Abstract
Context
Hormones regulate neuronal function in brain regions critical to cognition; however the cognitive effects of postmenopausal hormone therapy are controversial.
Objective
The goal was to evaluate the effect of postmenopausal hormone therapy on neural circuitry involved in spatial working memory.
Design
A randomized, double-blind placebo-controlled cross-over study was performed.
Setting
The study was performed in a tertiary care university medical center.
Participants
Ten healthy postmenopausal women of average age 56.9 years were recruited.
Interventions
Volunteers were randomized to the order they received hormone therapy, 5 ug ethinyl estradiol and 1 mg norethindrone acetate. Subjects received hormone therapy or placebo for 4 weeks, followed by a one month washout period with no medications, and then received the other treatment for 4 weeks. At the end of each 4 week treatment period a functional magnetic resonance imaging (fMRI) study was performed utilizing a nonverbal (spatial) working memory task, the Visual Delayed Matching to Sample task.
Main Outcome Measure
The effects of hormone therapy on brain activation patterns were compared to placebo.
Results
Compared to the placebo condition, hormone therapy was associated with a more pronounced activation in the prefrontal cortex (BA 44 and 45), bilaterally (p<0.001).
Conclusions
Hormone therapy was associated with more effective activation of a brain region critical in primary visual working memory tasks. The data suggest a functional plasticity of memory systems in older women that can be altered by hormones.
Key terms: Brain, women’s health, neuroimaging, fMRI, spatial memory, non-verbal memory, estrogen, progestin, hormones, menopause
INTRODUCTION
The role of estrogen in maintenance of neuronal integrity and cognitive function is of great significance in light of our aging population and the increasing incidence of dementias. Ovarian hormones are known to regulate neuronal function beyond the neuroendocrine reproductive axis, influencing brain regions critical to cognition (1). However, the specific cognitive effects in woman have been difficult to define. More pronounced age-related declines in complex visuospatial and motor function tasks have been identified in postmenopausal women compared to premenopausal women (2). Yet, while post-menopausal hormone therapy (HT) is suggested to improve verbal memory (3–5) and non-verbal memory (6–8), recent longitudinal data from the Nurses Health Study (9), as well as randomized clinical trial data from the Women’s Health Initiative Memory Study (10), have failed to confirm these potential cognitive benefits.
Non-invasive neuroimaging techniques are now providing mechanistic information concerning the brain aging process in women, allowing investigators to directly study the effects of hormones on various measures of neuronal function and neurochemistry (11,12). At present, the limited data suggest that HT in post-menopausal women may decrease brain white matter lesions (13), increase cerebral blood flow (14–16), have modulating effects on various neurotransmitter systems (17–19), increase glucose metabolism in certain brain regions (20), preserve hippocampal volume (21), and alter regional brain activation patterns during cognitive processes (22–25).
Studies of estrogen effects on brain activity during directed cognitive activity provide information on neural pathways utilized during memory tasks. Measuring regional cerebral blood flow (rCBF) patterns with PET and 15-oxygen-labeled water (H2150) in a cross-sectional study of postmenopausal women, differences among estrogen vs. non-estrogen users were demonstrated in the following regions: in the right parahippocampal gyrus, precuneus, inferior frontal cortex and dorsal frontal gyrus during verbal tasks, and in the right inferior parietal region, right parahippocampal gyrus, left visual association area, left anterior thalamus, and a region proximal to the right mammillary body during spatial tasks (22). Subsequent longitudinal data demonstrated an increase in rCBF in the hippocampus, parahippocampal gyrus and temporal lobe among hormone users (23).
Functional MRI (fMRI), another neuroimaging technique, provides measures of synaptic activity by measuring changes in the magnetic field associated with the deoxygenation of hemoglobin. Examining the effects of estrogen (conjugated equine estrogens, 1.25 mg) on performance of verbal and non-verbal working memory tasks, Shaywitz et al (24) demonstrated altered activation patterns using fMRI in areas of the parietal and frontal cortex that have been previously demonstrated to be involved in working memory (26). Working memory refers to the system which actively maintains and manipulates information over short time periods. This memory system is critical for many daily activities, such as remembering directions or multitasking short activities (27).
As our laboratory has previously identified an association between long-term postmenopausal HT and the preservation of cholinergic markers (17) and in measures of spatial memory (8), this study sought to further evaluate the effect of HT on neural circuitry involved in spatial working memory. We performed a randomized, double-blind, placebo controlled crossover study in postmenopausal women, utilizing a spatial memory task combined with fMRI. We hypothesized that the combined estrogen-progestin hormone therapy would be associated with increased activation in areas known to be involved in working memory.
MATERIALS AND METHODS
Subjects
A group of 10 healthy postmenopausal women, 50 – 60 years of age, were recruited by advertisement. Menopause was defined as the absence of menstrual periods for 1 year for those with intact reproductive organs or the time of hysterectomy with bilateral salpingo-oophorectomy. After an initial phone screening, the women had personal interviews in which medical and psychiatric histories, screening laboratory tests (fasting cholesterol, glucose, estradiol, complete blood count, TSH, electrolytes and liver function tests), and a physical exam including pelvic examination and pelvic ultrasound were obtained. All women had normal pap smears and mammograms within one year prior to participation in the study.
