Abstract
Bacterial flagellin is recognized by Toll-like receptor (TLR5) and activates NF-κB which leads to the induction of proinflammatory gene expression. Salmonella expresses two flagellin proteins, FliC and FljB. We purified FliC and FljB and examined the ability of the Salmonella flagellins to activate the NF-κB transcription factor in human embryonic kidney cells. We found that FliC and FljB as purified proteins possessed a comparable specific activity for activation of NF-κB-dependent gene expression in HEK293 cells. We also determined the ability of UV-inactivated bacteria, both wild-type and fliC and fljB mutant strains, to activate NF-κB. Wild-type fliC+/fljB+ Salmonella and the fliC+/fljB− mutant strain were robust activators, whereas the fliC−/fljB+ and flhC− mutant strains were very poor activators. The NF-κB activation capacity of bacterial strains correlated with their flagellin expression level. Finally, Salmonella cell wall-associated polymeric flagellin displayed greatly reduced ability to activate NF-κB compared to purified monomeric flagellin.
Keywords: flagellin, TLR5, Salmonella, NF-κB
INTRODUCTION
Salmonella enterica infections have the potential to cause diseases ranging from gastric enterocolitis to typhoid fever culminating in septic shock and death [1]. Bacterial flagellin is a virulence factor of S. enterica. Strains of Salmonella deficient in flagellin can exhibit defects in colonization and tissue invasion, as well as invasion of macrophages and epithelial cells in vitro [2, 3].
The host immune response against Salmonella includes innate immunity triggered by Toll-like receptor (TLR) 5 and ICE-protease-activating factor (Ipaf) recognition of flagellin [4–12]. TLR5 is one member of the TLR family of transmembrane receptors that recognize a broad variety of ligands termed pathogen associated molecular patterns (PAMPs) [13]. Recognition of Salmonella flagellin by TLR5, which is broadly expressed in many cell types including epithelial cells, results in the induction of proinflammatory cytokines and chemokines [14–17]. Cytosolic flagellin requires Ipaf for activation of caspase-1 and IL-1β secretion in Salmonella infected macrophages [11, 12].
Flagellin is the major protein component of bacterial flagellar filaments. Salmonella expresses two genetically distinct flagellin proteins, FliC (flagellin phase type 1/H1) and FljB (flagellin phase type 2/H2). Only one of the two flagellin types is produced at any given time, by virtue of a complex system of phase variation achieved by alternative orientation of the fljB promoter region and the production of a repressor of the fliC gene promoter [18]. The ability of the Salmonella FliC flagellin to activate the innate immune system via TLR5 is well established [19, 20]. Salmonella flagellin-dependent proinflammatory responses are mediated largely by the conserved amino and carboxyl terminal regions of the flagellar proteins [17]. Assembly of the monomer flagellin protein into a larger flagellar polymer mediates formation of the functional Salmonella flagellum. However, the assembly process results in an apparent functional shielding of the TLR5 recognition sequences, thereby decreasing recognition by TLR5 [14, 17, 21]. Although bacterial flagellin interaction with TLR5 is an important strategy of pathogen recognition by the host immune system, examples occur whereby changes in the flagellin sequence circumvent recognition by TLR5 but still retain bacterial motor activity [22, 23].
Here we show that purified FliC and FljB monomer proteins possess comparable ability to activate NF-κB-dependent gene expression in cultured HEK293 epithelial cells. However, the innate immune response mediated through TLR5 in response to infection with inactivated Salmonella strains deficient in either one or the other of the flagellin proteins, FliC or FljB, is vastly different. Greatly reduced levels of FljB protein expression in the absence of FliC accounts in part for the observed differences in NF-κB activation capacity seen between fliC−/fljB+ and fliC+/fljB− strains.
MATERIALS and METHODS
Bacterial strains
The Salmonella enterica serovar Typhimurium strains used in this study were generously provided by M. Mahan (University of California, Santa Barbara). They included the wild-type strain ATCC 14028 (fliC+/fljB+) [9] and mutant strains fliC+/fljB−, fliC−/fljB+, and flhC− [24]. Briefly, the flhC−, fliC+/fljB− and fliC−/fljB+ mutants were constructed by P22 phage transduction using TH3928 (flhC5456::MudJ-kanR), TH714 (fljB5001::MudJ-kanR) and TH1077 (fliC5050::MudJ-kanR) [25] respectively as the donor strain into the virulent recipient strain S. typhimurium ATCC 14028, followed by selection on Luria-Bertani (LB) media containing kanamycin (50 μg/ml) [24]. Bacterial inoculum for infection of animal cultures were grown overnight in LB broth at 37°C.
