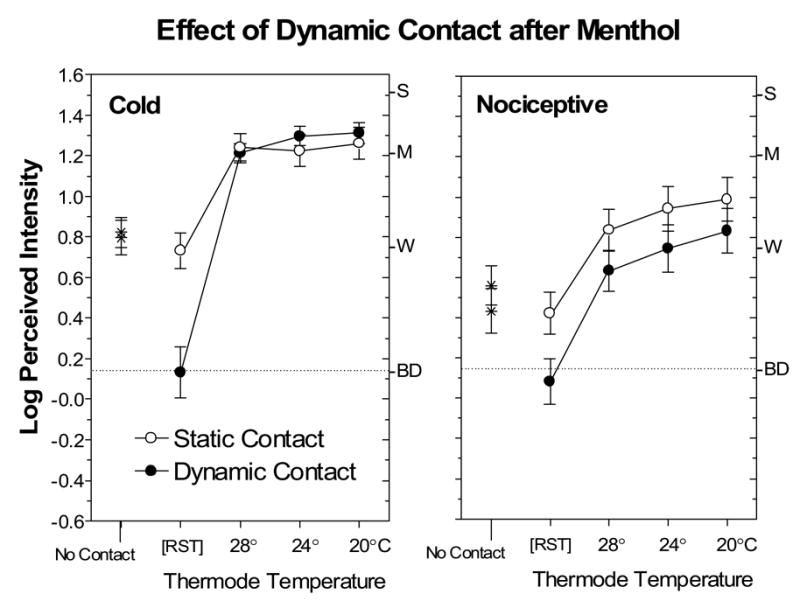
Log-mean perceived intensity ratings for thermal and nociceptive sensations after menthol application at RST prior to contact with the thermode (No Contact), after contact with the thermode set to RST, and with the thermode set to three temperatures. Open circles designate data obtained in the Static Contact condition; filled trials designate data obtained in the Dynamic Contact condition. The No Contact and RST ratings served as baseline measurements against which the effects of static and dynamic contact could be compared. Error bars indicate ± SEMs.
Fig. 4.
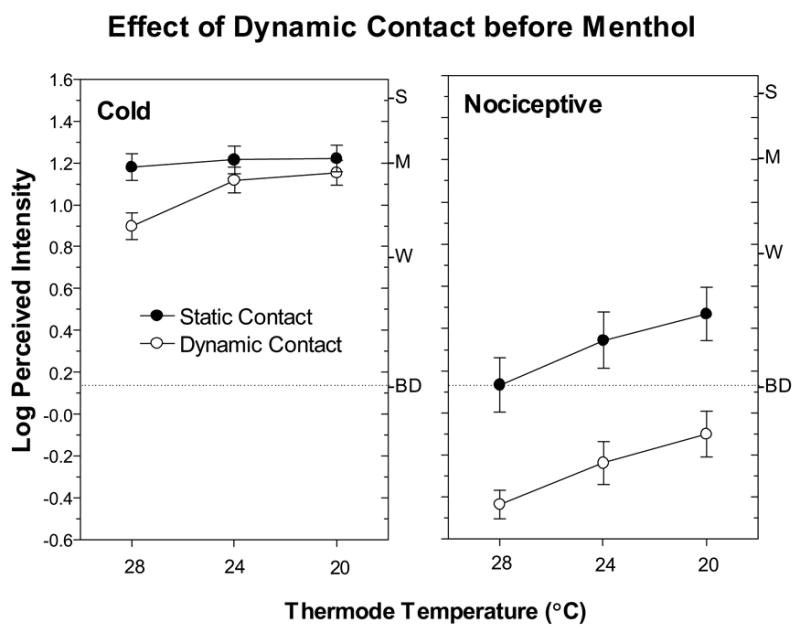
Log-mean perceived intensity ratings for thermal and nociceptive sensations obtained prior to menthol application in the Static Contact (filled circles) and Dynamic Contact (empty circles) conditions when the skin was actively cooled. Error bars indicate ± SEMs.
Fig. 5 displays the frequencies with which different qualities of sensation were reported during static and dynamic contact after menthol treatment. Consistent with reductions in perceived intensity of both cold and nociceptive sensations, dynamic contact reduced the number of reports of “cool”, “cold”, “burning” and “stinging/pricking”, with the biggest reduction in “cool” ratings. Although these reductions were accompanied by an increase in the number of subjects reporting “nothing” in the Dynamic Condition (from 1 to 6), a more notable increase occurred in reports of “warm” (from 2 to 16). This unexpected result led us to conduct a second experiment to rule out the possibility that during dynamic contact the thermode may have been slightly warmer than RST. It was possible that the different physical coupling between the skin and the surface thermocouple (lying atop the skin under a layer of cellophane) versus the thermode thermocouples (embedded in the copper plate attached to each Peltier module) might have led to disparate temperature readings. If the thermode was slightly warmer than the skin, suppression of cold sensations may have resulted from inhibition of ongoing cold fiber discharge rather than from dynamic contact per se.