Women were included who were free of significant general medical, neurological or psychiatric illness; had not received hormone therapy in the last 3 months; had never experienced a head injury with loss of consciousness; and had no history of drug or alcohol abuse or dependence. All participants were right handed, were nonsmokers, and were taking no medications with actions in the central nervous system. Exclusion criteria included an endometrial lining greater than 5mm, ovarian pathology on ultrasound, migraines, liver dysfunction, history of thromboembolic disease, uncontrolled thyroid disease, fasting cholesterol > 300 mg/dl, fasting triglycerides >300 mg/dl, and fasting glucose > 140 mg/dl. After a full description of the study, written informed consent was obtained. All procedures were approved by the University of Michigan Institutional Review Board.
Study protocol
The study design was a randomized, double-blind, placebo-controlled crossover design of hormone therapy versus placebo. Subjects were randomized to the order they received hormone therapy with 5 ug ethinyl estradiol and 1 mg norethindrone acetate (Femhrt, Warner Chilcott, PLC, United Kingdom). Randomization was performed with a computer generated random number list. Prior to beginning treatment the subjects had baseline neuropsychological testing. Subjects received hormone therapy or placebo for 4 weeks, followed by a one month washout period with no medications, and then received the other treatment for 4 weeks. At the end of each 4 week treatment period an fMRI study was performed. Pill counts were done after each treatment period to document compliance.
A neuropsychological battery of tests was given to exclude the presence of dementia or specific deficits in visual spatial skills. The battery included the following measures: 1) Mini-Mental State Examination (28) as a brief screening measure of dementia, 2) Shipley Institute of Livings Scale (29) that is a short two-subtest (vocabulary and verbal abstraction tasks) estimate of intellectual power (29), 3) Geriatric Depression Rating Scale (30) to exclude the presence of depression, 4) Benton Visual Retention Test-Revised (31) as a measure of visual memory ability and 5) Benton Visual Form Discrimination (32), as a measure of visual spatial perception.
FMRI Visual Delayed Matching to Sample Task
The fMRI paradigm to evaluate nonverbal (spatial) memory used a validated Visual Delayed Matching to Sample task (VDMT) (33,34). During this task, subjects were presented with complex visual stimuli through a set of RF-shielded goggles mounted to a headcoil (Resonance Technology Inc., Northridge, CA). The visual stimuli consisted of 9 x 9 grids containing 81 squares (Figure 1). For each stimulus a random pattern of 40 squares were darkened to form a pattern. The visual stimuli were presented under one of three conditions where the participant was asked to make a response by pressing one of two buttons on a MRI-compatible response pad.
Figure 1.
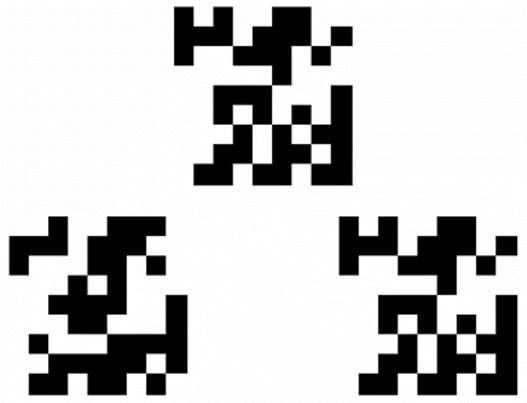
The sample visual working memory task stimulus, consisted of a 9 X 9 grid of pixels, half of which are darkened in a random fashion. The target stimulus at the top is displayed first, then removed, and the two test stimuli below are displayed following a 1 or 4 second delay. The test stimulus on the right matches the target stimuli at the top.
In the matching condition, subjects were presented a target stimulus at the top of the screen with 2 additional test items appearing simultaneously directly underneath the target. The women were asked to select which of the 2 test items matches the target. Stimuli were presented for 3 seconds in duration followed by a 7 second fixation cross before presentation of the next item. The other two conditions assessed visual working memory skills. Specifically, the target stimulus was presented alone for 1.5 seconds to be followed by either a 1 second or 4 second delay. Following the delay, test items were presented for 3 seconds during which time women were required to indicate the match. The 4 second delay condition engaged working memory processes to a greater extent and enabled the visual spatial information to be maintained in memory across the delay.
In the scanner, the stimuli were presented in a blocked design, with 4 trials from each of the 3 conditions presented in a counterbalanced fashion. Each run had a duration of approximately 6 minutes with a 30 second break between runs. In each session, blocks of stimuli for each condition were counterbalanced for a total of 108 trials over 3 runs. A commercial software package (E-Prime, Psychology Software Tools Inc, Pittsburg, PA) running on a computer in the MRI control room controlled the timing of the stimulus presentation. The total number of scans was 180 with an interscan interval of 2 seconds. Accuracy and response time was recorded. The overall task was preceded by the presentation of detailed instructions during which no data were acquired. To minimize performance differences between subjects, prior to fMRI data collection, participants practiced the task on a computer outside the scanner until they reached a level of at least 70% accuracy.