Mammalian cells
Human epithelial kidney cells stably expressing the firefly luciferase reporter under control of an NF-κB-dependent promoter (HEK293-Luc) [26], generously provided by S. Ghosh (Yale University), were maintained in DMEM (Invitrogen) supplemented with 10% (v/v) fetal bovine serum and 1 mM sodium pyruvate. Growth was at 37°C in a 5% CO2 atmosphere; monolayers of cells in 24-well plates were used for NF-κB activation analyses.
Infection of HEK293-Luc cells and luciferase assays
Monolayers of HEK293-Luc cells were treated for 4 h with either purified flagellin protein or UV-inactivated bacteria [10] as indicated. UV-killing of Salmonella was verified by loss of viability on LB plates. Extracts were prepared and luciferase activity was measured according to the manufacturer’s protocol (Promega) using a GEM Biomedical OPTOCOMP I luminometer. Protein measurements were determined by the Bradford method (BioRad).
Western immunoblot analysis
Proteins were fractionated by SDS-polyacrylamide gel electrophoresis (10% acrylamide) gels, transferred to nitrocellulose membranes, and probed with an anti-flagellin 15D8 monoclonal antibody (Bioveris #15D8) at a dilution of 1:500. Detection was with a secondary sheep anti-mouse antibody conjugated to horseradish peroxidase (Amersham). Visualization of antibody-antigen complex formation was accomplished with the Pierce reagent (Pierce), followed by imaging on a Versadoc 3000 imager (BioRad).
Isolation of Salmonella flagellin
Salmonella typhimurium flagellin proteins 1 (FliC) and 2 (FljB) were purified as monomeric flagellin from the Salmonella mutant strains fliC+/fljB− (flagellin 1) and fliC−/fljB+ (flagellin 2), respectively, as described [17]. Analysis of the purified flagellin proteins was performed by SDS-polyacrylamide gel electrophoresis fractionation and staining with Coomasie brilliant blue. The amount of flagellin was quantified using bovine serum albumin as the reference protein; quantification was performed with a BioRad VersaDoc imaging system and the Quantity One software program.
RESULTS and DISCUSSION
Salmonella flagellin acting through the TLR5 receptor has been established to mediate activation of transcription factor NF-κB [4, 7, 26, 27]. The robustness of the proinflammatory cellular program initiated by bacterial flagellin proteins varies; flagellins from different bacteria differentially activate the TLR5-dependent signaling cascade [22, 23]. The FliC and FljB Salmonella flagellin proteins display 78% sequence identity at the deduced amino acid level (Fig. 1A). Among the differences are variances that occur within the evolutionarily conserved N- and C-terminal regions. Therefore, we tested whether the two flagellin subtypes of Salmonella typhimurium (type 1, FliC; type 2, FljB) differed in their ability to activate the TLR5-dependent innate immune response.
Figure 1. Mutant strains of Salmonella deficient in expression of FliC andFljB flagellin proteins differentially activate NF-κB in HEK293 cells.
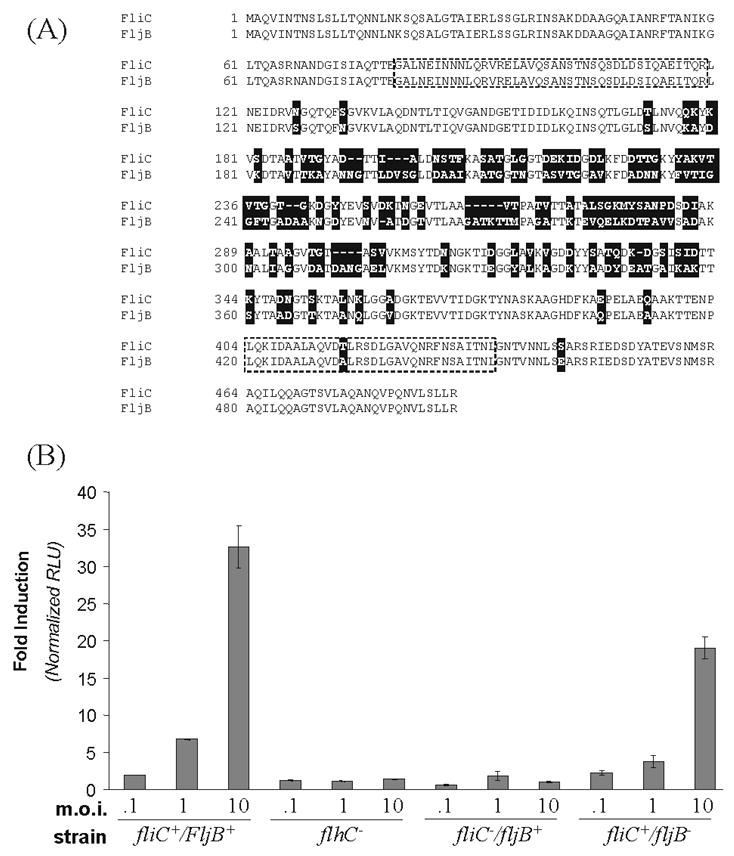
A. The Salmonella typhimurium FliC and FljB proteins were aligned using the SIM alignment software (http://www.expasy.ch/tools/sim-prot.html). Nonconserved residues are denoted with a shaded box. The TLR5 recognition site [17] is indicated by the dashed line. B. Human HEK293 cells stably transformed with a firefly luciferase reporter gene under control of the NF-κB promoter were infected in triplicate with wild-type Salmonella typhimurium (fliC+/fljB+), a FliC (flagellin 1) deficient strain (fliC−/fljB+), a FljB (flagellin 2) deficient strain (fliC+/fljB−), or the flhC− strain deficient in the global regulator of flagellin production. HEK293 cells were harvested at 4 h after infection and luciferase activity (RLU) determined. Luciferase activity from infected cells is expressed relative to that obtained from uninfected cells.