Fig. 5.
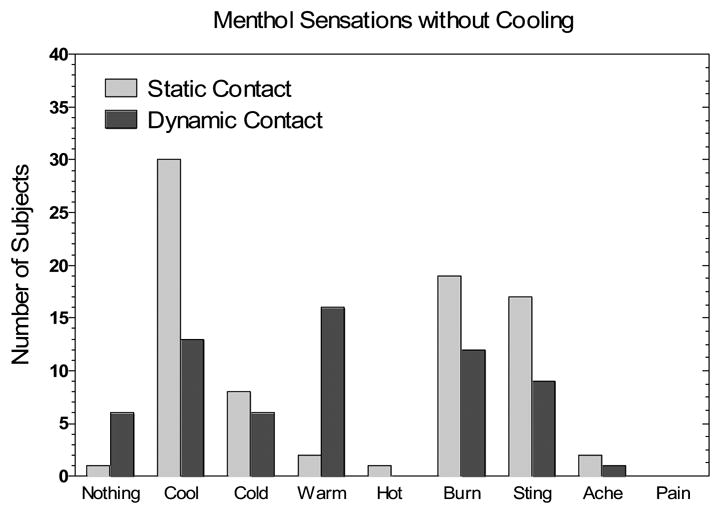
The number of subjects reporting each sensation quality in Exp. 1 after menthol application and during static (light gray bars) or dynamic contact (dark gray bars) with the thermode set to RST.
Experiment 2: Dynamic suppression of menthol sensations as a function of temperature offset
Shown in Fig. 6 are the log-mean intensity ratings of thermal sensations over time when the temperature of the thermode was the same, slightly cooler (top) or slightly warmer (bottom) than the skin. Consistent with the results of exp. 1, suppression was significant when the thermode was set to RST (filled circles). A repeated measures ANOVA on the data for cool offsets which included time and temperature as factors indicated there was a main effect of time [F(10,220)=6.9, p<0.0001] and an interaction between time and temperature [F(20,440)=3.2, p<0.0001]. The latter interaction indicated that contact suppression varied jointly as a function of thermode temperature and the time after skin contact. Suppression was transient, remaining significant for only 40 sec. Most important, a Tukey HSD test confirmed that when the thermode was 0.5°C cooler than the skin, thermal intensity was still rated significantly lower during dynamic contact (time 0) than before contact [p<0.05]. Cold sensations were therefore suppressed even when the thermode was slightly cooler than the skin, a condition which by itself should enhance cold sensations. On the other hand, warming the thermode deepened and prolonged cold suppression. An ANOVA on the data for warm offsets yielded main effects of time [F(10,220)=30.1, p<0.0001] and temperature [F(2,44)=8.8, p<0.001] as well as a time x temperature interaction [F(20,440)=5.4, p<0.0001]. Warming the thermode 0.5° and 1.0°C above the measured skin temperature caused cold suppression to last for 60 and 140 sec, respectively (Tukey HSD, p<0.05).
Fig. 6.
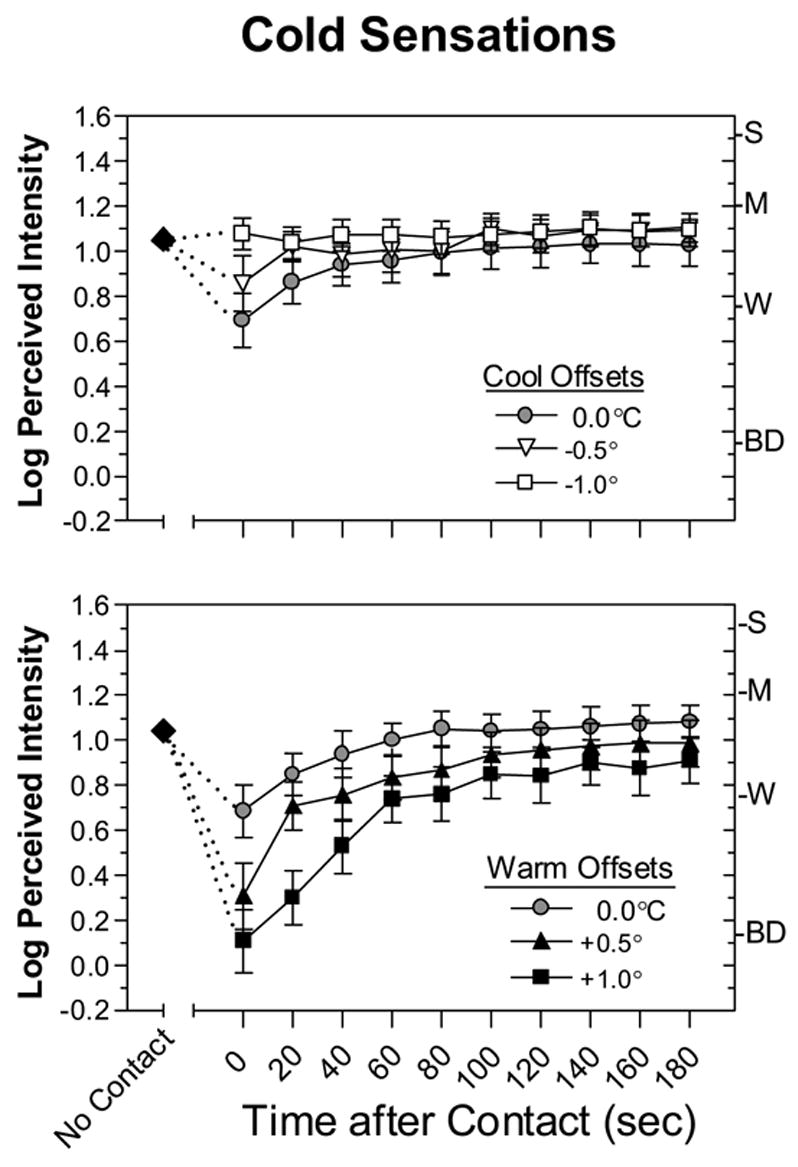
Log-mean perceived intensity ratings for cold sensations before (No Contact; filled diamonds), during (Time = 0) and at 20-sec intervals after dynamic contact. In the contact condition the thermode was adjusted to RST (0.0°C) or to one of two temperature offsets below (top graph) or above (bottom graph) RST. Error bars indicate ± SEMs.