FMRI Acquisition and Processing
All scans were acquired using a 3T whole body MRI scanner (General Electric, Milwaukee, WI) equipped with a standard head coil. Anatomical MRI scans were acquired axially in all subjects with an SPGR 3D volumetric acquisition (TR 9.6/TE 3.3/IR PREP 200ms/flip angle 17 degrees/bandwidth 15.63/24cm F.O.V./1.5mm slice thickness/106–110 slices/256 x 256 matrix/2 nex) for anatomical localization and coregistration to standardized stereotactic coordinates. FMRI acquisition was sensitized for the BOLD effect using a T2* weighted single-shot spiral pulse sequence (Noll et al 1995) with 32 oblique-axial slices prescribed to be approximately parallel to the AC-PC line (Spiral GRE, TE = 25, TR = 2000, FA = 60°, 4 mm-thick contiguous slices, 24 cm FOV, image matrix = 64 x 64). Image reconstruction included processing steps to remove distortions caused by magnetic field inhomogeneity and other sources of misalignment to the structural data (35). Data were sinc-interpolated in time, slice-by-slice, to correct for the staggered sequence of slice acquisition (36).
The first four functional volumes of each run were discarded to remove magnetic saturation effects. The remaining functional images were realigned to the first volume to eliminate movement artifacts using SPM2-based algorithms (37). Realignment parameters for each subject were carefully examined to ensure subject’s head movement did not exceed 2 mm. Subject’s MRI and functional images were coregistered to each other by way of rigid body affine transformation using a mutual information algorithm, described in detail elsewhere (38). The subject’s MRI was then spatially normalized into standard stereotactic space (International Conference on Brain Mapping (ICBM) atlas) via linear and non-linear warping (39). The transformation matrix was then applied to the functional images. Finally, a three-dimensional Gaussian smoothing kernel set at 8 mm full-width-half-maximum (FWHM) was applied to each subject’s functional data to accommodate for residual anatomical variability and to improve signal to noise ratios.
FMRI Data Analyses
All fMRI data analyses were conducted using the general linear model in SPM2 (Wellcome Department of Cognitive Neurology, London, UK) For the first level analyses, contrast images were generated for each subject to assess differences in activation between visual task performance at short delays and long delays and visual task performance during matching. These initial contrast images (4 sec minus matching and 1 sec delay minus matching) were subtracted from each other to isolate the visual working memory component. The effects of hormone therapy were then assessed on this component.
To evaluate the effect of hormone therapy the visual working memory contrast images for each subject during hormone therapy and placebo conditions were analyzed at the group-level using one-sample t-tests. A statistical threshold of p ≤0.001 uncorrected for multiple comparisons, standard in this field, was used to identify significant voxels for all comparisons. A minimum cluster size of 15 voxels was additionally employed as a criterion of significant activation. Additionally, numerical differences between groups were determined by averaging the values of voxels contained in an area of significant differences, down to a threshold of p=0.01. These data were then plotted and examined for regional differences between conditions to eliminate the possibility that significant effects in the voxel-by-voxel comparisons were caused by artifactual data or outliers.
RESULTS
The average age of the participants in this study was 56.9 years. All 10 subjects completed the study protocol and pill counts documented completion of the medication. The demographic and baseline information is described in Table 1. The baseline neuropsychological testing documented normal IQ and normal spatial perception and memory, and demonstrated that these volunteers were without evidence of dementia or depression (Table 1). No significant differences between conditions were obtained for neuropsychological test results in this small sample (paired t-tests, p > 0.05).
Table 1.
Demographic and Baseline Information
| Average (+/− SD) | |
|---|---|
| Age (years) | 56.9 (1.4) |
| Menopause Age (Years) | 50.0 (2.7) |
| Years Post-Menopause | 6.9 (2.8) |
| BMI | 25.5 (4.0) |
| Baseline Estradiol (pg/mL) | 17.2 (4.8) |
| Education (Years) | 16.3 (1.9) |
| Mini Mental Status Exam (MMSE)a | 28.6 (1.9) |
| Shipley Estimated IQb | 115.0 (12.0) |
| Geriatric Depression Rating Scalec | 0.4 (1.0) |
| Benton Visual Retention Test – Revised (# correct)d | 7.7 (1.0) |
| Benton Visual Form Discrimination (# correct)e | 30.9 (2.2) |
maximum score=30; cutoff of 24 and above is within normal limits
mean=100, S.D.=15
a score of 5 or higher indicates the presence of depressive symptoms
maximum score=10, mean=7.08, S.D=4.21 for this age group
maximum score =32; cutoff score of 24 and above is within normal limits
Neuroimaging
Performance data during the visual task at short (1 sec) and longer (4 sec) delays showed high levels of accuracy during both of the working memory tasks with no significant difference between the placebo and HT treatment conditions (accuracies of 86 ± 9 and 85 ± 7% during the placebo condition and 90 ± 5 and 86 ± 5% during HT paired, two-tailed t-tests, p>0.05).
A subtraction method was used in order to assess directly the working memory component of this visual task. The only difference between delay 1 and delay 4 conditions is the added working memory component. Therefore, subtracting the brain activation observed during the one second delay task from that of the four second delay task effectively isolates the activation that is due solely to areas involved in working memory. This analysis demonstrated activation in regions consistent with those areas that have been shown to be involved in working memory (Table 2, Figure 2), including the prefrontal cortex (BA 44 and 46), bilaterally, left medial frontal cortex (BA 6), inferior parietal cortex, bilaterally, and cerebellum, bilaterally, (p ≤ 0.001).