Activation of NF-κB-dependent gene expression by wild-type compared to flagellin-deficient Salmonella bacterial mutants
To assess the activation of the innate immune response by Salmonella flagellin mediated through TLR5, we utilized a HEK293 cell line that stably expresses an NF-κB dependant firefly luciferase reporter (HEK293-Luc). HEK293 cells are known to express the TLR5 protein and respond robustly to flagellin treatment [4]. By contrast, these cells lack a competent lipopolysaccharide (LPS) TLR4-mediated response [10, 26]. 293 cells also do not express IPAF [28], a pattern recognition receptor expressed in macrophages that recognizes cytosolic flagellin [11, 12]. Engagement of the TLR5 receptor by flagellin results in the recruitment of the MYD88 protein adaptor and subsequent activation of the NF-κB transcription factor [13].
HEK293-Luc cells were infected with UV-killed bacteria, either wild-type (WT) or mutant Salmonella strains deficient in either one or both flagellin proteins (Fig. 1B). UV-killed non-viable bacteria were used to minimize potential differences that might occur with live viable bacteria if the efficiency of infection or virulence differed between strains in cell culture. Infection with WT (fliC+/fljB+) Salmonella resulted in the activation of NF-κB reporter activity that was dependent upon the multiplicity of infection (m.o.i.). By contrast, infection with the non-flagellated flhC− strain lacking the global regulator of flagellin production gene, and hence both the FliC and FljB proteins, was unable to detectably activate NF-κB. Surprisingly, infection with the fliC−/fljB+ mutant also failed to significantly activate NF-κB, whereas the fliC+/fljB− mutant was able to achieve levels of NF-κB-dependent gene activation approaching those of WT Salmonella (Fig. 1B). Finally, as an added control, treatment of HEK293-Luc cells with purified Salmonella LPS did not mediate activation of the NF-κB dependant firefly luciferase reporter (data not shown).
Activation of NF-κB-dependent gene expression by purified FljB and FliC Salmonella flagellin proteins
One possible explanation for the finding of differential activation of NF-κB by the mutant Salmonella strains fliC−/fljB+ and fliC+/fljB− is that the two flagellin proteins, FliC (type 1) and FljB (type 2), differ quantitatively in their ability to activate TLR5 on HEK cells. To test this possibility, flagellin FliC was purified from the fliC+/fljB− mutant strain deficient in FljB protein. The FljB flagellin was purified from the fliC−/fljB+ strain (Fig. 2A). Finally, the flagellins were also purified from the WT strain fliC+/fljB+ that expresses both flagellin proteins. The electrophoretic mobility of the FliC and FljB proteins differs slightly from each other as previously described (Fig. 2A). Under the conditions that we used for growth of WT Salmonella in LB broth, the major flagellin type present in the flagellin preparation purified from WT was FljB (Fig. 2A).
Figure 2. Individual flagellin 1 (FliC) and 2 (FljB) proteins purified from Salmonella comparably activate NF-κB in treated HEK293 cells.