Separate ANOVAs on the intensity ratings for nociceptive sensations (burning/stinging/pricking) revealed similar results, with the only exception being that no significant interaction was detected between temperature and time after cooling offsets [F(20,240)=1.53, p=0.07]. The failure to find a differential effect over time may have been a consequence of the more limited degrees of freedom in the analysis, since only those individuals who rated burning/stinging/pricking sensations above “barely detectable” in the baseline condition (n=13) were included.
Fig. 7 also displays data central to the question of whether suppression of menthol cold was caused by a slightly warm thermode. The open symbols show that when the thermode was set to the measured skin temperature with no menthol present, subjects rated warmth and cold to be less than barely detectable, indicating that the skin-temperature thermode was perceptually neutral. However, when menthol was present, at Time 0 the thermode was rated both slightly warm and slightly cool. An examination of individual responses indicated that many subjects were confused about the temperature of the thermode when it contacted the skin. Of the 25 subjects, 12 rated it as both warm and cool, 8 rated it as only cool, 4 rated it as only warm, and 1 reported no thermal sensation. Dynamic contact by a neutral thermode therefore reduced the intensity of menthol’s ongoing cold sensations and sometimes evoked the perception of warmth.
Fig. 7.
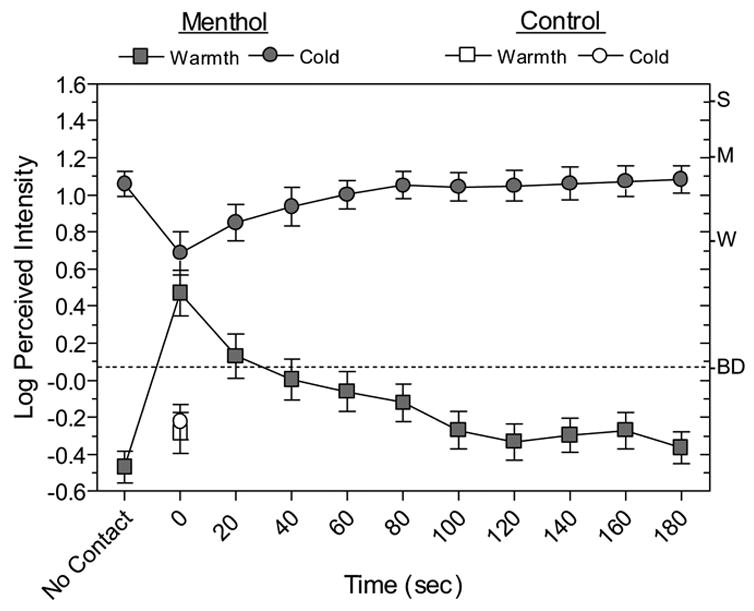
Log-mean perceived warmth and cold ratings obtained during and after contact with the thermode set to RST. The cold ratings after menthol exposure (filled circles) are the same as the 0.0°C offset data in Fig. 6, and have been combined across warming and cooling sessions. The warm ratings (filled squares) were made together with the cold ratings. The data obtained without menthol (open symbols) were collected in a third (control) session. Error bars indicate ± SEMs.
