Abstract
Understanding how vaccinia virus (VV) generates immunity necessitates an appreciation for how this virus interacts with dendritic cells (DC), which are the most potent activators of naïve CD8+ T cells. In order to optimally activate naïve CD8+ T cells, DC must undergo maturation, during which costimulatory molecules are upregulated and cytokines are produced. In this report we show that VV infection of immature murine bone marrow derived DC (BMDC) failed to induce maturation. Similar results were obtained when CD8+ DC were analyzed, a subset shown previously to be important in vivo in the generation of a vaccinia-specific response. The finding that VV infection of DC resulted in APC that were incapable of initiating T cell activation was surprising given the previously reported role for direct presentation in the generation of anti-VV CD8+ T cell responses in mice. To address the potential mechanism responsible for direct presentation, we tested the hypothesis that previously matured DC were susceptible to vaccinia virus infection and could present newly synthesized VV-derived epitopes for CD8+ T cell activation. Our results show that during VV infection of mature DC, threshold levels of viral protein are produced that promote T cell activation. These results suggest that even though VV cannot mature DC, previously matured DC exposed to VV can generate a VV-specific CD8+ T cell response providing a potential mechanism by which direct infection results in T cell activation in vivo.
Keywords: Dendritic Cells, Viral, Antigen Presentation, Cell Activation
INTRODUCTION
Although it has been almost 30 years since the eradication of smallpox (Henderson, 1976), there has been renewed interest in poxvirus pathogenesis because of the emerging threat of its use as an agent of bioterrorism (Henderson et al., 1999). Because of this threat, it is not only critical that we understand the pathogenesis of poxvirus infection, but also how immune responses are generated following infection. Vaccinia virus (VV) has been used extensively as a model for poxvirus infection (Kieny et al., 1984; Paoletti, 1996; Koszinowski and Thomssen, 1975; Smith and Kotwal, 2002); nonetheless, a number of gaps remain in our understanding of the precise mechanism by which VV generates a protective cellular immune response. One key to understanding the generation of anti-poxvirus immunity is the elucidation of how poxviruses interact with dendritic cells (DC).
Dendritic cells play a key role in the initiation of a CD8+ T cell response as they appear to be the antigen presenting cells that are capable of priming VV-specific CD8+ T cells in vivo (Banchereau and Steinman, 1998; Norbury et al., 2002). In order for DC to optimally prime naïve CD8+ T cells, DC must undergo a process termed maturation. Upon contact with an inflammatory stimulus such as TLR ligands, CD40 ligation, or cytokines (e.g. IL-1, type I IFN, or TNFα), DC migrate from the site of antigen contact to a draining lymph node where they are poised to activate naïve T cells (Akira et al., 2000; Medzhitov, 2001; Caux et al., 1994; Wesa and Galy, 2001; Luft et al., 1998; Montoya et al., 2002; Sallusto and Lanzavecchia, 1994; Cumberbatch et al., 1991; Cumberbatch and Kimber, 1992). During this migration, DC upregulate the expression of costimulatory molecules CD80 and CD86 (also known as B7.1 and B7.2) which bind CD28 on naïve T cells in an interaction necessary for optimal T cell activation (Harding et al., 1992; Harding and Allison, 1993; Freeman et al., 1993). Mature DC also secrete cytokines, such as IL-12 and Type I IFN, that provide the additional signal necessary for the acquisition of CD8+ T cell effector function (Curtsinger et al., 1999; Schmidt and Mescher, 1999; Schmidt and Mescher, 2002; Curtsinger et al., 2003; Curtsinger et al., 2005).
Previous studies of human DC have shown that VV fails to induce maturation, and therefore costimulatory molecule expression (Engelmayer et al., 1999; Drillien et al., 2000). These studies also showed that VV replication in immature human DC is abortive. Importantly, inhibition of human DC maturation occurs well before the virus kills the cell, suggesting that in addition to inducing death of infected DC, VV has mechanisms that can directly inhibit maturation. Moreover, infection of mature DC with VV only minimally altered costimulatory molecule expression an did not induce significant death (Engelmayer et al., 1999).
Vaccinia virus has a large arsenal of immune evasion proteins that counteract many of the anti-viral mechanisms of the innate and adaptive immune response. The VV proteins encoded by the K3L and E3L genes specifically affect the cell’s response to dsRNA and therefore the expression and function of type I IFN, a potent cytokine for DC maturation (Beattie et al., 1991; Chang et al., 1992; Paez and Esteban, 1984; Xiang et al., 2002). Other proteins have been found to interfere with TLR signaling, an additional mechanism by which DC can be matured. The protein encoded by the A46R gene blocks TLR signaling by interacting with TLR adaptor proteins MyD88, Toll-IL-1 receptor domain-containing adaptor-inducing IFN-beta (TRIF), and TRIF-related adapter molecule (TRAM) (Stack et al., 2005), while the A52R gene encodes a protein that has been implicated in blocking TLR signaling by blocking activation of NF-κB, a key transcription factor involved in the induction of inflammatory gene transcription (Bowie et al., 2000; Harte et al., 2003; Sen and Baltimore, 1986b; Sen and Baltimore, 1986a; Baeuerle and Henkel, 1994). In addition, proteins encoded by the N1L and K1L genes are also able to inhibit activation of NF-κB (DiPerna et al., 2004; Bartlett et al., 2002; Shisler and Jin, 2004). VV also produces receptor homologs for the cytokines type I IFN (encoded by the B18R gene) and IL-1β (encoded by the B15R gene) (Symons et al., 1995; Colamonici et al., 1995; Smith and Chan, 1991). Each of these proteins can potentially prevent dendritic cell maturation and therefore have a profound effect on the generation of an adaptive immune response.
Because VV has so many ways of potentially inhibiting DC maturation, it may be difficult to imagine how DC infected with VV could directly present antigen to and activate naïve CD8+ T cells. However, direct presentation of viral antigen has been shown to play a significant role in the priming of an anti-VV CD8+ T cell response in vivo (Basta et al., 2002; Shen et al., 2002). Using a VV that contains genes encoding human cytomegalovirus proteins US2 or US11, both of which prevent direct presentation of endogenous VV antigens, Basta et al. showed that approximately 40% of the CD8+ T cell response is due to direct presentation of VV antigens. Moreover, Shen et al. also used VV encoding US11 to show that anti-VV CD8+ T cell responses generated with intraperitoneal and intravenous routes of infection were, for the most part, a result of direct presentation (Shen et al., 2002).