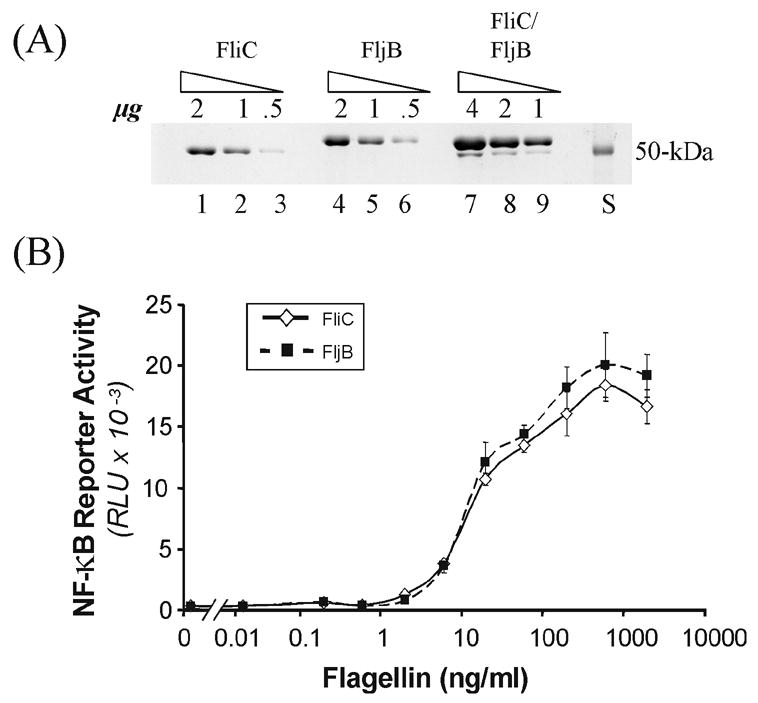
Flagellin 1 (FliC) and 2 (FljB) proteins were purified from Salmonella mutant strains expressing either the FliC protein (fljB−) or the FljB protein (fliC−), and from wild-type bacteria expressing both FliC and FljB. A. Varying amounts (μg) of purified flagellin protein preparations were fractionated by SDS polyacrylamide gel electrophoresis and stained with Coomasie brilliant blue. Lanes 1–3, FliC; lanes 4–6, FljB; lanes 7–9, flagellins FliC and FljB purified from wild-type Salmonella; and lane S, a 50-kDa protein size standard. B. HEK293 cells stably transformed with a firefly luciferase reporter gene under control of an NF-κB dependent promoter were treated in duplicate with increasing concentrations of purified monomeric FliC (◇) or FljB (■) flagellin protein. The HEK293 cells were harvested at 4h after flagellin treatment and luciferase activity (RLU) determined. Luciferase activity was normalized to HEK293 extract protein.
Flagellin-dependent NF-κB activation was determined by treatment of HEK293-Luc cells with increasing amounts of purified monomeric flagellin protein (Fig. 2B). Surprisingly, FljB and FliC proteins showed a comparable ability to mediate the activation of the NF-κB dependant reporter. The dose-response curves for the two Salmonella flagellin protein monomers were comparable in the HEK293-Luc cell assay system (Fig. 2B). In three independent experiments, the difference in NF-κB dependent luciferase reporter activity observed between the FljB and FliC proteins at 20 ng/ml was less than 20%.
Flagellin protein expression levels by wild-type and mutant Salmonella strains
Because the two purified Salmonella flagellins displayed a similar capacity to activate NF-κB in our reporter system, we considered the possibilities that the differential NF-κB activation observed following infection of HEK293 cells with fljB− and fliC− mutant strains might result from either a reduced effective specific activity of the natural FljB and FliC flagellin proteins when expressed by Salmonella and assembled into flagellar filaments, or alternatively because of a different expression level of the two flagellins. Using purified Salmonella flagellins to establish a standard curve for a western immunoblot assay, the relative abundance of the two flagellin proteins was determined immunochemically for WT Salmonella and for the mutant strains, fliC−/fljB+ and fliC+/fljB−. The monoclonal antibody utilized for flagellin detection recognized both the FliC protein and the FljB protein with comparable avidity as shown in Figure 3A. Strikingly, while the level of total flagellin expressed by the fliC+/fljB− mutant Salmonella strain was similar to the amount expressed by the fliC+/fljB− WT strain, the fliC−/fljB+ mutant expressed a substantially lower level of flagellin when cultured in LB broth (Fig. 3B). Thus, the differences seen between the fliC−/fljB+ and the fliC+/fljB− strains following infection of HEK293-Luc cells (Fig. 1B) can be explained by the significant difference in flagellin expression level between the two mutant strains (Fig. 3B). Interestingly, for the WT strain, the major flagellin subtype expressed was FljB (Fig. 3B).
Figure 3. Determination of flagellin FliC and FljB protein expression levels in mutant Salmonella strains.
