Abstract
Matrix metalloproteinase inhibitors (MMPIs) reduce blood-brain barrier (BBB) disruption and prevent cell death. Animal models of multiple sclerosis, cerebral ischemia and hemorrhage, and bacterial meningitis respond to treatment with MMPIs. We have used the intracerebral injection of lipopolysaccharide (LPS) in rat, which induces MMP production and results in a delayed opening of the BBB, to screen MMPIs to identify therapeutic agents. We hypothesized that the mouse would respond similarly to LPS and that the mouse/LPS model of BBB damage would be more useful for screening of MMPIs. Therefore, we adapted the rat LPS model to the mouse and compared the response to LPS and treatment with MMPIs. Wistar-Kyoto rats (WKY) and three strains of mice had stereotactic injections of LPS into the caudate. 14C-sucrose was used to measure permeability of the BBB 24 hours after injection. Initially, we tested three broad-spectrum MMPIs in the rat, BB-1101, BB-94, and BB-2293, and a MMP-2 selective inhibitor, IW449; both BB-1101 and BB-94 significantly suppressed LPS-induced BBB damage (p<0.05). In the 3 mouse strains, C57/BL6, C57/BL10, and C57/BL10HIIIR2, LPS significantly opened the BBB in C57/BL6, and it was the only strain that showed a reduction in BBB permeability with BB-94. Treatment with methylprednisolone and several broad spectrum MMPIs, including BB-1101, were ineffective in the C57/BL6. There was a significant reduction in BBB permeability seen with 10% dimethyl sulfoxide (DMSO) alone, which was used to dissolve the selective MMP-2 and -9 inhibitor, SB-3CT. The tetracycline derivative, minocycline, reduced the BBB injury in mouse by blocking the production of MMP-9. Our results show variability in rats and mice to LPS and MMPIs, which most likely is based on genetic make-up. Understanding these differences may provide important clues that could guide selection of MMPIs in treatment of neurological diseases.
1. INTRODUCTION
Opening of the blood-brain barrier (BBB) occurs when matrix metalloproteinases (MMPs) are expressed and activated in a number of neurological diseases, including bacterial meningitis, multiple sclerosis, and cerebral ischemia and hemorrhage (Rosenberg, 2002). Inhibitors to the MMPs (MMPIs) block the disruption of the BBB, and have been proposed to treat neuroinflammation (Yong et al., 2001). Several broad-spectrum inhibitors have been shown to block the opening of the BBB in experimental allergic encephalomyelitis, bacterial meningitis, cerebral ischemia, and intracerebral hemorrhage (Gijbels et al., 1994; Lapchak et al., 2000; Leib et al., 2001; Leppert et al., 2001; Wang et al., 2005). A number of tetracycline derivatives, such as minocycline and doxycycline, have been shown to reduce the production of the MMPs by blocking the inflammatory response of microglia, and these agents have been tested in several neurological diseases (Brundula et al., 2002; Chen et al., 2000; Du et al., 2001; Power et al., 2003; Yrjanheikki et al., 1999). Recently it was shown that treatment with broad-spectrum MMPIs for an extended period in rodents with stroke impaired healing probably by interfering with recovery through blocking of angiogenesis and neurogenesis; both of which are dependent on MMPs for movement through the extracellular matrix (Lee et al., 2006; Zhao et al., 2006). Because of the complex actions of the MMPs both during injury and recovery and the number of MMPs involved in these reactions, it is important to develop methods for screening potential therapeutic agents. We have used a method of inducing a focal area of inflammation that is induced by the microinjection of lipopolysaccharide (LPS) into the caudate of the rat. We have adapted that method to the mouse in order to study transgenically modified animals and used it to show that stromelysin-1 (MMP-3) is important in bringing neutrophils into the brain (Gurney et al., 2006) This report describes the effects of several MMPIs on LPS-induced BBB damage in rat and mouse brain. The goal is to identify a method to screen potential therapeutic agents in a small animal model.