In these studies we determined the effect of VV infection on mouse DC maturation. We found that VV did not induce mouse DC maturation as shown by the failure of bone marrow-derived DC (BMDC) or spleen-derived CD8+ DC to upregulate expression of the maturation markers. Interestingly however, UV-inactivated VV (UV-VV) did induce upregulation of these costimulatory molecules as well as the secretion of inflammatory cytokines. As a result, UV-VV treated BMDC, but not VV infected BMDC, were able to activate naïve CD8+ T cells in the presence of exogenous antigen. Infection of previously matured DC, while abortive, resulted in the presentation of adequate levels of newly synthesized viral protein for efficient T cell activation. Thus we propose that one mechanism by which direct presentation may occur in vivo is through virus infection of DC that were previously matured by a signal not associated with direct infection, e.g. cytokines or viral components released from neighboring infected cells.
MATERIALS AND METHODS
Mice and cell lines
C57BL/6 mice were purchased from the Frederick Cancer Research and Development Center (Frederick, MD). Rag−/− P-14 lymphocytic choriomeningitis virus (LCMV) gp33-41-specific T-cell receptor (TCR) transgenic mice on a C57BL/6 background were purchased from Taconic. NTCC L929 is a fibroblast cell line derived from C3H/An (H-2k) mice grown in Dulbecco’s modified Eagle’s medium (Invitrogen, Grand Island, NY) supplemented with 10% fetal calf serum (HyClone, Logan, UT), L-glutamine, sodium pyruvate, nonessential amino acids, HEPES, penicillin, streptomycin (BioWhittaker, Walkersville, MD), and 5 x 10−5M 2-mercaptoethanol. All research performed on mice in this study complied with federal and institutional guidelines set forth by the Wake Forest University Animal Care and Use Committee.
Generation of bone marrow-derived dendritic cells
The protocol used to generate BMDC was similar to that used by Inaba et al. (Inaba et al., 1992). Bone marrow was harvested from the femurs and tibias of mice and RBC were lysed with ammonium chloride buffer (BioWhittaker, Walkersville, MD). Bone marrow cells were then plated at 5 X 105 cells/well in a 24-well plate in RPMI 1640 medium (Invitrogen, Grand Island, N.Y.) supplemented with 10% fetal calf serum (HyClone, Logan, UT), HEPES, gentamicin sulfate (BioWhittaker, Walkersville, MD), 5 x 10−5 M 2-mercaptoethanol, 50 ng/ml recombinant granulocyte-macrophage colony-stimulating factor (GMCSF) (Biosource, Camarillo, CA). Every 2 days, the cell culture medium of the BMDC cultures was removed followed by the addition of fresh medium containing 50 ng/ml recombinant GMCSF.
Isolation of CD8+ DC from the spleen
Minced spleens from C57BL/6 mice were incubated in the presence 100μg/ml Collagenase D (Roche, Indianapolis, IN) for 30 minutes at 37°C. The tissue was then passed through a 70 μm filter. CD11c+ cells were enriched by negative selection using the using Miltenyi microbeads as per the manufacturer’s instructions (Miltenyi Biotec, Auburn, CA). In some cases CD8+ DC were subsequently isolated by positive selection.
Viruses
The recombinant vaccinia viruses expressing ovalbumin, VV-Ova and VV-EGFPOva, were a kind gift from J. Bennink (NIH). VV-vPE-16 used as a control virus is a recombinant vaccinia virus construct that expresses the gp160 protein from the IIIB strain of human immunodeficiency virus and was a kind gift of Patricia Earl and Bernard Moss (National Institute of Allergy and Infectious Diseases, Bethesda, Md)(Earl et al., 1991). VV-gp33-41, a recombinant VV expressing the lymphocytic choriomeningitis virus gp33-41 epitope, was a kind gift from J.M. Grayson and was prepared as described previously (Whitton et al., 1993). VV were sucrose gradient purified prior to infection of dendritic cells. Wild-type vesicular stomatitis virus (VSV) was a gift from Douglas Lyles (Wake Forest University School of Medicine) and was grown as previously described (Lyles et al., 1996).
DC infection and treatments
Virus infections and treatments were performed directly in the wells where the BMDC were generated to avoid maturation of cells as a result of manipulation. VV-Ova was diluted in RPMI and added to the BMDC cultures at a multiplicity of infection (MOI) of 0.1, 1, or 10 PFU/cell. BMDC treated with 300 ng/ml lipopolysaccharide (LPS) (Sigma, St. Louis, MO) were used as a positive control for fully matured cells. UV inactivation of virus was carried out using the standard 254nm, 115-V, 1A lamp (Ultra Lum, CA) for 20 min at a distance of 6.5 cm. These conditions destroyed all detectable infectious units. For infection of CD8+ DC, cells were cultured overnight (12 hours) in the presence of 100ng/ml rGM-CSF to increase viability (Rizzitelli et al., 2005).
Cell staining and flow cytometry
At various times following treatment, VV-Ova-infected, mock-infected, and LPS (300 ng/ml)-treated DC were stained with the following antibodies: allophycocyanine-conjugated anti-CD11c together with phycoerythrin-conjugated anti-CD40, anti-CD80, or anti-CD86 antibodies (PharMingen, San Diego, CA). The cells were blocked with Fc block prior to staining to prevent nonspecific binding of antibodies. Flow cytometry was performed using a FACSCalibur and the data analyzed with CellQuest Pro software (both from BD, San Diego, CA).
Cytokine detection
Supernatants from BMDC cultures that were VV-Ova-infected, mock-infected, or LPS-treated were analyzed at various times following treatment for the presence of cytokines. Interleukin- (IL-)12p40 was detected by capture enzyme-linked immunosorbent assay (ELISA). IL-12p70, tumor necrosis factor alpha (TNF-α), IL-10, IL-6, IFN-γ, and MCP-1 were detected using the cytometric bead array (CBA) kit as per the manufacturer’s instructions (BD, San Diego, CA).