Table 2.
Areas of Activation During Non-Verbal Working Memory: Effect of Task, Delay Four > Delay One
| Region | Coordinates (x, y, z)† | Z | Cluster Size‡ |
|---|---|---|---|
| Left Prefrontal Cortex (BA 44) | −42, 6, 29 | 4.17 | 810 |
| Left Prefrontal Cortex (BA 46) | −44, 30, 25 | 3.51 | 200 |
| Right Prefrontal Cortex (BA 44) | 45, 4, 31 | 3.89 | 522 |
| Left Medial Frontal Cortex (BA 6) | −5, 14, 47 | 5.74 | 3895 |
| Left Inferior Parietal Cortex | −31, −70, 43 | 6.88 | 7609 |
| Right Inferior Parietal Cortex | 27, −61, 48 | 6.08 | 8032 |
| Left Cerebellum | −12, −75, −14 | 3.81 | 168 |
| Right Cerebellum | 25, −75, −17 | 4.39 | 792 |
Coordinates (x,y,z) refer to the ICBM coordinates where the significant differences were centered in mm.
Cluster size is expressed in mm3.
All regions significant at p ≤ 0.001, uncorrected for multiple comparisons.
Figure 2.
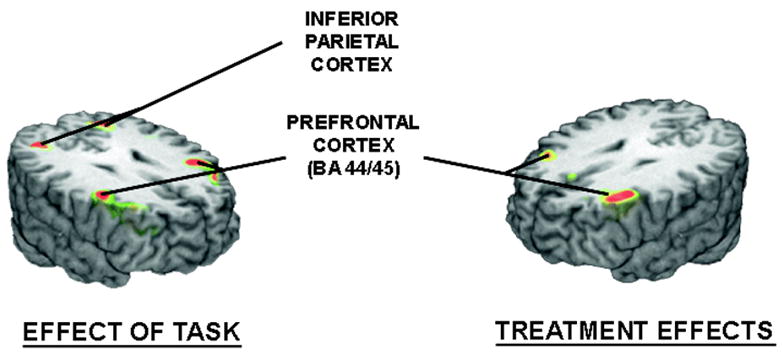
The left image shows the areas where significant effects of the visual working memory task (4 sec delay minus 1 second delay) were observed on BOLD-fMRI activation across conditions. The image on the right shows the areas where significantly higher levels of brain regional activation were observed during hormone treatment, compared to placebo.
Hormone therapy had significant effects on activation during performance of the visual working memory task when compared to placebo (Table 3). Hormone therapy was associated with a more pronounced activation in the prefrontal cortex (BA 44 and 45), bilaterally (Figure 2). No areas of significantly higher activation were observed during the placebo condition, compared to the hormone therapy condition.
Table 3.
Effect of Hormone Therapy, Delay Four > Delay One
| Region | Coordinates (x, y, z)† | Z | Cluster Size‡ |
|---|---|---|---|
| Hormones > Placebo | |||
| Left Prefrontal Cortex (BA 44/45) | −39, 24, 32
−44, 20, 15 |
3.25
2.99 |
276
195 |
| Right Prefrontal Cortex (BA 44/45) | 40, 11, 18 | 4.69 | 3931 |
| Placebo > Hormones | |||
| No significant regions detected | |||
Coordinates (x,y,z) refer to the ICBM coordinates where the significant differences were centered in mm.
Cluster size is expressed in voxels of 13 mm.
All regions significant at p ≤ 0.001, uncorrected for multiple comparisons.
DISCUSSION
The present study demonstrated that HT had significant effects on brain regional activation during performance of the visual working memory task when compared to placebo. Specifically, HT was associated with more pronounced activation of the prefrontal cortex, bilaterally at comparable levels of task performance. Therefore, differences in task performance could not account for the effects observed, an important confound in studies of aging or pathological cognitive processes. The results obtained in this study support the contention that the presence of higher levels of gonadal steroids is associated with more effective activation of brain regions involved in primary visual working memory tasks. On the other hand, it is possible that the finding of increased prefrontal cortical activation could simply reflect a requirement for more effort in the hormone group to maintain the same level of performance. However, the same activation pattern has been shown in young adults with behavioral changes, suggesting that this may be a real effect of the combined hormone treatment (26, 40, 41).
Working memory is a limited-capacity storage system to actively maintain and manipulate information over short time periods critical for many daily activities (27). The areas of activation/deactivation demonstrated in this study are consistent with known activation patterns usually found during visuospatial working memory tasks in healthy adults. These include the prefrontal cortex (involved in monitoring, organization and planning), parietal regions, medial frontal cortex, and cerebellum (27,42,43). Working memory has been demonstrated to be less efficient in older adults, and these age-related changes have often been linked to neuroanatomical and metabolic alterations in the prefrontal cortex (44,45). Our finding of increased activation in the prefrontal cortex in older women using hormone therapy is important and suggestive of potential cognitive therapeutic effects that need to be explored further.