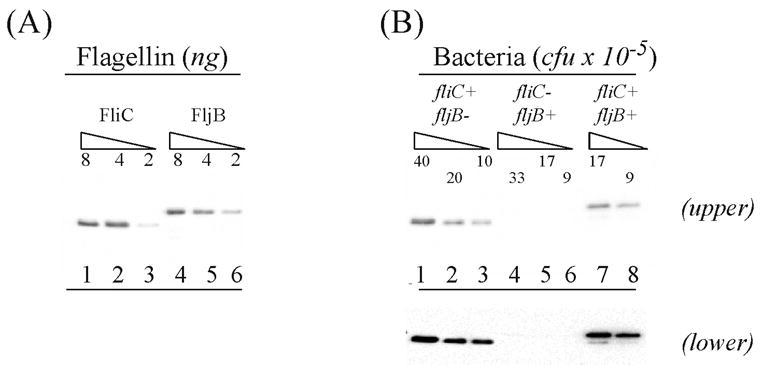
A. Flagellin detection standard. Varying amounts (ng) of purified FliC (lanes 1–3) and FljB (lanes 4–6) flagellin proteins were fractionated by SDS polyacrylamide gel electrophoresis and visualized by western immunoblot analysis with monoclonal antibody 15D8. B. Cultures of mutant Salmonella strains fliC+/fljB− (lanes 1–3) and fliC−/fljB+ (lanes 4–6) and fliC+/fljB+ wild-type Salmonella (lanes 7,8) were grown overnight in Luria broth and the bacteria (105 cfu) analyzed for flagellin expression by western immunoblot analysis with antibody 15D8. Upper and lower denote lighter and darker exposures, respectively, of the same blot.
From the standard curves for NF-κB activation established following treatment of HEK293-Luc cells with increasing amounts of purified monomeric flagellin (Fig. 2B) and from the standard flagellin quantitation by immunoblot analysis (Fig. 3A), we were able to estimate a value for NF-κB activation expected following HEK293-Luc infection with Salmonella. As summarized in Table I, about 5- to 8-fold less NF-κB activation was observed from that theoretically expected following infection based on the amount of flagellin present. This result suggests that the purified natural monomeric flagellin more efficiently activates the signal transduction cascade than the Salmonella cell wall-associated flagellin that is assembled into flagellar filament structures. Our findings are consistent with the conclusion that the TLR5 recognition motif encoded within the N- and C-terminal portions of the flagellin protein is masked following assembly of the flagellin subunits into the flagellar macromolecular filament complex [17]. Consequently, monomeric flagellin exhibits a greatly increased interaction with the TLR5 receptor and subsequent activation of downstream signaling relative to flagellin incorporated into flagellar filaments .
Table I.
Salmonella Strain Dependent Expression of Flagellin and NF-κB Activation
| NF-κB Activation (infected/uninfected) |
|||
|---|---|---|---|
| Strain | Flagellin | Observed | Theoretical1 |
| fljB−/fliC+ | 15.5 | 22 | 174 |
| fljB+/fliC− | 0.11 | 1 | 1 |
| fljB+/fliC+ | 26.1 | 37 | 237 |
Theoretical induction activity was calculated from the amount (ng) of flagellin protein detected in the inoculum bacteria (7.5 x 106 CPU) compared to the activity observed following treatment of HEK293-Luc cells with varying amounts of purified monomeric flagellin.
Summary
Considerable knowledge has been gained regarding the ability of bacterial flagellin to activate the host immune response, both by signaling through TLR5 and Ipaf and through the generation of adaptive immunity [7, 8, 11, 12, 17, 29]. Herein we establish that the two flagellin proteins, FliC and FljB, purified from Salmonella possess a similar ability to activate the proinflammatory transcription factor NF-κB. Analysis of Salmonella mutant strains deficient in one or the other or both flagellin proteins revealed that the ability to trigger NF-κB-dependant reporter expression in infected epithelial cells was dependent upon the expression level, but not the type, of Salmonella flagellin protein.
The possible evolutionary advantage gained by members of the Salmonella genus through expression of two separate flagellin proteins in the context of innate immunity is not completely clear. It has been hypothesized that switching of the flagellin subtypes provides protection in vivo from the host adaptive response [30]. Recent findings have shown that CD4+ T cells are able to recognize both flagellin types, although with a bias toward FliC, and furthermore that Salmonella locked in expression of FljB are attenuated in vivo [5, 30]. We show here that in culture, FljB is the primary flagellin type expressed by wild-type Salmonella grown in LB. By contrast, following oral infection of mice with a mixed population of Salmonella, the overwhelming majority of recovered bacteria expressed FliC [30]. The significance of the differential expression of FliC in the mouse is not obvious in the context of TLR5 signaling, as both flagellins were able to stimulate NF-κB activation with similar specific activity as shown herein. Conceivably the FljB and FliC flagellins may differ in their ability to signal via the cytosolic Ipaf sensor in infected macrophages [11, 12].