IFN-α/β was measured using the VSV-based IFN-α/β bioassay. This assay measures the amount of IFN-α/β present based on the decreased susceptibility of L929 cells to VSV-mediated cytolysis. Briefly, infectious virus in the supernatants from BMDC cultures was inactivated at 4°C overnight by acid treatment at pH 2, the acid was neutralized, and serial dilutions were incubated with L929 cells in 96-well plates overnight at 37°C. As a standard, cells were incubated with serial fivefold dilutions of IFN (Universal IFN-α/β; PBL Biomedical Laboratories, New Brunswick, NJ). The samples were aspirated, and cells were challenged with wild-type VSV at an MOI of 8 in 100 μl of medium. Controls included cells infected with wild-type VSV alone and cells that were not challenged with wild-type VSV. Cells were incubated overnight at 37°C, medium was aspirated, and cells were fixed with 95% ethanol. Cells were then stained with a 0.1% crystal violet solution in methanol. Absorbance was read at 540 nm on an ELISA reader (Ahmed et al., 2003).
Assessment of naïve T-cell proliferation and function
On day 6 post-culture, BMDC were mock-treated, LPS-treated, UV-VV-Ova-treated, or infected with VV-Ova. At the indicated times following treatment the cells were pulsed with (LCMV) gp33-41 peptide. These cells were then washed to remove soluble peptide. Spleens were harvested from P14 mice (Rag−/−) and processed to yield single cell suspensions. Splenocyte populations were at least 88% CD8+ T cells. CFSE (5 μM) (5- and 6-carboxyfluorescein diacetate, succinimidyl ester, Molecular Probes, Eugene, OR)-labeled, antigen-specific P14 LCMV gp33-41-specific TCR transgenic splenocytes were then co-cultured with DC pulsed with gp33-41 peptide for 3 days at a ratio of 10:1 at 37°C in 96-well plates. Alternatively, CFSE-labeled P-14 gp33-41-specific TCR transgenic splenocytes were co-cultured with DC matured with LPS and either infected with VV-gp33-41 or pulsed with gp33-41 peptide for 3 days at a ratio of 10:1. On d3 of culture, cells were harvested and stimulated with 10−6M peptide for 5h in the presence of GolgiPlug (BD PharMingen, San Diego, CA). Cells were then stained with anti-CD8 antibody, fixed and permeabilized, and IFN production assessed by intracellular staining. Proliferation was determined by the decrease in CFSE intensity as measured by flow cytometry. Analysis and quantitation of data was performed using FlowJo software (FlowJo, Ashland, OR).
Assessment of antigen presentation
On day 6 post-culture, BMDC were mock-treated, LPS-treated or infected with VV-Ova. Twenty-two hours following treatment the cells were pulsed with (LCMV) gp33-41 peptide. These cells were then washed to remove soluble peptide and co-cultured at a CTL to DC ratio of 2:1 for 5 hours with a gp33-41-specific CTL line in the presence of GolgiPlug (BD PharMingen, San Diego, CA). Cells were then stained with anti-CD8 antibody, fixed and permeabilized, and IFN production assessed by intracellular staining.
Statistical analysis
A paired Student’s t test was used to compare significance of individual time points and BMDC treatments. P≤0.05 was considered statistically significant.
RESULTS
VV infection of immature mouse BMDC did not induce upregulation of costimulatory molecules
We first determined the effect of VV infection on the upregulation of costimulatory molecules CD40, CD80, and CD86 on murine DC. Mouse BMDC were infected with sucrose gradient purified VV-Ova at an MOI of 0.1, 1, or 10 for 24 hours. We first looked at 24 hours post-infection so that we could directly compare our results with those obtained by others in the human system. Expression of maturation markers on the infected cells was compared to DC treated with LPS or UV inactivated virus (UV-VV) for 24 hours. As expected, treatment of mouse BMDC with LPS resulted in significant upregulation of CD40, CD80, and CD86 expression at 24 hours (Fig. 1). However, infection of mouse BMDC with VV did not induce upregulation of any of these markers, regardless of MOI (Fig. 1). In contrast, treatment with UV-VV resulted in a dose dependent increase CD40, CD80, and CD86 expression. CD40 was the least sensitive to upregulation by UV-VV, whereas CD86 expression was upregulated to a level that was greater than that observed with LPS. Of note, increased expression of these markers following treatment with UV-VV was absent at 8 hr post-treatment but maximal at 18h, and thus was delayed compared to LPS-induced maturation (data not shown). It was possible that the kinetics with which VV induced up-regulation of these markers was delayed compared to LPS or alternatively that there was a transient upregulation of costimulatory markers early following infection that had returned to baseline by 24 hrs. However, this was not the case as analyses at 6 and 48 still showed a failure of VV to induce these molecules (data not shown). Together these results show that UV-VV was a strong maturation stimulus, yet live virus was not capable of inducing maturation of mouse BMDC.
Figure 1. VV infection fails to induce upregulation of maturation markers on murine BMDC.
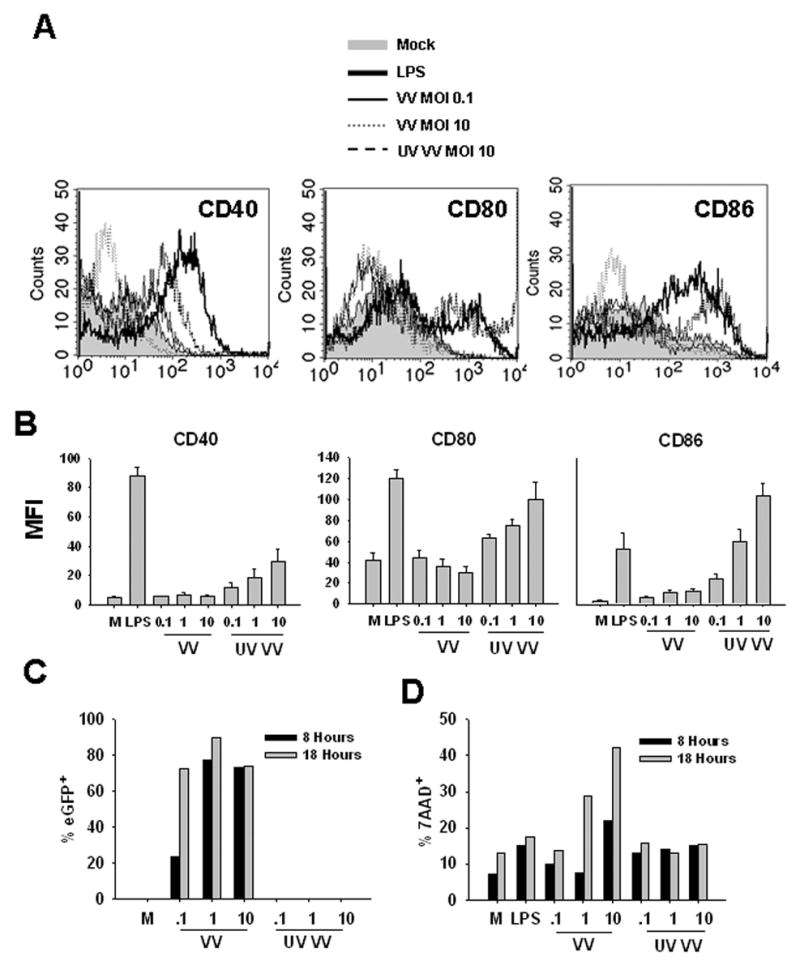
Mouse bone marrow cells were cultured in the presence of GM-CSF for 6 days and then treated with 300ng/ml LPS, VV, or UV-VV for the indicated time or were mock treated (M). The maturation phenotype of BMDC was determined by staining with anti-CD11c in combination with antibodies to the maturation markers CD40, CD80, and CD86. A, Histograms showing CD40, CD80, and CD86 expression levels on CD11c+ BMDC that were mock-treated, LPS treated, infected with VV (MOI 0.1 or 10), or treated with UV-VV (MOI 10) for 24 hours. B, Average geometric mean fluorescence intensities of CD40, CD80, and CD86 expression on BMDC treated for 24 hours. C, BMDC were infected with VV-eGFP and percent eGFP+ was measured at 8 or 18 hours to determine infectivity. D, BMDC were stained with 7AAD to assess death. Data are representative of at least 4 independent experiments. Cells in all analyses are following gating on the CD11c+ population.