Discussion
The primary aim of this study was to use contact suppression of ICN to investigate whether the menthol and cold-sensitive cation channel TRPM8 might be involved in perception of nociceptive sensations during mild cooling. As well as providing evidence consistent with such an involvement, new findings were obtained regarding menthol’s effects on the perception of cold vs. nociception, and on the effects of dynamic contact on perception of nonpainful cold.
Menthol Selectively Enhances Nociceptive Sensations During Cooling
Menthol induced both cold and nociceptive sensations at RST, but enhanced only nociceptive sensations during physical cooling. This outcome was surprising in view of menthol’s reputation as a cooling agent and prior evidence that pre-treating the mouth with menthol intensifies the perceived cold of sipped solutions [25]. However, other studies of menthol on hairy skin have also found greater effects on nociception than on cold perception. The first study to measure both types of sensations after topical application [12] found that 5% and 10% solutions of menthol produced larger increases in the frequency of reports of nociceptive sensations than cold sensations. More recently, high concentrations of menthol have been reported to produce painful burning sensations [10] and cold hyperalgesia without altering the detection threshold for cooling [13]. Why menthol might have different sensory effects on different body sites is unclear. Because human cold perception is mediated chiefly by A-δ fibers [26;27], and menthol’s nociceptive sensations appear to result primarily from stimulation of C-fibers [10], differences in expression of TRPM8 in these two classes of fibers throughout the body could lead to regional differences in the relative strength of menthol-induced cold and nociceptive sensations. TRPM8 has been found in both classes of fibers in rats [28] and in a higher percentage of trigeminal ganglion neurons (particularly those in the region serving the tongue) than DRG neurons in both rats and mice [7;28]. However, the latter finding merely predicts menthol should be a more effective stimulus in the mouth than on the skin, independent of its relative effects on perception of cold and nociception.
The ability of menthol to stimulate nociceptive sensations, cold pain and hyperalgesia implies that TRPM8 is co-expressed with receptors that are sensitive to painfully cold or hot temperatures (e.g., TRPV1, TRPA1). Although as mentioned earlier TRPM8 has been reported to be expressed with TRPV1 [15;16] in rat DGR neurons, negative results from other studies [15;28–30] leaves open the possibility that such co-expression may not occur in humans. Alternatively possibility is that mild cooling may stimulate the nociceptive pathway via afferent fibers which, because of their low thermal thresholds, have not been classified as nociceptors. This possibility is consistent with the cold sensitivity of wide dynamic range (WDR) and heat-pinch-cold (HPC) spinal-thalamic tract (STT) neurons. Both types of STT neurons have been implicated in encoding thermal pain [31;32], and both have cold thresholds [31;33;34] in the same temperature range as TRPM8 [6–8;35]. If C-fibers that express TRPM8 do account for the cold sensitivity of WDR and/or HPC neurons, the intensity of nociceptive and cold sensations should vary with the strength of these inputs. However, because stimulation of cold fibers can inhibit cold-induced nociception [36–40], the intensity of nociceptive sensations during menthol exposure (as well as during innocuous cooling below 30°C) is probably a joint function of the level of stimulation in the nociceptive and cold pathways [31].
Contact Suppression of Nociceptive Sensations from Menthol
The demonstration that nociceptive sensations from menthol can be suppressed by dynamic contact throughout the range of temperatures tested provides further evidence that TRPM8 plays a role in ICN: ICN and menthol’s nociceptive sensations appear to be mediated by a common central pathway that can be inhibited by dynamic contact. This conclusion is complicated, however, by differences in the qualitative attributes of nociceptive sensations produced by menthol and cooling. Fig. 5 shows that at RST, menthol evoked slightly more reports of burning than stinging/pricking, and that dynamic contact reduced the frequency of reports of both qualities. In contrast, two prior studies [1;2] have shown that ICN is characterized by more frequent reports of stinging/pricking than burning. The latter findings were replicated in exp. 1, in which cooling to all three temperatures without menthol yielded a higher percentage of stinging/pricking sensations: e.g., at 24°C stinging/pricking was reported on 44% of trials, compared to burning on 26% of trials. Because burning has been associated with stimulation of C-fibers and stinging/pricking with stimulation of Aδ-fibers [41–43], these differences suggest that menthol and innocuous cooling may stimulate somewhat different (but overlapping) subsets of nociceptive fibers. If so, contact suppression must take place at a point within the nociceptive pathway that affects input from both classes of fibers. This raises the possibility that contact suppression may not be specific to cold stimulation. A study is planned that will address this possibility by determining whether suppression occurs when the skin is heated to temperatures below the heat pain threshold that also stimulate nociceptive sensations (e.g., 38°–43°C) [44].