Broad-spectrum MMPIs have been tested in patients with advanced cancer; while these agents reduced the growth in the tumors, they caused fibrosis of joints with arthritic-like symptoms (Nelson et al., 2000). This has lead to a search for selective inhibitors that retain the tumor reducing effects without the joint problems (Overall et al., 2002). One of the main actions of the MMPIs is to reduce the inflammatory response in brain that leads to disruption of the BBB. Intracerebral injection of LPS induces MMPs and opens the BBB in rodents (Andersson et al., 1992). We have measured BBB permeability with intravenous injection of radiolabeled sucrose followed by analysis of the brain and blood samples for radioactivity that allows for the calculation of a sucrose uptake index (Rosenberg et al., 1998). There is an opening of the BBB after 24 hrs that is mediated by the expression of cytokines, free radicals and MMPs (Mun-Bryce et al., 1998). Therefore, we hypothesized that LPS-induced BBB disruption could be used to screen potential therapeutic agents. To test the hypothesis, we used the intracerebral injection of LPS with the sucrose uptake index to test several MMPIs in rat and mouse. We adapted the sucrose uptake method used in the rat to the mouse. In this report we compare the effect of broad spectrum and selective MMPIs in the Wistar-Kyoto rat (WKY) and different strains of mice. We report that broad-spectrum MMPIs with similar inhibitory profiles behave differently in the rat and the mouse, and that there are differences in the mouse with different genetic backgrounds. In addition, we found that the solvent, dimethyl sulfoxide (DMSO), which is used to dissolve the MMPIs, reduces BBB permeability when used alone. We conclude that responses in the mouse are too variable for use as a biological screening method, but that the LPS method can be used in studies of transgenic animals.
2. RESULTS
Broad spectrum MMPIs close BBB in Rat
Both of the broad-spectrum inhibitors, BB-1101 and BB-94, significantly reduced the opening of the BBB (FIGURE 1). However, the third broad-spectrum inhibitor, BB-2983, which has a profile of inhibitory activity very close to that of BB-1101 (P. Brown, British Biotech, personal communication), showed no effect on the LPS-induced BBB opening. Sucrose space in the rats given the selective MMP-2 inhibitor, IW499, was similar to saline-injected controls (TABLE 2).
Figure 1.

Effect of MMPIs on sucrose space in LPS-mediated BBB opening in the rat brain. LPS was injected into the caudate along with Evans blue for localization of the injection site. The broad-spectrum MMPIs, BB-1101 and BB-94, significantly reduced the BBB in the rat caudate. Asterisk indicate statistical significance at the level shown. All groups had 6 animals except for BB-1101, which was 5.
TABLE 2.
Effect of Various MMPIs on 14C-Sucrose Uptake in Rat Brain Induced by an Intracerebral Injection of Lipopolysaccharide.
| AGENT | Dose (mg/kg) | N | SUCROSE SPACE (%) | P |
|---|---|---|---|---|
| Control | 6 | 7.18±0.36 | n.s. | |
| IW449 | 5 | 6 | 7.30±0.55 | n.s. |
| BB-2983 | 50 | 6 | 8.93±0.69 | n.s. |
| BB-1101 | 30 | 5 | 3.68±0.29 | <0.01 |
| BB-94 | 30 | 6 | 4.6±0.31 | <0.05 |
MMPIs Show Strain Differences in Mouse Model of LPS-Induced BBB Injury
Preliminary studies were done to characterize the BBB permeability in the mouse with the 14C-sucrose method. The minimal dose necessary to obtain significant BBB opening was determined to be 5 ng (FIGURE 2). Earlier, we showed with this model that the BBB was maximally opened between 16 and 24 hours after injection of LPS (Gurney et al., 2006).
Figure 2.

Dose response of lipopolysaccharide (LPS) in mouse brain. LPS was injected into the caudate and the animals were sacrificed 24 hrs after injection. Both 5 and 24 ng of LPS caused a significant opening of the blood-brain barrier compared to saline. ANOVA with Bonferroni correction showed statistically significant differences. * (p<0.05).
Strain differences were found in the response to LPS injection. The same dose of LPS produced markedly different effects on the BBB in different strains. LPS had the maximal effect on BBB opening in C57/BL6. A response to LPS occurred in C57/BL10HIIIR2. However, the C57/BL10 mouse stain had a response similar to saline (FIGURE 3).
Figure 3.

LPS intracerebral injection in different mouse strains. The inflammatory opening of the BBB was significantly different than saline in the C57/BL6 strain (n=10), but not in the C57/BL10 (n=8) or the C57/BL10RIIIH2 (n=11). * (p<0.05).
Effect of MMPIs in different mouse strains
We tested the inhibitors in the mouse LPS model. BB-94, which produced a significant response to LPS injection in the rat, had a similar effect only in the C57/BL6 strain; no effect was seen for BB-94 in the C57/BL10HIIIR2 strain (FIGURE 4). Other agents that had worked in the rat, such as the BB-1101, failed to show an effect in the mouse model. Several other agents were tested in the C57/BL6 mice. TABLE 3 shows the results of the various agents tested. Only minocycline affected BBB damage. Other agents tested, including BB-1101, GM6001, and methylprednisolone, were ineffective.