We next sought to determine the percentage of BMDC that became infected following exposure to VV. Experiments performed using a polyclonal anti-VV antibody following infection with a vaccinia virus expressing eGFP (VV-EGFPOva) suggested that the antibody was not sensitive enough to detect the majority of infected cells. Thus we performed these analyses with a VV expressing eGFP to quantitate infection. Importantly, VV-eGFP infection did not induce BMDC maturation, similar to the results seen with VV-Ova (data not shown). Using an MOI of 1 or 10, approximately 80% of BMDC were infected at 8 hours as measured by eGFP expression (Fig. 1C). At an MOI of 0.1, the number of infected cells at 8 hours was less (20%) as would be expected. By 18 hours post-infection with this low MOI, however, the percentage of infected cells increased to approximately 75%. No eGFP expression was detected in BMDC treated with UV-inactivated VV-eGFP. To determine the extent of cell death, we stained cells with 7AAD at 8 and 18 hours postinfection. At 8 hours pi, increased death was only seen after infection with the high MOI (Fig. 1D). By 18 hours p.i. there was a dose dependent increase in the number of cells that were dying. As would be expected, there was no increase in death in cells that were treated with UV-inactivated virus.
VV infection of mouse BMDC fails to induce secretion of inflammatory cytokines
Another property associated with DC maturation is the ability to secrete pro-inflammatory cytokines. We used a cytometric bead array to determine the presence of IL-6, MCP-1, and TNFα in the supernatants of mouse BMDC infected with VV or treated with LPS or UV-VV. In addition, we also determined the levels of IL-12p40 in BMDC supernatants by ELISA and IFN-α/β using a VSV-based bioassay. In these analyses we found that infection with VV did not result in significant TNFα, IL-12p40, or IFNα/β secretion (Fig. 2). While BMDC infected with VV did secrete MCP-1, the level produced was much less than that secreted by LPS-treated BMDC and was inversely proportional to MOI. A very low but detectable level of IL-6 was detected in BMDC infected with VV (MOI-10), but this value was minimal compared to that secreted by LPS-treated BMDC, and IL-6 secretion was not detected following infection at an MOI of 1 or 0.1.
Figure 2. VV infection fails to induce inflammatory cytokine secretion in murine BMDC.
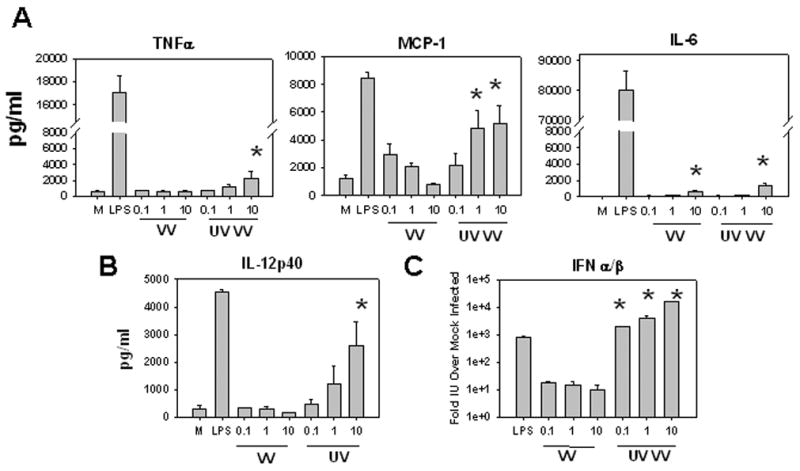
Supernatants from treated BMDC were harvested at 24 hours and analyzed for the presence of inflammatory cytokines. A, Levels of TNFα, MCP-1, and IL-6 in BMDC supernatants as measured by CBA (see Materials and Methods). B, IL-12p40 levels in BMDC supernatants were measured by ELISA. C, Type I IFN levels in BMDC supernatants relative to mock treated BMDC as measured by the VSV-based IFN-α/ß bioassay. Levels of IFNα/β in polyI:C treated BMDC supernatants were similar to LPS. * P< .05 compared with mock-infected BMDC (A and B) or compared with VV-infected BMDC (C). Values are an average of 3 experiments.
In contrast exposure of BMDC to UV-VV at an MOI of 10 induced detectable secretion of both TNFα and IL-6, moderate production of MCP-1 and IL-12p40, and robust production of IFNα/β. These results show that infection of BMDC with VV resulted in minimal cytokine production; however, treatment with UV-VV resulted in production of a similar profile of cytokines as compared to LPS-treated cells, albeit at lower levels. The exception to this was IFNα/β, where the level exceeded that of LPS-treated cells.