Contact Suppression of Menthol Cold
The almost complete suppression of menthol’s cold sensations at RST was unexpected. In previous studies of ICN, cold sensations were unaffected by dynamic contact except at the mildest temperature tested (31°C; [1]). However, the same pattern of results was obtained in the present study, with cold sensations significantly suppressed at 28° but not at 24° or 20°C. In contrast to the consistent suppression of nociceptive sensations across temperatures and intensities (Figs. 3–5), the results for cold imply that dynamic contact exerts a weak inhibitory effect on stimulation in the cold pathway that quickly becomes insignificant at higher levels of stimulation. These different patterns of suppression may reflect different inhibitory mechanisms: a subtractive mechanism for cold versus a proportional mechanism for nociception. Because menthol stimulates cold sensations by raising the static discharge rate of cold fibers [45;46], the virtually complete suppression of menthol cold in experiment 1 could be explained by a mechanism that selectively inhibits steady-state activity in the cold pathway. A subtractive mechanism of this kind, which has been described in other neural systems as a high-pass filter of synaptic activity [47–49], should not affect perception of stimuli that produce a strong dynamic discharge, but might reduce perception of weak stimuli that cause only marginal increases in firing rate above resting levels. However, this interpretation cannot easily explain the absence of suppression at 28°C following menthol treatment (Fig. 5), since cold ratings during static contact were not significantly higher after menthol treatment than before (Fig. 2). One possibility is that during physical cooling menthol produced a slight increase in cold stimulation that was enough to overcome the inhibitory process but insufficient to produce significantly higher cold intensity ratings. As was mentioned earlier in regard to the unusually low slope of the psychophysical function for cold, the use of only three “moderately” cold temperatures may have reduced the sensitivity of the scaling task. Further study with a wider range of temperatures and menthol concentrations will be necessary to test the subtractive inhibition hypothesis of contact suppression of menthol cold.
Induction of Warm Sensations during Suppression of Menthol Cold
Also unexpected were the reports of warmth that accompanied suppression of menthol cold at RST (Figs. 3 and 5). Warmth was not reported during contact suppression in prior experiments that did not include menthol (e.g., [1], Fig. 3B). This difference may be a byproduct of the unusual excitatory conditions produced by menthol. In the earlier study, dynamic contact suppressed cold sensations under conditions in which warm fibers would be simultaneously inhibited by cooling. In the present study cold sensations were suppressed when skin temperature remained constant at approximately 33°C, a temperature at which warm fibers continue to discharge statically [50–52]. It is possible the static activity in warm fibers was adequate to induce warmth after the corresponding static activity in the cold pathway had been inhibited. The possibility that inhibition of cold stimulation contributes to warm sensations must also be considered. A comparative study of menthol’s effects on corneal sensitivity in humans and sensory afferent fibers in cats [53] indicated that application of menthol to the human cornea produced sensations of “freshness” followed by sensations of warmth that were correlated with stimulation and subsequent inhibition of cold-sensitive corneal fibers. Because warm fibers have not been reported in the cornea [53], the authors concluded that inhibition of cold fibers is sufficient to induce warmth. However, if the “off-response” of cutaneous cold fibers alone could stimulate warmth, cold spots that do not coincide spatially with warm spots should yield sensations of warmth as the skin re-warms following cold stimulation. Although the phenomenon of “paradoxical heat” or warmth has been reported during cold stimulation (e.g., [54–57]), no reports of warmth specific to re-warming cold spots have appeared in the literature.
Summary and Implications
The present results are consistent with the hypothesis that TRPM8 plays a role in perception of nociceptive sensations when the skin is cooled to innocuous temperatures. In addition, the suppression of menthol cooling at RST means that input from all afferent fibers that express TRPM8, whether they encode nociceptive or cold sensations, can be transiently inhibited by dynamic contact. The finding that dynamic contact can suppress steady-state cold sensations from menthol provides additional evidence that nonpainful cold perception does not depend solely on the response characteristics of cold fibers and the neurons to which they project in the cold pathway [58;59]. Instead, perception of cold from menthol and from temperatures as mild as 31°C [2;44] involves bimodal stimulation in the cold and nociceptive pathways that can be inhibited by contact with a surface.