Figure 4.
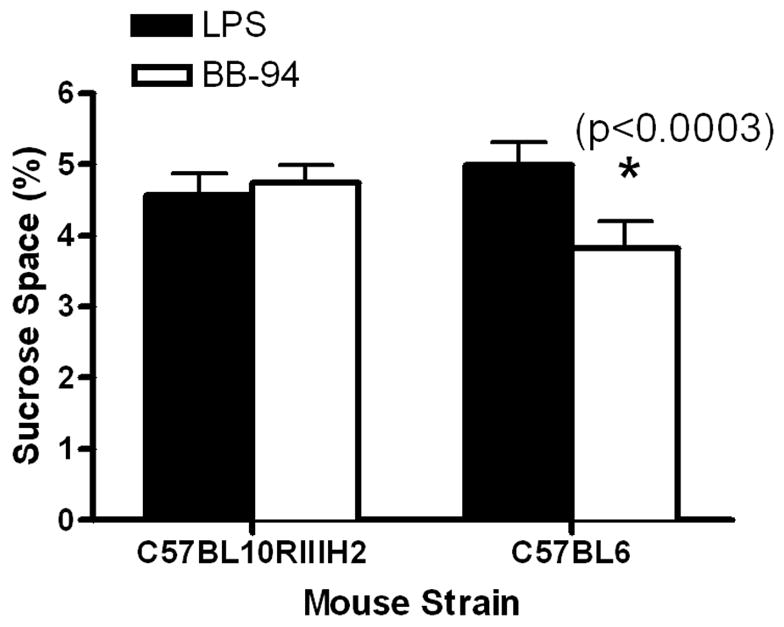
Treatment with BB-94 of LPS-induced BBB opening is shown for two strains of mice. In the C57BL10RIIIH2 strain there was no effect seen, but a significant reduction in sucrose space was found in the C57/BL6 mice (p<0.0003).
TABLE 3.
Effect of Various MMPIs on 14C-Sucrose Uptake in Mouse Brain (C57/BL6) Induced by an Intracerebral Injection of Lipopolysaccharide
| AGENT | N | DRUG SUCROSE SPACE (%) | N | SALINE SUCROSE SPACE (%) | P |
|---|---|---|---|---|---|
| GM6001 (65mg/kg) | 6 | 4.35±0.17 | 6 | 4.92±0.55 | n.s. |
| BB1101 (30mg/kg) (Time=0) | 5 | 5.76±0.58 | 11 | 5.36±0.51 | n.s. |
| BB1101 (60mg/kg) (Time=4hrs) | 4 | 6.85±0.25 | “ | “ “ | n.s. |
| Methylprednisolone (50mg/kg) | 4 | 5.35±0.30 | 12 | 5.08±0.28 | n.s. |
| Methylprednisolone (100mg/kg) | 6 | 5.80±0.59 | “ | “ “ | n.s. |
| BB-94 (30mg/kg) | 10 | 3.82±0.38 | 10 | 4.99±0.33 | <0.0003 |
| Minocycline (150mg/kg) | 10 | 4.09±0.32 | 12 | 5.08±0.028 | <0.05 |
Effect of the solvent DMSO
DMSO was used as a solvent for the selective MMP-2 and MMP-9 inhibitor. We tested both a one and a ten percent DMSO solution. Ten percent DMSO significantly reduced the BBB injury, while the 1% solution showed no effect (FIGURE 5). In an earlier study, we showed that another commonly used solvent, propylene glycol, blocked the opening of the BBB in a model of focal ischemia (R. Sood et al., in press).
Figure 5.

Effect of a selective MMP-2/-9 inhibitor on LPS disruption of the BBB in mouse. The inhibitor, SB-3CT, was dissolved in 1 or 10% DMSO. No effect was seen at 1% DMSO (n=11). The agent was effective when dissolved 10% DMSO (n=10), but the solvent alone (n=11) a significant reduction in sucrose space was seen, indicating that the main effect was from the DMSO, which would have masked an effect of the agent. * (p<0.05).
Effect of Minocycline
In addition to the MMPIs, we tested the tetracycline derivative, minocycline, which reduces the inflammatory response. Minocycline significantly reduced the BBB opening in the mouse. To identify the role of the MMPs in the reaction, we performed gel zymography on the LPS injected animals with and without treatment with minocycline. Minocycline significantly (p<0.02) lowered the MMP-9 levels in the tissues (FIGURE 6).