Vaccinia infection of CD8+ DC results a failure to mature
A recent study has shown that CD8+ DC are the predominant DC that present VV antigen in vivo following infection by the subcutaneous or intravenous routes (Belz et al., 2004). Given this, we investigated the effect of vaccinia infection on CD8+ DC as it was possible that the result of VV infection of this DC subset was distinct from that observed in myeloid DC. As a method for the in vitro differentiation of bona fide CD8+ DC is lacking, we isolated splenic CD8+ DC for our studies. Isolated DC were infected with recombinant vaccinia virus expressing eGFP (VV-EGFPOva) at increasing MOI and viability and maturation was assessed at 12h. This virus also allowed direct determination of infected cells. As expected, there was a dose-dependent increase in the percent of eGFP+ CD8α+ DC 12 hours p.i. (Fig. 3A). VV infection did not result in death at levels significant above mock infection CD8α+ DC. However others have noted high levels of non-specific death in uninfected splenic CD8α+ DC after overnight culture (Rizzitelli et al., 2005) and thus the death of infected cells is hard to assess above this background (Fig. 3B). As has been shown previously (Rizzitelli et al., 2005), isolated CD8+ DC cultured overnight upregulated CD86 compared to cells tested directly ex vivo (data not shown). Addition of LPS resulted in further upregulation of this maturation marker (Fig. 3). When CD86 expression was analyzed on the infected cells, a significantly reduced maturation was observed, consistent not only with a failure of vaccinia virus infection to induce maturation, but also a block in the spontaneous maturation that results from overnight culture. At 18 hours p.i., CD8α+ DC treated with LPS have even further upregulated CD86 expression, while VV-EGFPOva-infected cells still exhibit an immature phenotype (data not shown). Of note a small fraction of cells do undergo limited maturation. These cells, however, are a very small percentage of the starting DC population. Together these data suggest that, similar to the in vitro generated myeloid DC, VV fails to mature the vast majority of CD8+ DC.
Figure 3. Infection of CD8+ DC with vaccinia virus fails to induce maturation.
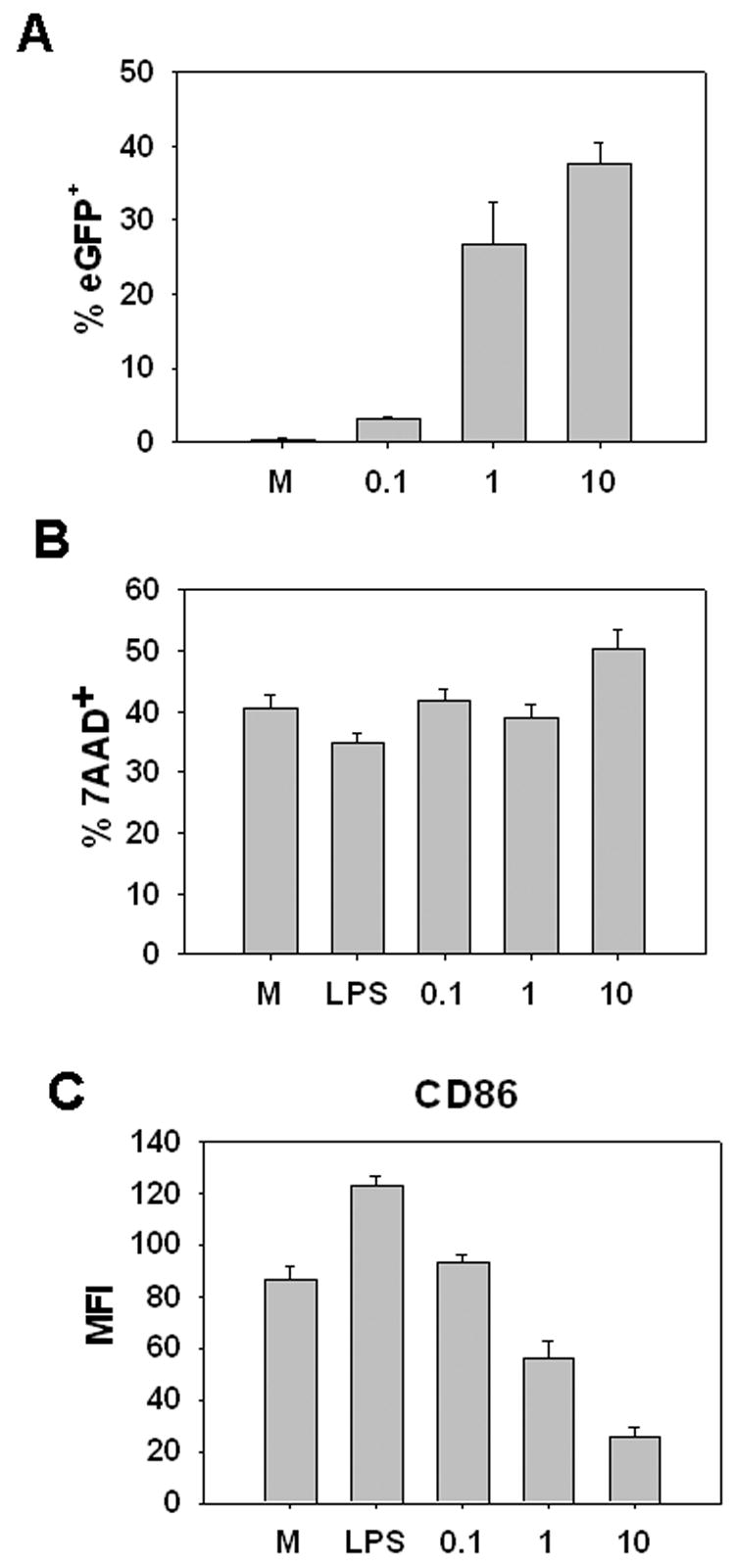
CD8+ DC enriched from the spleen of C57BL/6 mice were infected with VV-EGFPOva (MOI= 0.1, 1, or 10) for 12h. Cells were then stained with antibodies to CD11c, CD8, and CD86 or with 7AAD. Data are obtained following gating on the CD11c+ CD8+ cells in the live cell gate at 12 hours post-infection. A, percent eGFP+ B, percent 7AAD+ and C, MFI of CD86 expression are shown. Data are average of 3 experiments.