Evidence suggests that the prefrontal cortex may be a major target of estrogen action. In the ovariectomized rhesus monkey model cyclic estradiol improved response on a spatial working memory task compared to placebo treatment, and also increased the dendritic spine number in the prefrontal cortex (46,47). In addition, estrogen regulates neurotransmitter activities in the primate prefrontal cortex by altering cholinergic, serotonergic, noradrenergic, and dopaminergic innervation (48–50). The present study expands the body of knowledge and demonstrates a functional change in the prefrontal cortex of post-menopausal women exposed to a short course of estrogen-progestin hormone therapy.
Brain activation patterns during performance of spatial memory tasks are known to change with aging. In younger adults activation is predominantly right hemispheric, while older adults often demonstrate a pattern of bilateral activation (51), despite showing similar behavioral performances on the task. This bilateral activation has been interpreted as a reflection of recruitment to compensate for loss of neural integrity. In the working memory fMRI study of older women by Shaywitz et al (24) estrogen therapy was associated with increased asymmetry of the hemispheric encoding/retrieval function for both verbal and nonverbal tasks. During encoding, the left hemisphere showed greater activation than the right hemisphere, while during retrieval, the right hemisphere showed greater activation than the left hemisphere during retrieval, a pattern characteristic of younger adults.
In the present study, using a combined estrogen-progestin preparation, we demonstrated more focused activation in the prefrontal cortex; however, we did not observe alterations in the bilaterality of brain activation. Bilateral activation during this working memory task may represent compensation for age-related neuronal decline, and/or verbal strategies employed by the subjects to help with the recall of the shapes presented. Another consideration is that estrogen alone may have different brain effects than estrogen combined with a progestin. Regardless, these studies suggest a functional plasticity of memory systems in older women that can be altered by hormones. Furthermore, they suggest the need for the direct examination of brain function with imaging techniques as a more sensitive measure of cognitive aging than that afforded by standard neuropsychological tests.
With the small sample size employed we did not observe significant differences in neuropsychological test performance within or outside the scanner. However, demonstrable effects of short term HT on measures of neuronal activity as reflected by the fMRI-BOLD signal were obtained. These may simply reflect early changes in neuronal activity that with either longer treatment or larger samples are translated into differences in testing performance, albeit this hypothesis would have to be corroborated in larger scale, longitudinal studies.
This study has particular strengths and limitations. The intrasubject design increased power and removed the need to closely match treatment and control groups according to chronological age, education, and other characteristics that may influence neuropsychological function. While cross-over designs always carry a risk of carry over effects, a wash out period was included in the protocol and the order of estrogen or placebo treatment was randomized. In addition, the protocol utilized a combined estrogen and progestin hormone therapy which does not allow for discrimination between the effects of estrogen and progestin, and this issue requires further investigation.
While it is clear that long-term HT is not beneficial for prevention of chronic illnesses (52,53), the effects of short-term HT on brain circuitry and function warrant further study. The present study suggests that even relatively short periods of HT administration have demonstrable effects on neuronal function that may be of benefit to some women during the perimenopausal transition or early postmenopause. Understanding the actions of estrogens in the brain may not only assist in counseling perimenopausal and early menopausal women about short-term estrogen use, but may facilitate the development of both appropriate alternatives to standard HT and medications targeted to prevent cognitive aging.
Acknowledgments
We thank the fMRI staff (Eve Gochis, Keith Newnham, Luis Hernandez, Ph.D. and Douglas Noll, Ph.D) at the University of Michigan, Ann Arbor for their assistance in this study. We would also like to thank Robert Bilder, Ph.D. at U.C.L.A. for providing us with the Delayed Visual Matching to Sample task that was used in this study.
Footnotes
Publisher's Disclaimer: "This is an un-copyedited author manuscript copyrighted by The Endocrine Society. This may not be duplicated or reproduced, other than for personal use or within the rule of “Fair Use of Copyrighted Materials” (section 107, Title 17, U.S. Code) without permission of the copyright owner, The Endocrine Society. From the time of acceptance following peer review, the full text of this manuscript is made freely available by The Endocrine Society at http://www.endojournals.org/. The final copy edited article can be found at http://www.endojournals.org/. The Endocrine Society disclaims any responsibility or liability for errors or omissions in this version of the manuscript or in any version derived from it by the National Institutes of Health or other parties. The citation of this article must include the following information: author(s), article title, journal title, year of publication and DOI.”
Disclosure statement: Y.R.S. received an investigator-initiated grant from Pfizer Pharmaceuticals Group; T.L., C.C.P. and A.T. have nothing to declare; T.E.N. has consulted for GlaxoSmithKline Inc.; J.K.Z. received lecture fees from GlaxoSmith Kline Inc., Eli Lilly and Co., and Forest Laboratories.
Supported in part by Grant K23RR017043 from the National Center for Research Resources and an investigator initiated grant from Pfizer Pharmaceuticals Group.