Our results are consistent with the earlier report that Salmonella FliC and FljB proteins comparably induced TNF-α production in monocytes and THP-1 cells [31]. Curiously, for TNF-α induction, the central hypervariable region of Salmonella flagellin was reported to be essential [31]. By contrast, recombinant FliC with the T416A substitution to encode an alanine at position 416, as found in FljB within the conserved C-terminal region (Fig. 1), significantly reduced recognition of mutant FliC by TLR5 [17]. These two studies [17, 31] that quantified gene expression as a function of flagellin concentration utilized Salmonella flagellins expressed as recombinant proteins in E. coli, whereas we purified flagellin directly from Salmonella bacteria. Possibly, post translational modifications such as lysine methylation or glycosylation may differ between bacterial flagellins produced by Salmonella compared to a heterologous host in a manner that affects signaling pathways leading to NF-κB activation and induction of proinflammatory gene expression.
Interestingly, disruption of the ability to produce the FliC protein in the fliC−/fljB+ Salmonella strain reduced expression of FljB protein, but the converse was not seen with the fliC+/fljB− strain. It has been reported that following apical infection of human intestinal epithelial cells in vitro, the appearance of basolateral flagellin precedes that of intact Salmonella bacterium, thus priming the host innate immune response characterized by the production of proinflammatory chemokines [4]. Furthermore, bacterial recognition of host lysophospholipids triggers secretion of monomeric flagellin protein [32] and cytosolic flagellin can activate caspase-1 and secretion of IL-1β [11, 12]. Modulation of both the protein level and polymerization state of secreted bacterial flagellin, and flagellin phase type, has the potential both to initiate the production of proinflammatory chemokines leading to influx of host immune cells susceptible to Salmonella invasion, and conversely to limit the amplitude of the cytokine storm by shielding of the key recognition sequence within the polymerized flagellar structure.
Acknowledgments
We thank M. Mahan, D. Heithoff and D. Low for helpful discussions. This work was supported in part by research grant AI-20611 from the National Institute of Allergy and Infectious Diseases, U.S. Public Health Service.
Abbreviations used
- FliC
flagellin 1
- FljB
flagellin 2
- LPS
lipopolysaccharide
- MOI
multiplicity of infection
- TLR5
Toll-like receptor 5
- WT
wild-type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final cis form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wick MJ. Living in the danger zone: innate immunity to Salmonella. Curr Opin Microbiol. 2004;7:51–57. doi: 10.1016/j.mib.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 2.Robertson JMC, McKenzie NH, Duncan M, Allen-Vercoe E, Woodward MJ, Flint HJ, Grant G. Lack of flagella disadvantages Salmonella enterica serovar Enteritidis during the early stages of infection in the rat. J Med Microbiol. 2003;52:91–99. doi: 10.1099/jmm.0.04901-0. [DOI] [PubMed] [Google Scholar]
- 3.Schmitt CK, Ikeda JS, Darnell SC, Watson PR, Bispham J, Wallis TS, Weinstein DL, Metcalf ES, O'Brien AD. Absence of All Components of the Flagellar Export and Synthesis Machinery Differentially Alters Virulence of Salmonella enterica Serovar Typhimurium in Models of Typhoid Fever, Survival in Macrophages, Tissue Culture Invasiveness, and Calf Enterocolitis. Infect Immun. 2001;69:5619–5625. doi: 10.1128/IAI.69.9.5619-5625.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gewirtz AT, Navas TA, Lyons S, Godowski PJ, Madara JL. Cutting Edge: Bacterial Flagellin Activates Basolaterally Expressed TLR5 to Induce Epithelial Proinflammatory Gene Expression. J Immunol. 2001;167:1882–1885. doi: 10.4049/jimmunol.167.4.1882. [DOI] [PubMed] [Google Scholar]