Figure 6.

Effect of minocycline on LPS induced BBB opening. A) Minocycline (n=11) significantly reduced the BBB damage compared to control (n=12). B) The 92-kDa MMP-9 was reduced by minocycline.
3. DISCUSSION
Our results show variability in the response of different genetic strains of mice to the proinflammatory agent, LPS. Two mice strains reacted robustly to the intracerebral injection, while the third was similar to saline controls. Different genetic strains that reacted to LPS similarly showed different reactions to the drug, BB-94, which worked well in the rat. Furthermore, BB-1101, which had been effective in several rat disease models, was ineffective in C57/BL6, which had shown a response to BB-94. We found the most consistent reduction in BBB permeability with BB-94, which is a broad-spectrum, hydroxymate-based inhibitor of the MMPs. It was effective in both the rat and the mouse, but not in all species of mice tested. This compound is relatively lipid soluble with less effect on TACE, indicating that it may cross the BBB and that it would not suppress TNF-α. The other broad-spectrum inhibitors surprisingly had little effect in the strains of mice tested. Neither the selective MMP-2 nor the selective MMP-2 and -9 inhibitors showed an effect on BBB permeability. Interestingly, the tetracycline derivative, Minocycline, was effective in reducing the BBB damage by reducing the production of MMP-9. Our results do not support the use of mice models for the testing of MMPIs.
The lack of uniformity in the response to different MMPIs may be due to multiple factors. The agents tested that had been used in clinical trials were studied for blood levels and toxicity, but had not been tested for brain penetration. The fact that they worked in the brain suggests that they are getting into the brain since it is unlikely that all of the effect is due to peripheral actions. Surprisingly, agents that worked in the rat failed to show an effect in the mouse. The C57/BL10HIIIR2 stain, which is derived from the C57/BL10, was tested because it was used to develop a knock out mouse for MMP-3, which was used in a recent study (Gurney et al., 2006). The lack of a response in the C57/BL10 was known and was the reason that the MMP-3 knock out mouse, which was originally bred for arthritis studies using the C57/BL10, had the background switched to the C57/BL10HIIIR2 (J. Mudgett, personal communication).
We showed that the 14C-sucrose method of quantifying BBB permeability could be adapted to the mouse, making it possible for use with genetically manipulated animals. Intracerebral injection of LPS causes a discreet disruption of the BBB. The inflammatory response is complex and involves induction of multiple inflammatory markers including other proteases and free radicals (Perry et al., 1992). LPS acts through Toll-like receptors, which trigger a large number of responses (Lehnardt et al., 2002). The use of sucrose, which is normally excluded from the brain, is a sensitive marker to show subtle disturbances of the BBB. The assumption with the use of sucrose is that very little enters the brain in the ten minutes of circulation, allowing for the use of the amount in the blood without resort to differential equations and multiple samples (Ohno et al., 1978).
There was a strong response to inflammation in the WKY rat and the C57/BL6 strain of mice. Both are commonly used in experimental studies of injury. Opening of the BBB occurred in the C57/BL6, while the response to LPS in the C57/BL10 mouse strain was similar to saline injection. The surprising finding was a response to LPS in the C57/BL6 and in the C57/BL10HIIIR2, but only the former showed an effect with treatment with BB-94. Other agents, such as GM6001 and methylprednisone were ineffective in the mouse brain in this model. These results show that the responses of the MMPIs will depend to a large extent on the rodent species and strains, which will increase the complexity of the testing of these agents. The responses in a single species or strain will need to be verified in other ones.
Another unexpected result in this study was the ability of the solvent, DMSO, to control the BBB damage. When a lower dose of the solvent was used, the effect was lost. This could have been due to a lack of a response of the drug or to an inability of the smaller amount of solvent to dissolve the drug. DMSO has been shown to be beneficial in several models of brain injury (Bardutzky et al., 2005; Farkas et al., 2005). Because of the response to DMSO it was not possible to determine the effect of the SB-3CT, which was poorly soluble and needed a solvent. DMSO is a viscous substance with antioxidant effects, but its protective properties are not well understood.
We found that Minocycline reduced BBB damage by reducing the production of MMP-9. Minocycline is an anti-inflammatory agent that reduces the response to inflammation by blocking the action of the microglia cells, which are a source of MMP-9. However, another agent with anti-inflammatory effect, methylprednisolone, was ineffective. Minocycline is a pleuripotential drug that has effects other than suppression of MMP-9, and one of these effects could have contributed to the beneficial effect of the agent.