References
- 1.McEwen BS. Estrogen actions throughout the brain. Recent Prog Horm Res. 2002;57:357–384. doi: 10.1210/rp.57.1.357. [DOI] [PubMed] [Google Scholar]
- 2.Halbreich U, Lumley LA, Palter S, Manning C, Gengo F, Joe SH. Possible acceleration of age effects on cognition following menopause. J Psychiatr Res. 1995;29:153–163. doi: 10.1016/0022-3956(95)00005-p. [DOI] [PubMed] [Google Scholar]
- 3.Jacobs M, Tang MX, Stern Y, Sano M, Marder K, Bell KL, Schofield P, Dooneief G, Gurland B, Mayeux R. Cognitive function in nondemented older women who took estrogen after menopause. Neurology. 1998;50:368–373. doi: 10.1212/wnl.50.2.368. [DOI] [PubMed] [Google Scholar]
- 4.Phillips SM, Sherwin BB. Effects of estrogen on memory function in surgically menopausal women. Psychoeuroendocrinol. 1992;17:485–495. doi: 10.1016/0306-4530(92)90007-t. [DOI] [PubMed] [Google Scholar]
- 5.Sherwin BB. Estrogen and/or androgen replacement therapy and cognitive functioning in surgically menopausal women. Psychoneuroendocrinology. 1988;13:345–357. doi: 10.1016/0306-4530(88)90060-1. [DOI] [PubMed] [Google Scholar]
- 6.Resnick SM, Metter EJ, Zonderman AB. Estrogen replacement therapy and longitudinal decline in visual memory. Neurology. 1997;49:1491–1497. doi: 10.1212/wnl.49.6.1491. [DOI] [PubMed] [Google Scholar]
- 7.Kimura D. Estrogen replacement therapy may protect against intellectual decline in postmenopausal women. Hormones and Behavior. 1995;29:312–321. doi: 10.1006/hbeh.1995.1022. [DOI] [PubMed] [Google Scholar]
- 8.Smith YR, Giordani B, O'Neill RL, Zubieta JK. Long-term estrogen replacement is associated with improved non-verbal memory and attentional measures in postmenopausal women. Fertil Steril. 2001;76(6):1101–1107. doi: 10.1016/s0015-0282(01)02902-8. [DOI] [PubMed] [Google Scholar]
- 9.Kang JH, Weuve J, Grodstein F. Postmenopausal hormone therapy and risk of cognitive decline in community-dwelling aging women. Neurology. 2004;63:101–107. doi: 10.1212/01.wnl.0000132522.13574.67. [DOI] [PubMed] [Google Scholar]
- 10.Shumaker SA, Legault C, Rapp SR, Thal L, Wallace RB, Ockene JK, Hendrix SL, Jones BN, 3rd, Assaf AR, Jackson RD, Kotchen JM, Wassertheil-Smoller S, Wactawski-Wende J. WHIMS Investigators. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women's Health Initiative Memory Study: a randomized controlled trial. JAMA. 2003;289:2651–2662. doi: 10.1001/jama.289.20.2651. [DOI] [PubMed] [Google Scholar]
- 11.Smith YR, Zubieta JK. Neuroimaging of aging and estrogen effects on central nervous system physiology. Fertil Steril. 2001;76(4):651–659. doi: 10.1016/s0015-0282(01)01985-9. [DOI] [PubMed] [Google Scholar]
- 12.Maki PM, Resnick SM. Effects of estrogen on patterns of brain activity at rest and during cognitive activity: A review of neuroimaging studies. NeuroImage. 2001;14:789–801. doi: 10.1006/nimg.2001.0887. [DOI] [PubMed] [Google Scholar]
- 13.Schmidt R, Fazekas F, Reinhart B, Kapeller P, Fazekas G, Offenbacher H, Eber B, Schumacher M, Freidl W. Estrogen replacement therapy in older women: a neuropsychological and brain MRI study. J Amer Geriatrics Soc. 1996;44(11):1307–1313. doi: 10.1111/j.1532-5415.1996.tb01400.x. [DOI] [PubMed] [Google Scholar]
- 14.Slopien R, Junik R, Meczekalski B, Halerz-Nowakowska B, Maciejewska M, Wareni-Szymankiewicz A, Sowinski J. Influence of hormonal replacement therapy on the regional cerebral blood flow in postmenopausal women. Maturitas. 2003;46:255–262. doi: 10.1016/s0378-5122(03)00144-0. [DOI] [PubMed] [Google Scholar]
- 15.Greene RA. Estrogen and cerebral blood flow: A mechanism to explain the impact of estrogen on the incidence and treatment of Alzheimer’s disease. Int J Fertil Menopausal Stud. 2000;45:253–257. [PubMed] [Google Scholar]
- 16.Okhura T, Teshima Y, Isse K, Matsuda H, Inoue T, Sakai Y, Iwasaki N, Yaoi I. Estrogen increases cerebral and cerebellar blood flow in postmenopausal women. Menopause. 1995;2:13–18. [Google Scholar]
- 17.Smith YR, Minoshima S, Kuhl DE, Zubieta JK. Effects of long-term hormone replacement therapy on cholinergic synaptic concentrations in healthy postmenopausal women. J Clin Endo Metab. 2001;86:679–684. doi: 10.1210/jcem.86.2.7222. [DOI] [PubMed] [Google Scholar]
- 18.Moses EL, Drevets WC, Smith G, Mathis CA, Kalro BN, Butters MA, Leondires MP, GReeg PJ, Lopresti B, Loucks TL, Berga SL. Effects of estradiol and progesterone administration on human serotonin 2A receptor binding: A PET study. Biol Psychiatry. 2000;48:854–860. doi: 10.1016/s0006-3223(00)00967-7. [DOI] [PubMed] [Google Scholar]