VV-infected BMDC fail to activate naïve CD8+ T cell proliferation and function
As it is well established that the increased expression of costimulatory molecules and the secretion of inflammatory cytokines is essential for DC to effectively prime a CD8+ T cell response (Harding et al., 1992; Harding and Allison, 1993; Freeman et al., 1993; Curtsinger et al., 1999; Curtsinger et al., 2003; Schmidt and Mescher, 1999; Schmidt and Mescher, 2002; Curtsinger et al., 2005), we tested the ability of VV infected DC to activate naïve CD8+ T cells. Given the similarity in the maturation of CD8+ DC and the BMDC following infection with VV, we used BMDC for these assays because of the difficulty in obtaining adequate numbers of CD8+ DC for our studies. Treated BMDC were co-cultured with LCMVgp33-41 specific CD8+ T cells from P14 TCR transgenic mice. Use of this system allowed us to determine the functional significance of changes in the maturation state of the DC that resulted from VV exposure while keeping constant the amount of exogenous peptide (LCMV gp33-41). Figure 4A shows a representative experiment from our analyses of CFSE-labeled LCMVgp33-41-specific CD8+ T cells that were cultured for 3 days with BMDC previously treated with LPS, VV, or UV-VV. Figure 4B shows the averages from 3 independent experiments. At limiting concentrations of gp33-41 peptide (10−10M), LPS treated BMDC induced significant proliferation and function of naïve gp33-41-specific CD8+ T cells. On average approximately 70% of cells entered division following stimulation with BMDC matured by exposure to LPS and of the dividing cells at least 65% produced IFNγ.
Figure 4. VV infected murine BMDC do not activate naïve CD8+ T cells in the presence of limiting concentrations of exogenous peptide antigen.
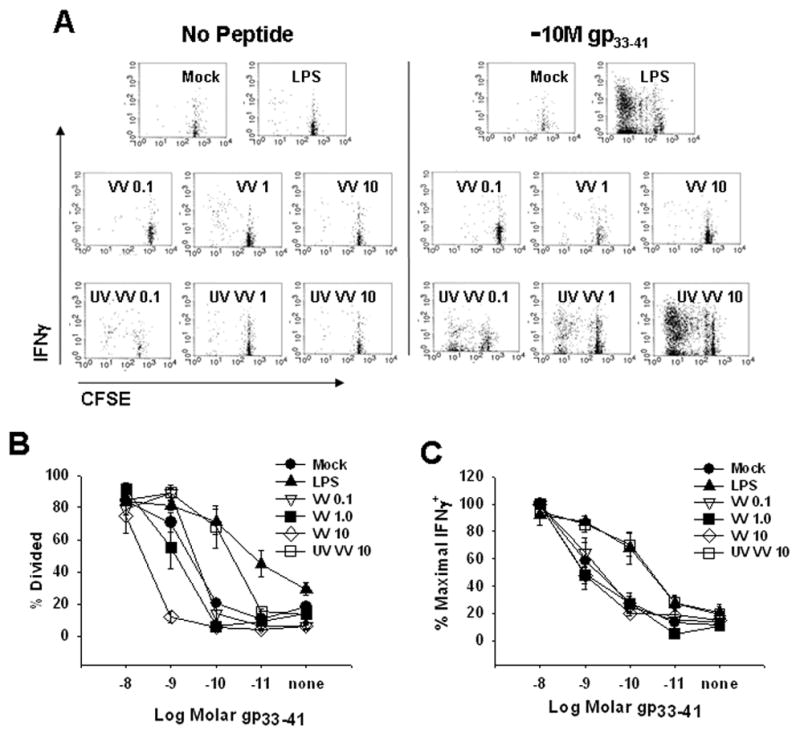
Mouse BMDC were treated with LPS or UV VV or infected with VV for 24 hours. DC were then pulsed with titrated concentrations of LCMV gp33-41 peptide. Pulsed BMDC were washed and co-cultured with CFSE-labeled splenocytes from naïve Rag−/− gp33-41-specific P14 TCR transgenic mice for 3 days. A, Proliferation and function of P14 CD8+ T cells after co-culture with unpulsed or 10−10M pulsed BMDC treated under the designated conditions. B, Percent of P14 CD8+ T cells that divided after co-culture with BMDC pulsed with varying concentrations of gp33-41 peptide. C, Percent maximal IFNγ production by divided P14 CD8+ T cells after co-culture with BMDC pulsed with titrated concentrations of gp33-41 peptide. BMDC infected with VV (MOI= 0.1, 1, or 10) or UV VV (MOI=10) are shown. Values in B and C are an average of 3 experiments.
In contrast, BMDC infected with VV induced very minimal proliferation of naïve gp33-41-specific CD8+ T cells; the percentage of cells that had divided following co-culture with BMDC infected with an MOI of 0.1 or 1 was similar to that observed following culture with mock-infected cells. For cells infected with the highest MOI (10) the percent divided was below that of mock-infected BMDC when the 10−9M peptide concentration was used. Furthermore, CD8+ T cells cultured with VV-infected BMDC pulsed with limiting peptide concentrations were not functional as shown by their failure to produce IFNγ following peptide stimulation. Mock treated BMDC or those infected with VV were all similar in their ability to promote the acquisition of effector function in naïve T cells. Of note, at high concentrations of peptide (10−8M), T cells co-cultured with VV-infected BMDC underwent proliferation and gained function to a level similar to that observed following culture with LPS-treated BMDC, demonstrating that the failure of T cells to proliferate when co-cultured with VV-infected BMDC was not the result of the presence of virus that infected the T cells or another negative signal that prevented T cell activation.
Similar to LPS-treated cells, BMDC exposed to UV-VV induced T cell proliferation and function. Proliferation was similar in both the percentage of cells that entered division and the proliferation index, which is a measure of the average number of divisions undergone by the T cells that entered division (2.6 ± SE 0.46 for LPS and 2.7 ± SE 0.27 for UV-VV). Additionally, a similar percentage of CD8+ T cells cultured with BMDC treated with UV-VV produced IFNγ compared with cultures stimulated with LPS matured BMDC (70% vs. 68%) (Fig. 4C). This is consistent with our results from figures 1 and 2 showing BMDC treated with UV-VV at an MOI of 10 exhibited high expression of the costimulatory molecules CD80 and CD86 and secreted inflammatory cytokines. Together these data show that VV infection rendered BMDC incapable of stimulating naïve CD8+ T cell proliferation and function at limiting concentrations of peptide antigen that were capable of promoting high levels of proliferation and function when LPS-matured or UV-VV-matured DC were used as APC.