A major hurdle for the use of the MMPIs in treatment is their beneficial role in the recovery phase. MMPIs interfere with angiogenesis and neurogenesis in ischemia (Zhao et al., 2006). In addition, there may be beneficial effects of disruption of the BBB in terms of initiating a conditioning response. When the BBB is the critical pathological change, such as occurs in the bacterial infections, agents that block the BBB damage may be useful. When there is extensive tissue damage, as is seen in ischemic injury, the need for a robust recovery phase with new vessels and cells growing into the damaged area, the use of these agents may need to be limited to early after injury when the proteases are damaging the tissues.
In conclusion, screening of potential MMPIs with the mouse model of inflammation will be difficult to translate to the rat and ultimately to human unless a species of mice can be identified that reacts similarly as man to the inflammatory stimulus. It will still be possible to use the mouse for BBB studies in the knockout animals, using the sucrose uptake index, which is an accurate method for quantification of the changes in the BBB. The selective agents that we tested were disappointing, and the best response against LPS-induced neuroinflammatory BBB disruption was found with broad-spectrum MMPIs and the anti-inflammatory tetracycline derivative, Minocycline. Finally, when the compounds tested are poorly soluble and solvents such as DMSO are used, the effects of the solvents alone will need to be carefully monitored.
4. EXPERIMENTAL PROCEDURE
Measurement of BBB Permeability with 14C-Sucrose in Rodents
Studies in the rat were done as previously described (Mun-Bryce et al., 2002). In brief, Wistar-Kyoto rats, weighing 250 to 300 gms, were anesthetized with 2% halothane. A 26 gauge needle was stereotacticaly inserted into the caudate. Lipopolysaccharide (5 ng) was injected over 5 minutes and the needle was removed. Evans blue dye was injected with the LPS to allow identification of the site of injection. Twenty-four hours after injection the animal was anesthetized and injected intravenously in the femoral vein with 14C-sucrose (10 μCi; New England Nuclear). Ten minutes after injection the animal was euthanized with an injection of saturated potassium into the heart. A sample of blood was removed from the heart and the brain was removed. Both the plasma and brain tissues were prepared for liquid scintillation counting. Tissues were extracted at the time of death and weighed. The tissue sample was solublized in 0.5 mL of Protosol (Dupont). Plasma and brain samples were prepared in 4 mL Aquasol (Beckman) for liquid scintillation counting (Beckman Instruments). The ratio of the radioactivity in the brain to that in the blood was used to calculate a sucrose uptake index, which is an estimation of the transport of sucrose from the blood to the brain.
Testing of MMPIs in the Rat
Four MMPIs were tested in the rat, including the MMP-2 selective inhibitor, IW449 (5 mg/kg; Dupont Corp., Wilmington, DE; gift of G. Feuerstein), and the broad-spectrum agents, BB-2983 (50 mg/kg), BB-1101 (30 mg/kg), and BB-94 (30 mg/kg) (British Biotechnology, Oxon, UK; gifts from A. Gearing and P. Brown). The inhibitory constants, Ki, for the various inhibitors are given in TABLE 1. The doses selected were provided by the company or were used in previously published papers, but the ability to act in the brain was not tested by the companies. All drugs were injected intraperitoneally shortly after the injection of LPS. The anti-inflammatory agent, Minocycline, which interferes with the function of microglia and reduces the production of MMP-9, was tested. In the Minocycline studies, the levels of gelatinases (MMP-2 and MMP-9) were measured by gelatin zymography as previously described.(Rosenberg et al., 1998) We tested the broad-spectrum MMPI, GM6001 (65 mg/kg), which was effective in several mouse models of neurological diseases. In addition, we studied a selective MMP-2 and MMP-9 inhibitor, SB-3CT, which was dissolved in either 1% or 10% dimethyl sulfoxide (DMSO) prior to injection. Animals with only the solvent were studied as controls.
TABLE 1.