- 19.Kugaya A, Epperson CN, Zoghbi S, van Dyck CH, Hou Y, Fujita M, Stanley JK, Garg PK, Seibyl JP, Inis RB. Increase in prefrontal cortex serotonin2A receptors following estrogen treatment in postmenopausal women. Am J Psychiatry. 2003;160:1522–1524. doi: 10.1176/appi.ajp.160.8.1522. [DOI] [PubMed] [Google Scholar]
- 20.Rasgon NL, Silverman D, Siddarth P, Miller K, Ercoli LM, Elman S, Lavretsky H, Huang S-C, Phelps ME, Small GW. Estrogen use and brain metabolic change in postmenopausal women. Neurobiology of Aging. 2005;26:229–235. doi: 10.1016/j.neurobiolaging.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 21.Eberling JL, Wu D, Hann MN, Mungas D, Buonocore M, Jagust WJ. Preliminary evidence that estrogen protects against age-related hippocampal atrophy. Neurobiology of Aging. 2003;24:725–732. doi: 10.1016/s0197-4580(02)00056-8. [DOI] [PubMed] [Google Scholar]
- 22.Resnick SM, Maki PM, Golski S, Kraut MA, Zonderman AB. Effects of estrogen replacement therapy on PET cerebral blood flow and neuropsychological performance. Hormones and Behavior. 1998;34:171–182. doi: 10.1006/hbeh.1998.1476. [DOI] [PubMed] [Google Scholar]
- 23.Maki PM, Resnick SM. Longitudinal effects of estrogen replacement therapy on PET cerebral blood flow and cognition. Neurobiol Aging. 2000;21:373–383. doi: 10.1016/s0197-4580(00)00123-8. [DOI] [PubMed] [Google Scholar]
- 24.Shaywitz SE, Shaywitz BA, Pugh KR, Fulbright RK, Skudlarski P, Menel WE. Effect of estrogen on brain activation patterns in postmenopausal women during working memory tasks. JAMA. 1999;281:1197–1202. doi: 10.1001/jama.281.13.1197. [DOI] [PubMed] [Google Scholar]
- 25.Stevens MC, Clark VP, Prestwood KM. Low-dose estradiol alters brain activity. Psychiatry Res. 2005;139:199–217. doi: 10.1016/j.pscychresns.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 26.Smith EE, Jonides J. Neuroimaging analyses of human working memory. Proc Natl Acad Sci U S A. 1998;95:12061–12068. doi: 10.1073/pnas.95.20.12061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wager TD, Smith EE. Neuroimaging studies of working memory: A meta-analysis. Cognitive, Affective and Behavioral Neuroscience. 2003;3:255–274. doi: 10.3758/cabn.3.4.255. [DOI] [PubMed] [Google Scholar]
- 28.Folstein MF, Folstein SE, McHugh PR. "Mini-mental state." A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 29.Shipley WC. Institute of Living Scale. Los Angeles: Western Psychological Services; 1946. [Google Scholar]
- 30.Yesavage JA, Rose TL, Lapp D. Validity of the Geriatric Depression Scale in subjects with senile dementia. Palo Alto, CA: Clinical Diagnostic and Rehabilitation Unit, Veterans Administration Medical Center; 1981. [Google Scholar]
- 31.Benton AL. Revised Visual Retention Test. New York: The Psychological Corporation; 1974. [Google Scholar]
- 32.Benton AL, Hamsher KdeS, Varney NR, Spreen O. Contributions to neuropsychological assessment. New York: Oxford University Press; 1983. [Google Scholar]
- 33.Lencz T, Bilder RM, Turkel E, Goldman RS, Robinson D, Kane JM, Lieberman JA. Impairments in perceptual competency and maintenance on a visual delayed match-to-sample test in first episode schizophrenia. Arch Gen Psych. 2003;60:238–243. doi: 10.1001/archpsyc.60.3.238. [DOI] [PubMed] [Google Scholar]
- 34.Phillips WA. On the distinction between sensory storage and short-term visual memory. Percep Psychophys. 1974;16:283–290. [Google Scholar]
- 35.Noll DC, Stenger VA, Vazquez AL, Peltier SJ. Spiral Scanning in Functional MRI. In: CTW M, PA B, editors. Medical Radiology: Functional MRI. Heidelberg: Springer-Verlag; 1999. pp. 149–169. [Google Scholar]
- 36.Acquirre GK, D'Esposito M. Experimental Design for Brain fMRI. In: CTW M, PA B, editors. Medical Radiology: Functional MRI. Heidelberg: Springer-Verlag; 1999. pp. 369–380. [Google Scholar]
- 37.Friston KJK, Ashburner J, Frith CD, Poline J-B, Heather JD, Frackowiak RSJ. Spatial registration and normalization of images. Hum Brain Map. 1995;2:165–189. [Google Scholar]
- 38.Meyer CR, Boes JL, Kim B, Bland PH, Zasadny KR, Kison PV, Koral K, Frey KA, Wahl RL. Demonstration of accuracy and clinical versatility of mutual information for automatic multimodality image fusion using affine and thin-plate spline warped geometric deformations. Med Image Anal. 1997;1:195–206. doi: 10.1016/s1361-8415(97)85010-4. [DOI] [PubMed] [Google Scholar]