Acknowledgments
This research was supported in part by a grant from the National Institutes of Health (RO1 NS038463).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Green BG, Pope JV. Innocuous cooling can produce nociceptive sensations that are inhibited during dynamic mechanical contact. Exp Brain Res. 2003;148:290–299. doi: 10.1007/s00221-002-1280-9. [DOI] [PubMed] [Google Scholar]
- 2.Green BG, Schoen KL. Evidence that tactile stimulation inhibits nociceptive sensations produced by innocuous contact cooling. Behav Brain Res. 2005;162:90–98. doi: 10.1016/j.bbr.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 3.Gallar J, Pozo MA, Tuckett RP, Belmonte C. Response of sensory units with unmyelinated fibres to mechanical, thermal and chemical stimulation of the cat's cornea. J Physiol. 1993;468:609–622. doi: 10.1113/jphysiol.1993.sp019791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Georgopoulos AP. Functional properties of primary afferent units probably related to pain mechanisms in primate glabrous skin. J Neurophysiol. 1976;39:71–83. doi: 10.1152/jn.1976.39.1.71. [DOI] [PubMed] [Google Scholar]
- 5.Campero M, Serra J, Bostock H, Ochoa JL. Slowly conducting afferents activated by innocuous low temperature in human skin. J Physiol. 2001;535:855–865. doi: 10.1111/j.1469-7793.2001.t01-1-00855.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nealen ML, Gold MS, Thut PD, Caterina MJ. TRPM8 mRNA is expressed in a subset of cold-responsive trigeminal neurons from rat. J Neurophysiol. 2003;90:515–520. doi: 10.1152/jn.00843.2002. [DOI] [PubMed] [Google Scholar]
- 7.McKemy DD, Neuhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature. 2002;416:52–58. doi: 10.1038/nature719. [DOI] [PubMed] [Google Scholar]
- 8.Peier AM, Moqrich A, Hergarden AC, Reeve AJ, Andersson DA, Story GM, Earley TJ, Dragoni I, McIntyre P, Bevan S, Patapoutian A. A TRP Channel that Senses Cold Stimuli and Menthol. Cell. 2002;108:705–715. doi: 10.1016/s0092-8674(02)00652-9. [DOI] [PubMed] [Google Scholar]
- 9.Green BG. Lingual heat and cold sensitivity following exposure to capsaicin or menthol. Chem Senses. 2005;30 (Suppl 1):i201–i202. doi: 10.1093/chemse/bjh184. [DOI] [PubMed] [Google Scholar]
- 10.Wasner G, Schattschneider J, Binder A, Baron R. Topical menthol--a human model for cold pain by activation and sensitization of C nociceptors. Brain. 2004;127:1159–1161. doi: 10.1093/brain/awh134. [DOI] [PubMed] [Google Scholar]
- 11.Dessirier JM, O'Mahony M, Carstens E. Oral irritant properties of menthol: sensitizing and desensitizing effects of repeated application and cross-desensitization to nicotine. Physiol Behav. 2001;73:25–36. doi: 10.1016/s0031-9384(01)00431-0. [DOI] [PubMed] [Google Scholar]
- 12.Green BG. The sensory effects of l-menthol on human skin. Somatosens Mot Res. 1992;9:235–244. doi: 10.3109/08990229209144774. [DOI] [PubMed] [Google Scholar]
- 13.Namer B, Seifert F, Handwerker HO, Maihofner C. TRPA1 and TRPM8 activation in humans: effects of cinnamaldehyde and menthol. Neuroreport. 2005;16:955–959. doi: 10.1097/00001756-200506210-00015. [DOI] [PubMed] [Google Scholar]
- 14.McKemy DD. How cold is it? TRPM8 and TRPA1 in the molecular logic of cold sensation. Mol Pain. 2005;1:16. doi: 10.1186/1744-8069-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Okazawa M, Inoue W, Hori A, Hosokawa H, Matsumura K, Kobayashi S. Noxious heat receptors present in cold-sensory cells in rats. Neurosci Lett. 2004;359:33–36. doi: 10.1016/j.neulet.2004.01.074. [DOI] [PubMed] [Google Scholar]
- 16.Xing H, Ling J, Chen M, Gu JG. Chemical and cold sensitivity of two distinct populations of TRPM8-expressing somatosensory neurons. J Neurophysiol. 2006;95:1221–1230. doi: 10.1152/jn.01035.2005. [DOI] [PubMed] [Google Scholar]
- 17.Green BG, Zaharchuk R. Spatial variation in sensitivity as a factor in measurements of spatial summation of warmth and cold. Somatosens Mot Res. 2001;18:181–190. doi: 10.1080/01421590120072178. [DOI] [PubMed] [Google Scholar]
- 18.Green BG, Shaffer GS, Gilmore MM. Derivation and evaluation of a semantic scale of oral sensation magnitude with apparent ratio properties. Chem Senses. 1993;18:683–702. [Google Scholar]