The Kis of Different Metalloproteinase Inhibitors (nM) are Given for Individual Matrix Metalloproteinases (MMPs) and Tumor Necrosis Factor-α Converting Enzyme (TACE)
| DRUG | BB-94* | BB-2983* | BB-1101* | IW449+ | GM6001# |
|---|---|---|---|---|---|
| MP | |||||
| −1 | 5 | 10 | 8 | 8,000 | 0.4 |
| −2 | 4 | 10 | 4 | 12 | 0.34 |
| −3 | 20 | 20 | 30 | 1,438 | 26 |
| −7 | 6 | 3 | 60 | 9,000 | - |
| −8 | 4 | 10 | 3 | - | 0.18 |
| −9 | 1 | 30 | 3 | 894 | 0.57 |
| −12 | 1 | 2 | 5 | - | - |
| −13 | 2 | 5 | 7 | 43 | - |
| −14 | 5 | - | 10 | 406 | - |
| TACE (ADAM-17) | 4166 | 425 | 550 | >1000 | - |
British Biotechnology, Oxon, UK
DuPont Pharmaceuticals, Wilmington, Delaware
Chemicon
Adaptation of the 14C-sucrose method to mice
We measured sucrose space in mice, weighing 25–30 gms, by adapting the 14C-sucrose method from the rat to the mouse. Several LPS doses were used to obtain the minimal dose sufficient to open the BBB. We studied the BBB damage 24 hrs after injection because this was found to be the optimal time for maximal opening in an earlier report (Gurney et al., 2006). Evans blue was added to the injectate to visualize the site of injection. Twenty-four hours after LPS injection, the mice were anesthetized with 2% halothane and an incision was made in the midline of the neck to expose the jugular vein. 14C-sucrose (2 μCi) was injected into the jugular vein. Ten minutes later the animal was euthanized. The brain was removed and a sample of blood was obtained from the heart. Brain tissue was sectioned, weighed, and dissolved in Solvable (Dupont/NEN) for radioactive counting in a liquid scintillation counter (Packard). The doses of LPS (Sigma) tested were none (saline control), 5 and 25 ng.
Agents Tested in the C57/BL6 mice
We tested six agents in the C57/BL6, which is a commonly used mouse stain. Agents tested included: 1) GM6001 (65mg/kg; Chemicon); 2) BB-1101 (30mg/kg) given at the time of LPS injection; 3) BB-1101 (60mg/kg) given at 4 hrs after LPS; 4) methylprednisone (50mg/kg and 100 mg/kg); 5) BB-94 (30 mg/kg); and 6) Minocycline (150 mg/kg). The effect of the drugs was compared to saline-injected controls. All agents were injected intraperitoneally shortly after LPS injection except for the delayed use of BB-1101.
Strain Differences in LPS-Injected Mice
Three strains were tested: C57/BL10, C57/BL10HIIIR2, and C57/BL6. The effect of LPS in each of the strains was tested using the methods described above.
Gelatin gel zymography for MMP-2 and -9
An affinity column was used to separate out the gelatinases. The extracted tissue was dissolved in an extraction buffer containing 2.5% Triton X-100 and incubated with gelatin-linked sepharose beads (Pharmacia) for one hour at 4° C (Zhang et al., 1997). MMP-2 and MMP-9 were then removed from the beads with an elution buffer containing DMSO and stored at −80° C. Protein samples were separated by electrophoresis on a 12% polyacrylamide gel with gelatin substrate suspended within the matrix (Zhang et al., 1997). All samples were run in triplicate and protein standards were included on multiple gels to verify the molecular weight of the lysis bands. SDS, which prevents proteolysis during electrophoresis, was removed by rinsing the gels in 2.5% Triton X-100. To allow enzyme activity to progress, gels were incubated in LCSB buffer (0.05 M Tris, pH 7.6, 0.2 M NaCl2, 0.005 M CaCl2, 0.02% Brij-35l, and 0.1% sodium azide) for 72 hours at 37° C. To visualize the lysis bands, gels were stained with Coomassie blue stain (0.125% Coomassie G-250, 50% methanol, and 1% acetic acid). This was followed by 3 days of destaining in 10 % acetic acid. The pro-form of MMP-9 at 92 kDa and the active form at 84-kDa, were quantified by densitometry, using a transparency scanner (AGFA) and AlphaEase software (Alpha Innotech) running on a PC computer (Kleiner et al., 1994). All measurements were adjusted for protein levels by dividing the density values by protein concentrations. Results are reported as relative density units.
Statistical Analysis
The effect of the MMPIs in the LPS and stroke models in the rodents was tested statistically by either one-way analysis of variance with the Bonferroni correction for multiple comparisons or Student unpaired t-tests (GraphPad, Version 4, Prizm Corp., and San Diego, CA). Results are expressed as mean ± standard error of the mean (SEM). Significance was less than 0.05.