- 5.Bergman MA, Cummings LA, Alaniz RC, Mayeda L, Fellnerova I, Cookson BT. CD4+-T-Cell Responses Generated during Murine Salmonella enterica Serovar Typhimurium Infection Are Directed towards Multiple Epitopes within the Natural Antigen FliC. Infect Immun. 2005;73:7226–7235. doi: 10.1128/IAI.73.11.7226-7235.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hayashi F, Smith KD, Ozinsky A, Hawn TR, Yi EC, Goodlett DR, Eng JK, Akira S, Underhill DM, Aderem A. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature. 2001;410:1099–1103. doi: 10.1038/35074106. [DOI] [PubMed] [Google Scholar]
- 7.Tallant T, Deb A, Kar N, Lupica J, de Veer M, DiDonato J. Flagellin acting via TLR5 is the major activator of key signaling pathways leading to NF-kappaB and proinflammatory gene program activation in intestinal epithelial cells. BMC Microbiol. 2004;4:33. doi: 10.1186/1471-2180-4-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salazar-Gonzalez RM, McSorley SJ. Salmonella flagellin, a microbial target of the innate and adaptive immune system. Immunol Letters. 2005;101:117–122. doi: 10.1016/j.imlet.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 9.Shtrichman R, Heithoff DM, Mahan MJ, Samuel CE. Tissue Selectivity of Interferon-Stimulated Gene Expression in Mice Infected with Dam+ versus Dam- Salmonella enterica Serovar Typhimurium Strains. Infect Immun. 2002;70:5579–5588. doi: 10.1128/IAI.70.10.5579-5588.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simon R, Samuel CE. Innate Interferon Response in Macrophage and Epithelial Cells Infected with Wild-type compared to DNA Adenine Methylase and Flagellin Mutant Salmonella enterica Serovar Typhimurium. J Interferon & Cytokine Res. 2006 doi: 10.1089/jir.2006.0141. in press. [DOI] [PubMed] [Google Scholar]
- 11.Franchi L, Amer A, Body-Malapel M, Kanneganti TD, Ozoren N, Jagirdar R, Inohara N, Vandenabeele P, Bertin J, Coyle A, Grant EP, Nunez G. Cytosolic flagellin requires Ipaf for activation of caspase-1 and interleukin 1[beta] in salmonella-infected macrophages. Nat Immunol. 2006;7:576–582. doi: 10.1038/ni1346. [DOI] [PubMed] [Google Scholar]
- 12.Miao EA, Alpuche-Aranda CM, Dors M, Clark AE, Bader MW, Miller SI, Aderem A. Cytoplasmic flagellin activates caspase-1 and secretion of interleukin 1[beta] via Ipaf. Nat Immunol. 2006;7:569–575. doi: 10.1038/ni1344. [DOI] [PubMed] [Google Scholar]
- 13.Kawai T, Akira S. TLR signaling. Cell Death Differ. 2006;13:816–825. doi: 10.1038/sj.cdd.4401850. [DOI] [PubMed] [Google Scholar]
- 14.Mizel SB, West AP, Hantgan RR. Identification of a Sequence in Human Toll-like Receptor 5 Required for the Binding of Gram-negative Flagellin. J Biol Chem. 2003;278:23624–23629. doi: 10.1074/jbc.M303481200. [DOI] [PubMed] [Google Scholar]
- 15.Ogushi K-i, Wada A, Niidome T, Mori N, Oishi K, Nagatake T, Takahashi A, Asakura H, Makino S-i, Hojo H, Nakahara Y, Ohsaki M, Hatakeyama T, Aoyagi H, Kurazono H, Moss J, Hirayama T. Salmonella enteritidis FliC (Flagella Filament Protein) Induces Human beta -Defensin-2 mRNA Production by Caco-2 Cells. J Biol Chem. 2001;276:30521–30526. doi: 10.1074/jbc.M011618200. [DOI] [PubMed] [Google Scholar]
- 16.Zeng H, Carlson AQ, Guo Y, Yu Y, Collier-Hyams LS, Madara JL, Gewirtz AT, Neish AS. Flagellin Is the Major Proinflammatory Determinant of Enteropathogenic Salmonella. J Immunol. 2003;171:3668–3674. doi: 10.4049/jimmunol.171.7.3668. [DOI] [PubMed] [Google Scholar]
- 17.Smith KD, Andersen-Nissen E, Hayashi F, Strobe K, Bergman MA, Barrett SLR, Cookson BT, Aderem A. Toll-like receptor 5 recognizes a conserved site on flagellin required for protofilament formation and bacterial motility. Nat Immunol. 2003;4:1247–1253. doi: 10.1038/ni1011. [DOI] [PubMed] [Google Scholar]