- 19.Bartoshuk LM, Duffy VB, Fast K, Green BG, Prutkin J, Snyder DJ. Labeled scales (e.g, category, Likert, VAS) and invalid across-group comparisons: what we have learned from genetic variation in taste . Food Quality and Preference. 2003;14:125–138. [Google Scholar]
- 20.Borg G. A category scale with ratio properties for intermodal and interindividual comparisons. In: Geissler H-G, Petxold P, editors. Psychophysical judgment and the process of perception. Berlin: VEB Deutxcher Verlag der Wissenschaften; 1982. pp. 25–34. [Google Scholar]
- 21.Greenspan JD, Roy EA, Caldwell PA, Farooq NS. Thermosensory intensity and affect throughout the perceptible range. Somatosens Mot Res. 2003;20:19–26. doi: 10.1080/0899022031000083807. [DOI] [PubMed] [Google Scholar]
- 22.Sarlani E, Farooq N, Greenspan JD. Gender and laterality differences in thermosensation throughout the perceptible range. Pain. 2003;106:9–18. doi: 10.1016/s0304-3959(03)00211-2. [DOI] [PubMed] [Google Scholar]
- 23.Stevens JC. Thermal sensibility. In: Heller MA, Schiff W, editors. The psychology of touch. New York: Lawrence Erlbaum Assoc; 1991. pp. 61–90. [Google Scholar]
- 24.Green BG, George P. 'Thermal taste' predicts higher responsiveness to chemical taste and flavor. Chem Senses. 2004;29:617–628. doi: 10.1093/chemse/bjh065. [DOI] [PubMed] [Google Scholar]
- 25.Green BG. Menthol modulates oral sensations of warmth and cold. Physiol Behav. 1985;35:427–434. doi: 10.1016/0031-9384(85)90319-1. [DOI] [PubMed] [Google Scholar]
- 26.Fruhstorfer H. Thermal sensibility changes during ischemic nerve block. Pain. 1984;20:355–361. doi: 10.1016/0304-3959(84)90112-X. [DOI] [PubMed] [Google Scholar]
- 27.Fruhstorfer H, Zenz M, Nolte H, Hensel H. Dissociated loss of cold and warm sensibility during regional anaesthesia. Pflugers Arch. 1974;493:73–82. doi: 10.1007/BF00587918. [DOI] [PubMed] [Google Scholar]
- 28.Kobayashi K, Fukuoka T, Obata K, Yamanaka H, Dai Y, Tokunaga A, Noguchi K. Distinct expression of TRPM8, TRPA1, and TRPV1 mRNAs in rat primary afferent neurons with adelta/c-fibers and colocalization with trk receptors. J Comp Neurol. 2005;493:596–606. doi: 10.1002/cne.20794. [DOI] [PubMed] [Google Scholar]
- 29.Abe J, Hosokawa H, Okazawa M, Kandachi M, Sawada Y, Yamanaka K, Matsumura K, Kobayashi S. TRPM8 protein localization in trigeminal ganglion and taste papillae. Brain Res Mol Brain Res. 2005;136:91–98. doi: 10.1016/j.molbrainres.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 30.Tominaga M. Molecular mechanisms of thermosensation. Nippon Yakurigaku Zasshi. 2004;124:219–227. doi: 10.1254/fpj.124.219. [DOI] [PubMed] [Google Scholar]
- 31.Craig AD, Krout K, Andrew D. Quantitative response characteristics of thermoreceptive and nociceptive lamina i spinothalamic neurons in the cat. J Neurophysiol. 2001;86:1459–1480. doi: 10.1152/jn.2001.86.3.1459. [DOI] [PubMed] [Google Scholar]
- 32.Price DD, Greenspan JD, Dubner R. Neurons involved in the exteroceptive function of pain. Pain. 2003;106:215–219. doi: 10.1016/j.pain.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 33.Khasabov SG, Cain DM, Thong D, Mantyh PW, Simone DA. Enhanced responses of spinal dorsal horn neurons to heat and cold stimuli following mild freeze injury to the skin. J Neurophysiol. 2001;86:986–996. doi: 10.1152/jn.2001.86.2.986. [DOI] [PubMed] [Google Scholar]
- 34.Dostrovsky JO, Craig AD. Cooling-specific spinothalamic neurons in the monkey. J Neurophysiol. 1996;76:3656–3665. doi: 10.1152/jn.1996.76.6.3656. [DOI] [PubMed] [Google Scholar]
- 35.De La PE, Malkia A, Cabedo H, Belmonte C, Viana F. The contribution of TRPM8 channels to cold sensing in mammalian neurones. J Physiol. 2005;567:415–426. doi: 10.1113/jphysiol.2005.086546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Craig AD, Reiman EM, Evans A, Bushnell MC. Functional imaging of an illusion of pain. Nature. 1996;384:258–260. doi: 10.1038/384258a0. [DOI] [PubMed] [Google Scholar]
- 37.Craig AD, Bushnell MC. The thermal grill illusion: Unmasking the burn of cold pain. Science. 1994;265:252–255. doi: 10.1126/science.8023144. [DOI] [PubMed] [Google Scholar]
- 38.Yarnitsky D, Ochoa JL. Release of cold-induced burning pain by block of cold-specific afferent input. Brain. 1990;113:893–902. doi: 10.1093/brain/113.4.893. [DOI] [PubMed] [Google Scholar]