Acknowledgments
Studies were supported by a grant from the National Institute of Neurological Disorders and Stroke (RO1 NS045847).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andersson PB, Perry VH, Gordon S. The acute inflammatory response to lipopolysaccharide in CNS parenchyma differs from that in other body tissues. Neuroscience. 1992;48:169–186. doi: 10.1016/0306-4522(92)90347-5. [DOI] [PubMed] [Google Scholar]
- Bardutzky J, Meng X, Bouley J, Duong TQ, Ratan R, Fisher M. Effects of intravenous dimethyl sulfoxide on ischemia evolution in a rat permanent occlusion model. JCerebBlood Flow Metab. 2005;25:968–977. doi: 10.1038/sj.jcbfm.9600095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brundula V, Rewcastle NB, Metz LM, Bernard CC, Yong VW. Targeting leukocyte MMPs and transmigration: Minocycline as a potential therapy for multiple sclerosis. Brain. 2002;125:1297–1308. doi: 10.1093/brain/awf133. [DOI] [PubMed] [Google Scholar]
- Chen M, Ona VO, Li M, Ferrante RJ, Fink KB, Zhu S, Bian J, Guo L, Farrell LA, Hersch SM, Hobbs W, Vonsattel JP, Cha JH, Friedlander RM. Minocycline inhibits caspase-1 and caspase-3 expression and delays mortality in a transgenic mouse model of Huntington disease. NatMed. 2000;6:797–801. doi: 10.1038/77528. [DOI] [PubMed] [Google Scholar]
- Du Y, Ma Z, Lin S, Dodel RC, Gao F, Bales KR, Triarhou LC, Chernet E, Perry KW, Nelson DL, Luecke S, Phebus LA, Bymaster FP, Paul SM. Minocycline prevents nigrostriatal dopaminergic neurodegeneration in the MPTP model of Parkinson’s disease. ProcNatlAcadSciUSA. 2001;98:14669–14674. doi: 10.1073/pnas.251341998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farkas E, Annahazi A, Institoris A, Mihaly A, Luiten PG, Bari F. Diazoxide and dimethyl sulphoxide alleviate experimental cerebral hypoperfusion-induced white matter injury in the rat brain. Neurosci Lett. 2005;20(373):195–199. doi: 10.1016/j.neulet.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Gijbels K, Galardy RE, Steinman L. Reversal of experimental autoimmune encephalomyelitis with a hydroxamate inhibitor of matrix metalloproteases. JClinInvest. 1994;94:2177–2182. doi: 10.1172/JCI117578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurney KJ, Estrada EY, Rosenberg GA. Blood-brain barrier disruption by stromelysin-1 facilitates neutrophil infiltration in neuroinflammation. NeurobiolDis. 2006;23:87–96. doi: 10.1016/j.nbd.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Kleiner DE, Stetler-Stevenson WG. Quantitative zymography: Detection of picogram quantities of gelatinases. Analytical Biochemistry. 1994;218:325–329. doi: 10.1006/abio.1994.1186. [DOI] [PubMed] [Google Scholar]
- Lapchak PA, Chapman DF, Zivin JA. Metalloproteinase inhibition reduces thrombolytic (Tissue plasminogen Activator)-induced hemorrhage after thromboembolic stroke. Stroke. 2000;31:3034–3040. doi: 10.1161/01.str.31.12.3034. [DOI] [PubMed] [Google Scholar]
- Lee SR, Kim HY, Rogowska J, Zhao BQ, Bhide P, Parent JM, Lo EH. Involvement of matrix metalloproteinase in neuroblast cell migration from the subventricular zone after stroke. J Neurosci. 2006;26:3491–3495. doi: 10.1523/JNEUROSCI.4085-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehnardt S, Lachance C, Patrizi S, Lefebvre S, Follett PL, Jensen FE, Rosenberg PA, Volpe JJ, Vartanian T. The toll-like receptor TLR4 is necessary for lipopolysaccharide-induced oligodendrocyte injury in the CNS. J Neurosci. 2002;22:2478–2486. doi: 10.1523/JNEUROSCI.22-07-02478.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leib SL, Clements JM, Lindberg RL, Heimgartner C, Loeffler JM, Pfister LA, Tauber MG, Leppert D. Inhibition of matrix metalloproteinases and tumour necrosis factor alpha converting enzyme as adjuvant therapy in pneumococcal meningitis. Brain. 2001;124:1734–1742. doi: 10.1093/brain/124.9.1734. [DOI] [PubMed] [Google Scholar]