- 39.Meyer CR, Boes JL, Kim B, Bland PH, Frey KA. Warping normal patients into the ICBM atlas by maximizing MI; International Conference of Functional Mapping of the Human Brain; Montreal: Quebec. 1998. [Google Scholar]
- 40.Ranganath C. Working memory for visual objects: Complementary roles of inferior temporal, medial temporal, and prefrontal cortex. Neuroscience. 2006;139:277–289. doi: 10.1016/j.neuroscience.2005.06.092. [DOI] [PubMed] [Google Scholar]
- 41.Curtis CE, D’Esposito M. Persistent activity in the prefrontal cortex during working memory. Trends in Cognitive Sciences. 2003;7:415–423. doi: 10.1016/s1364-6613(03)00197-9. [DOI] [PubMed] [Google Scholar]
- 42.Cabeza R, Nyberg L. Imaging cognition II: An empirical review of 275 PET and fMRI studies. J of Cognitive Neuroscience. 2000;12:1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- 43.Habeck D, Rakitin BC, Moeller J, Scarmeas N, Zarahn E, Brown T, Stern Y. An event-related fMRI study of the neural networks underlying the encoding, maintenance, and retrieval phase in a delayed-match-to-sample task. Cognitive Brain Research. 2005;23:207–220. doi: 10.1016/j.cogbrainres.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 44.Gunning-Dixon FM, Raz N. Neuroanatomical correlates of selected executive functions in middle-aged and older adults: a prospective MRI study. Neuropsychologia. 2003;41:1929–1941. doi: 10.1016/s0028-3932(03)00129-5. [DOI] [PubMed] [Google Scholar]
- 45.Reuter-Lorenz PA, Marshuetz C, Jonides J, Hartley A, Smith EE. Neurocognitive aging of storage and executive processes. European Journal of Cognitive Psychology. 2001;13:257–278. [Google Scholar]
- 46.Rapp PR, Morrison JH, Roberts JA. Cyclic estrogen replacement improves cognitive function in aged ovariectomized rhesus monkeys. J Neurosci. 2003;23:5708–5714. doi: 10.1523/JNEUROSCI.23-13-05708.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tang Y, Janssen WGM, Hao J, Roberts JA, McKay H, Lasley B, Allen PB, Greengard P, Rapp PR, Kardower JH, Hof PR, Morrison JH. Estrogen replacement increases spinophilin-immunoreactive spine number in the prefrontal cortex of female rhesus monkeys. Cereb Cortex. 2004;14:215–223. doi: 10.1093/cercor/bhg121. [DOI] [PubMed] [Google Scholar]
- 48.Kritzer MF, Kohama SG. Ovarian hormones influence the morphology, distribution, and density of tyrosine hydroxylase immunoreactive axons in the dorsolateral prefrontal cortex of adult rhesus monkeys. J Comp Neurol. 1998;395:1–17. [PubMed] [Google Scholar]
- 49.Kritzer MF, Kohama SG. Ovarian hormones differentially influence immunoreactivity for dopamine beta-hydroxylase, choline acetyltransferase, and serotonin in the dorsolateral prefrontal cortex of adult rhesus monkeys. J Comp Neurol. 1999;409:438–451. doi: 10.1002/(sici)1096-9861(19990705)409:3<438::aid-cne8>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 50.Tinkler GP, Tobin JR, Voytko ML. Effects of two years of estrogen loss or replacement on nucleur basalis cholinergic neurons and cholinergic fibers to the dorsolateral prefrontal and inferior parietal cortex of monkeys. J Comp Neurol. 2004;469:507–521. doi: 10.1002/cne.11028. [DOI] [PubMed] [Google Scholar]
- 51.Reuter-Lorenz PA, Jonides J, Smith EE, Hartley A, Miller A, Marshuetz AM, Koeppe RA. Age differences in the frontal lateralization of verbal and spatial working memory revealed by PET. J Cognitive Neuroscience. 2000;12:174–87. doi: 10.1162/089892900561814. [DOI] [PubMed] [Google Scholar]
- 52.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SAA, Howard BV, Johnson KC, Kotchen JM, Ockene J Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 53.Anderson GL, Limacher M, Assaf AR, Bassford T, Beresford SAA, Black H, Bonds D, Brunner R, Brzyski R, Caan B, Chlebowski R, Curb D, Gass M, Hays J, Heiss G, Hendrix S, Howard BV, Hsia J, Hubbell A, Jackson R, Johnson KC, Judd H, Kotchen JM, Kuller L, LaCroix AZ, Lane D, Langer RD, Lasser N, Lewis CE, Manson J, Margolis K, Ockene J, O’Sullivan MJ, Phillips L, Prentice RL, Ritenbaugh C, Robbins J, Rossouw JE, Sarto G, Stefanick ML, Van Horn L, Wactawski-Wende J, Wallace R, Wassertheil-Smoller S Women’s Health Initiative Steering Committee. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trail. JAMA. 2004;291:1701–1712. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]