- 18.Bonifield HR, Hughes KT. Flagellar Phase Variation in Salmonella enterica Is Mediated by a Posttranscriptional Control Mechanism. J Bacteriol. 2003;185:3567–3574. doi: 10.1128/JB.185.12.3567-3574.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Uematsu S, Jang MH, Chevrier N, Guo Z, Kumagai Y, Yamamoto M, Kato H, Sougawa N, Matsui H, Kuwata H, Hemmi H, Coban C, Kawai T, Ishii KJ, Takeuchi O, Miyasaka M, Takeda K, Akira S. Detection of pathogenic intestinal bacteria by Toll-like receptor 5 on intestinal CD11c+ lamina propria cells. Nat Immunol. 2006;7:868–874. doi: 10.1038/ni1362. [DOI] [PubMed] [Google Scholar]
- 20.Feuillet V, Medjane S, Mondor I, Demaria O, Pagni PP, Galan JE, Flavell RA, Alexopoulou L. Involvement of Toll-like receptor 5 in the recognition of flagellated bacteria. Proc Natl Acad Sci USA. 2006;103:12487–12492. doi: 10.1073/pnas.0605200103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yonekura K, Maki-Yonekura S, Namba K. Complete atomic model of the bacterial flagellar filament by electron cryomicroscopy. Nature. 2003;424:643–650. doi: 10.1038/nature01830. [DOI] [PubMed] [Google Scholar]
- 22.Andersen-Nissen E, Smith KD, Strobe KL, Barrett SLR, Cookson BT, Logan SM, Aderem A. Evasion of Toll-like receptor 5 by flagellated bacteria. Proc Natl Acad Sci USA. 2005;102:9247–9252. doi: 10.1073/pnas.0502040102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee SK, Stack A, Katzowitsch E, Aizawa SI, Suerbaum S, Josenhans C. Helicobacter pylori flagellins have very low intrinsic activity to stimulate human gastric epithelial cells via TLR5. Microbes & Infect. 2003;5:1345–1356. doi: 10.1016/j.micinf.2003.09.018. [DOI] [PubMed] [Google Scholar]
- 24.Badie G, Heithoff DM, Sinsheimer RL, Mahan MJ. Altered levels of Salmonella DNA adenine methylase are associated with defects in gene expression, motility, flagellar synthesis, and bile resistance in pathogenic strain 14028, but not in laboratory strain, LT2. J Bacteriol. 2006 doi: 10.1128/JB.01580-06. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chilcott GS, Hughes KT. Coupling of Flagellar Gene Expression to Flagellar Assembly in Salmonella enterica Serovar Typhimurium and Escherichia coli. Microbiol Mol Biol Rev. 2000;64:694–708. doi: 10.1128/mmbr.64.4.694-708.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang D, Zhang G, Hayden MS, Greenblatt MB, Bussey C, Flavell RA, Ghosh S. A Toll-like Receptor That Prevents Infection by Uropathogenic Bacteria. Science. 2004;303:1522–1526. doi: 10.1126/science.1094351. [DOI] [PubMed] [Google Scholar]
- 27.Eaves-Pyles T, Szabo C, Salzman AL. Bacterial invasion is not required for activation of NF-kappaB in enterocytes. Infect Immun. 1999;67:800 – 804. doi: 10.1128/iai.67.2.800-804.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Poyet JL, Srinivasula SM, Tnani M, Razmara M, Fernandes-Alnemri T, Alnemri ES. Identification of Ipaf, a Human Caspase-1-activating Protein Related to Apaf-1. J Biol Chem. 2001;276:28309–28313. doi: 10.1074/jbc.C100250200. [DOI] [PubMed] [Google Scholar]
- 29.Cummings LA, Barrett SLR, Wilkerson WD, Fellnerova I, Cookson BT. FliC-Specific CD4+ T Cell Responses Are Restricted by Bacterial Regulation of Antigen Expression. J Immunol. 2005;174:7929–7938. doi: 10.4049/jimmunol.174.12.7929. [DOI] [PubMed] [Google Scholar]
- 30.Ikeda JS, Schmitt CK, Darnell SC, Watson PR, Bispham J, Wallis TS, Weinstein DL, Metcalf ES, Adams P, O'Connor CD, O'Brien AD. Flagellar Phase Variation of Salmonella enterica Serovar Typhimurium Contributes to Virulence in the Murine Typhoid Infection Model but Does Not Influence Salmonella-Induced Enteropathogenesis. Infect Immun. 2001;69:3021–3030. doi: 10.1128/IAI.69.5.3021-3030.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McDermott PF, Ciacci-Woolwine F, Snipes JA, Mizel SB. High-Affinity Interaction between Gram-Negative Flagellin and a Cell Surface Polypeptide Results in Human Monocyte Activation. Infect Immun. 2000;68:5525–5529. doi: 10.1128/iai.68.10.5525-5529.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Subramanian N, Qadri A. Lysophospholipid sensing triggers secretion of flagellin from pathogenic salmonella. Nat Immunol. 2006;7:583–589. doi: 10.1038/ni1336. [DOI] [PubMed] [Google Scholar]