- Leppert D, Lindberg RL, Kappos L, Leib SL. Matrix metalloproteinases: multifunctional effectors of inflammation in multiple sclerosis and bacterial meningitis. Brain ResBrain ResRev. 2001;36:249–257. doi: 10.1016/s0165-0173(01)00101-1. [DOI] [PubMed] [Google Scholar]
- Mun-Bryce S, Lukes A, Wallace J, Lukes-Marx M, Rosenberg GA. Stromelysin-1 and gelatinase A are upregulated before TNF-alpha in LPS-stimulated neuroinflammation. Brain Res. 2002;933:42–49. doi: 10.1016/s0006-8993(02)02303-x. [DOI] [PubMed] [Google Scholar]
- Mun-Bryce S, Rosenberg GA. Gelatinase B modulates selective opening of the blood-brain barrier during inflammation. American Journal of Physiology. 1998;274:R1203–11. doi: 10.1152/ajpregu.1998.274.5.R1203. [DOI] [PubMed] [Google Scholar]
- Nelson AR, Fingleton B, Rothenberg ML, Matrisian LM. Matrix metalloproteinases: biologic activity and clinical implications. J ClinOncol. 2000;18:1135–1149. doi: 10.1200/JCO.2000.18.5.1135. [DOI] [PubMed] [Google Scholar]
- Ohno K, Pettigrew KD, Rapoport SI. Lower limits of cerebrovascular permeability to nonelectrolytes in the conscious rat. AmJ Physiol. 1978;235:299–307. doi: 10.1152/ajpheart.1978.235.3.H299. [DOI] [PubMed] [Google Scholar]
- Overall CM, Lopez-Otin C. Strategies for MMP inhibition in cancer: innovations for the post-trial era. NatRevCancer. 2002;2:657–672. doi: 10.1038/nrc884. [DOI] [PubMed] [Google Scholar]
- Perry VH, Andersson PB. The inflammatory response in the CNS. Neuropathology & Applied Neurobiology. 1992;18:454–459. doi: 10.1111/j.1365-2990.1992.tb00811.x. [DOI] [PubMed] [Google Scholar]
- Power C, Henry S, Del Bigio MR, Larsen PH, Corbett D, Imai Y, Yong VW, Peeling J. Intracerebral hemorrhage induces macrophage activation and matrix metalloproteinases. AnnNeurol. 2003;53:731–742. doi: 10.1002/ana.10553. [DOI] [PubMed] [Google Scholar]
- Rosenberg GA. Matrix metalloproteinases in neuroinflammation. Glia. 2002;39:279–291. doi: 10.1002/glia.10108. [DOI] [PubMed] [Google Scholar]
- Rosenberg GA, Estrada EY, Dencoff JE. Matrix metalloproteinases and TIMPs are associated with blood-brain barrier opening after reperfusion in rat brain. Stroke. 1998;29:2189–2195. doi: 10.1161/01.str.29.10.2189. [DOI] [PubMed] [Google Scholar]
- Sood R, Taheri S, Estrada E, Rosenberg G. Quantitative evaluation of the effect of propylene glycol on BBB opening. J Mag Res Imag. doi: 10.1002/jmri.20802. in press. [DOI] [PubMed] [Google Scholar]
- Wang J, Tsirka SE. Neuroprotection by inhibition of matrix metalloproteinases in a mouse model of intracerebral haemorrhage. Brain. 2005;128:1622–1633. doi: 10.1093/brain/awh489. [DOI] [PubMed] [Google Scholar]
- Yong VW, Power C, Forsyth P, Edwards DR. Metalloproteinases in biology and pathology of the nervous system. NatRevNeurosci. 2001;2:502–511. doi: 10.1038/35081571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yrjanheikki J, Tikka T, Keinanen R, Goldsteins G, Chan PH, Koistinaho J. A tetracycline derivative, minocycline, reduces inflammation and protects against focal cerebral ischemia with a wide therapeutic window. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:13496–13500. [Google Scholar]
- Zhang JW, Gottschall PE. Zymographic measurement of gelatinase activity in brain tissue after detergent extraction and affinity-support purification. Journal of Neuroscience Methods. 1997;76:15–20. doi: 10.1016/s0165-0270(97)00065-4. [DOI] [PubMed] [Google Scholar]
- Zhao BQ, Wang S, Kim HY, Storrie H, Rosen BR, Mooney DJ, Wang X, Lo EH. Role of matrix metalloproteinases in delayed cortical responses after stroke. NatMed. 2006;12:441–445. doi: 10.1038/nm1387. [DOI] [PubMed] [Google Scholar]