VV-infected BMDC exhibit decreased antigen presentation
While our data from figures 1 and 2 would suggest that the failure of VV-infected DC to activate naïve T cells was because of the lack of costimulatory molecule expression, it was possible that VV infection resulted in decreased antigen presentation following peptide loading that also contributed to this effect. To address this issue, BMDC were infected with VV or matured with LPS were pulsed with varying concentrations of LCMV gp33-41 peptide. BMDC were then co-cultured with an established gp33-41-specific CTL line and IFNγ production assessed as a readout for peptide presentation. LPS-treated BMDC induced half-maximal IFNγ production at approximately a half-log lower concentration of antigen compared to mock-infected BMDC. This is consistent with previous studies showing that MHC class I expression is increased on mature DC (Kukutsch et al., 2000; Li et al., 2001; MacAry et al., 2001). Infection with VV resulted in a dose dependent increase in the amount of peptide required to induce half-maximal IFNγ production in the CTL lines, in that the higher the MOI the greater the amount of peptide required (Fig. 5). This suggested that VV infection of resulted in a partial block in antigen presentation in the context of MHC class I on the surface of BMDC.
Figure 5. Decreased antigen presentation by VV-infected BMDC.
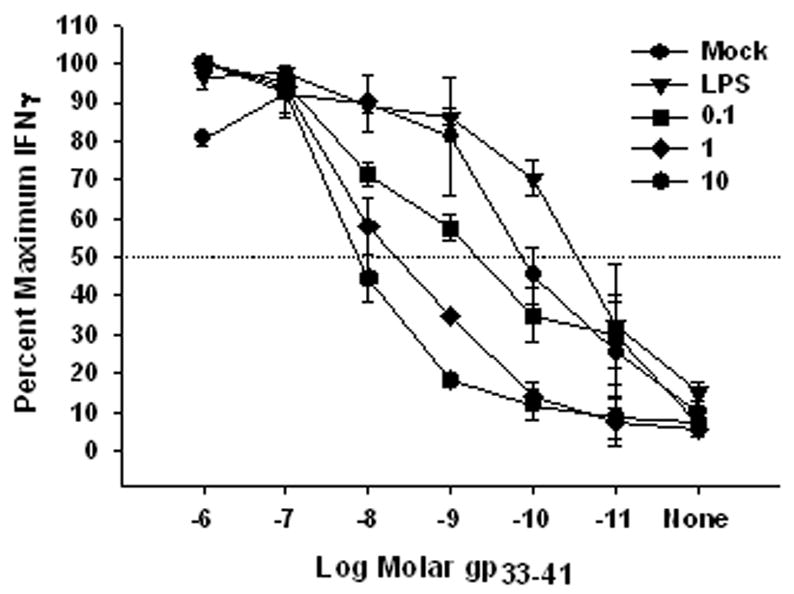
Mouse BMDC were mock-treated, treated with LPS, or infected with VV (MOI = 0.1, 1, or 10) for 18 hours and then pulsed with varying concentrations of gp33-41. Following washing to remove unbound peptide, BMDC were co-cultured with a gp33-41-specific CTL line for 5 hours in the presence of GolgiPlug. IFNγ production was determined by FACS analysis and expressed as percent maximal IFNγ production. Data are the average of 3 independent experiments.
While VV infection resulted in a decrease in antigen presentation in BMDC, this does not fully explain the inability of VV-infected BMDC to activate naïve CD8+ T cells in the presence of limiting concentrations of exogenous antigen. The pattern of induction of proliferation in naïve gp33-41-specific CD8+ T cells followed the pattern of antigen presentation as readout by the CTL line suggesting that the level of presented peptide is playing a large role in controlling the entry of cells into division. However, this is not the case with elicitation of effector function, since mock- and VV-infected BMDC were equally incapable of eliciting IFNγ production from naïve CD8+ T cells, despite the fact that mock-infected BMDC were technically presenting more peptide (Fig. 4C). This result suggested that the lack of costimulatory molecule expression was playing a more dominant role in the acquisition of effector function in this assay, although peptide levels may still be contributing to the effect. These data make the important point that vaccinia virus utilizes a number of mechanisms to undermine generation of the immune response.
Mature BMDC infected with VV are capable of activating naïve CD8+ T cells
It has been shown that a significant amount (~40%) of the CD8+ T cell response to VV in vivo is due to direct priming of naïve CD8+ T cells by infected DC (Basta et al., 2002). Our findings raised the question of how this could occur if infected DC undergo maturation as has been shown to be necessary for efficient naïve CD8+ T cell activation. We hypothesized that DC matured via an alternative signal could become infected with VV leading to presentation of viral antigen. To test this possibility, BMDC were treated with LPS for 20 hours, a period of time which should allow for maximal maturation. Matured DC were then infected with VV for 12 hours and the expression of CD40, CD80, and CD86 was determined. We found that BMDC pre-treated with LPS retained high expression of these markers following infection with VV at an MOI of 0.1, 1, and 10 (data not shown). We also found that VV did not induce significant death (as shown by staining for active caspase 3) of BMDC pre-treated with LPS (data not shown). These results showed that VV did not exhibit cytopathic effects and had minimal impact on maturation marker expression on murine DC that were mature at the time of infection. When mature BMDC were infected with VV-eGFP at MOIs of 10, 1, or 0.1– 10%, 12% and 5% of cells were eGFP+ at 20 hours p.i., in stark contrast to immature BMDC (MOI=10), where approximately 80% were eGFP+ at these timepoints (data not shown). These data suggest that mature BMDC relatively non-permissive to infection.
We next wanted to determine if previously matured mouse BMDC infected with VV were capable of activating naïve CD8+ T cells. To test this possibility, BMDC previously matured by LPS-treatment were infected with VV-gp33-41 and cultured with naïve P14 gp33-41-specific CD8+ T cells for 3 days (similar to what was done in figure 4) (Fig. 6). Proliferation and function of these CD8+ T cell populations was compared to naïve P14 T cells cultured with mock or LPS treated BMDC pulsed with 10−10M gp33-41. BMDC matured with LPS and subsequently infected with VV-gp33-41 (MOI=1 or 10) were capable of inducing proliferation and function of P14 T cells similar to LPS-matured BMDC pulsed with 10−10M gp33-41 (Fig. 6). Similar percentages of cells entered division and the average number of divisions the T cell population underwent (division index) was similar (1.3±0.1 (MOI=1), 1.2±0.1 (MOI=10), and 1.7±0.1 (LPS)). As expected, LPS-matured BMDC treated with UV-VV did not induce proliferation or function of P14 CD8+ T cells, demonstrating the requirement for VV protein expression following infection in order for presentation of gp33-41 to occur. Further no activation was apparent when LPS matured BMDC infected with VV-VPE16, a recombinant VV expressing the HIV gp160 protein was used (data not shown). Together these data suggest that VV protein, albeit at low levels, is being produced in previously matured BMDC exposed to VV. Moreover, mature DC exposed to VV are capable of activating naïve CD8+ T cells to proliferate and acquire effector function.