- 39.Wahren LK, Torebjork E, Jorum E. Central suppression of cold-induced C fibre pain by myelinated fibre input. Pain. 1989;38:313–319. doi: 10.1016/0304-3959(89)90218-2. [DOI] [PubMed] [Google Scholar]
- 40.Kanui TI. Thermal inhibition of nociceptor-driven spinal cord neurones in the cat: a possible neuronal basis for thermal analgesia. Brain Res. 1987;402:160–163. doi: 10.1016/0006-8993(87)91060-2. [DOI] [PubMed] [Google Scholar]
- 41.Bishop GH, Landau WM. Evidence for a double peripheral pathway for pain. Science. 1958;128:712–713. doi: 10.1126/science.128.3326.712. [DOI] [PubMed] [Google Scholar]
- 42.Zotterman Y. Studies in the peripheral nervous mechanisms of pain. Acta Med Scand. 1933;80:185–242. [Google Scholar]
- 43.Price DD, McHaffie JG, Stein BE. The psychophysical attributes of heat-induced pain and their relationships to neural mechanisms. J Cognit Neurosci. 1992;4:1–14. doi: 10.1162/jocn.1992.4.1.1. [DOI] [PubMed] [Google Scholar]
- 44.Green BG. Synthetic heat at mild temperatures. Somatosens Mot Res. 2002;19:130–138. doi: 10.1080/08990220220220131524. [DOI] [PubMed] [Google Scholar]
- 45.Hensel H, Zotterman Y. The effect of menthol on thermoreceptors. Acta Physiol Scand. 1951;24:27–34. doi: 10.1111/j.1748-1716.1951.tb00824.x. [DOI] [PubMed] [Google Scholar]
- 46.Schafer K, Braun HA, Isenberg C. Effect of menthol on cold receptor activity. Analysis of receptor processes. J Gen Physiol. 1986;88:757–776. doi: 10.1085/jgp.88.6.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ohliger-Frerking P, Wiebe SP, Staubli U, Frerking M. GABA(B) receptor-mediated presynaptic inhibition has history-dependent effects on synaptic transmission during physiologically relevant spike trains. J Neurosci. 2003;23:4809–4814. doi: 10.1523/JNEUROSCI.23-12-04809.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bertram R. Differential filtering of two presynaptic depression mechanisms. Neural Comput. 2001;13:69–85. doi: 10.1162/089976601300014637. [DOI] [PubMed] [Google Scholar]
- 49.Nishimura T, Yoshida M, Nagatsu I, Akasu T. Frequency dependent inhibition of the nicotinic transmission by serotonin in vesical pelvic ganglia of the rabbit. Neurosci Lett. 1989;103:179–184. doi: 10.1016/0304-3940(89)90572-7. [DOI] [PubMed] [Google Scholar]
- 50.Dodt E, Zotterman Y. The mode of action of warm receptors. Acta Physiol Scand. 1952;26:345–357. doi: 10.1111/j.1748-1716.1952.tb00916.x. [DOI] [PubMed] [Google Scholar]
- 51.Sumino R, Dubner R. Response characteristics of specific thermoreceptive afferents innervating monkey facial skin and their relationship to human thermal sensitivity. Brain Research Reviews. 1981;3:105–122. [Google Scholar]
- 52.Darian-Smith I, Johnson KO, LaMotte C, Shigenaga Y, Kenins P, Champness P. Warm fibers innervating palmar and digital skin of the monkey: responses to thermal stimuli. J Neurophysiol. 1979;42:1297–1315. doi: 10.1152/jn.1979.42.5.1297. [DOI] [PubMed] [Google Scholar]
- 53.Acosta M, Belmonte C, Gallar J. Sensory experiences in humans and single-unit activity in cats evoked by polymodal stimulation of the cornea. J Physiol. 2001;534:511–525. doi: 10.1111/j.1469-7793.2001.t01-1-00511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Susser E, Sprecher E, Yarnitsky D. Paradoxical heat sensation in healthy subjects: peripherally conducted by A delta or C fibres? Brain. 1999;122 ( Pt 2):239–246. doi: 10.1093/brain/122.2.239. [DOI] [PubMed] [Google Scholar]
- 55.Hamalainen M, Vartiainen L, Karvanen L, Jarvilehto T. Paradoxical heat sensations during moderate cooling of the skin. Brain Res. 1982;251:77–81. doi: 10.1016/0006-8993(82)91275-6. [DOI] [PubMed] [Google Scholar]
- 56.Pavlicek G, Jenkins JG. Paradoxical warmth. Am J Psychol. 1933;45:353. [Google Scholar]
- 57.Greenspan JD, Taylor DJ, McGillis SLB. Body site variation of cool perception thresholds, with observations on paradoxical heat. Somatosens Mot Res. 1993;10:467–474. doi: 10.3109/08990229309028851. [DOI] [PubMed] [Google Scholar]
- 58.Gracely RH, Farrell MJ. Perception of pain and temperature. In: Yantis S, Pashler H, editors. Steven's Handbook of Experimental Psychology. Vol. 1. New York: John Wiley & Sons, Inc; 2002. pp. 619–653. Sensation and Perception. [Google Scholar]
- 59.Hensel H. In: Temperature receptors in the skin. Hardy JD, Gagge AP, Stolwijk JA, editors. Springfield, IL: Charles C. Thomas; 1970. pp. 442–453. [Google Scholar]