Figure 6. Murine BMDC previously matured with LPS and infected with VV-gp33-41 are capable of activating naïve P14 gp33-41-specific CD8+ T cells.
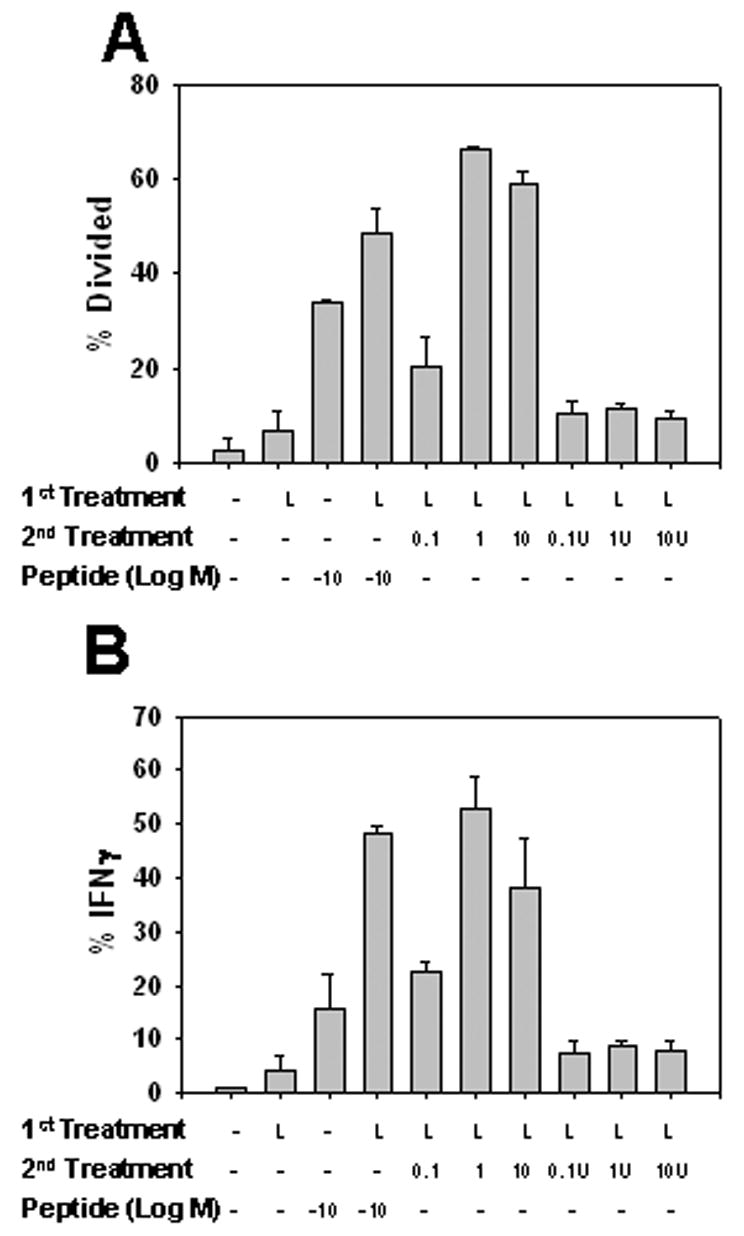
Mouse BMDC were treated with LPS (L) for 18 hours followed by pulsing with 10−10M gp33-41, infection with VV-gp33-41, or exposure to UV VV-gp33-41 for 12 hours. Virus treatments were performed with MOIs of 0.1, 1, and 10. Treated BMDC were co-cultured for 3 days with CFSE-labeled splenocytes from naïve gp33-41-specific P14 TCR transgenic mice. A, Percent of gp33-41-specific CD8+ T cells that entered division. B, Percent of P14 T cells that produced IFNγ+ following restimulation at the end of the three day co-culture period. Values are the average of 3 experiments.
DISCUSSION
Previous studies in mice suggested DC are the relevant APC for priming a VV-specific CD8+ T cell response in vivo (Banchereau and Steinman, 1998; Norbury et al., 2002). In this report we investigated the capacity of VV-infected murine DC to undergo maturation as a result of infection and subsequently to activate naïve CD8+ T cells. We demonstrated that VV infected mouse BMDC did not undergo maturation as measured by the upregulation of costimulatory markers and the production of cytokines and were thus not capable of activating naïve CD8+ T cells in the presence of limiting concentrations of exogenous antigen. The majority of our analyses of were performed using immature BMDC, which are predominantly of myeloid phenotype, as large numbers of these cells can be readily generated. However, as a recent study suggested that CD8α+ DC are the predominant activators of vaccinia-specific CD8+ T cells in vivo following infection by the intravenous or subcutaneous routes (Belz et al., 2004), we also tested the ability of vaccinia infection to induce maturation of CD8α+ DC isolated from the spleen. As with the in vitro generated myeloid DC, CD8α+ DC infected with vaccinia virus failed to undergo maturation. In our analysis of the infected cells that survived after overnight culture, we did observe a small percentage of cells that underwent limited maturation. Given the small percentage of the initial population that these would have constituted, one possibility is that these cells were farther along in their maturation at the time of infection, thereby limiting the cytopathic effect of the virus as was observed for myeloid DC. However, determining whether this is the case will require further investigation. Nonetheless, in general the effect of VV infection on the maturation of myeloid and CD8+ DC appeared similar.
Previous studies in mice have shown that direct presentation by infected APC contributes significantly to the generation of the VV-specific CD8+ T cell response (Basta et al., 2002; Shen et al., 2002). This finding was seemingly contradictory to our studies showing that infection with VV failed to trigger DC maturation to become proficient activators of naïve T cells. Of note, the failure to undergo maturation was observed at times postinfection between 6 and 48hr (data not shown and Fig 1), encompassing the timeframe during which Norbury et al. reported presentation of vaccinia virus proteins in the draining lymph node (Norbury et al., 2002).
Given the failure of VV-infected immature DC to activate CD8+ T cells, we tested the possibility that previously matured DC were susceptible to infection with VV and could thereby act directly as APC capable of activating naive CD8+ T cells. In support of this hypothesis we found that BMDC matured by LPS and subsequently infected with VV-gp33-41 retained expression of maturation markers and were able to activate naïve gp33-41-specific CD8+ T cells. This ability of previously matured DC to present vaccinia peptides is in agreement with a recent report form Wilson et al., which showed previously matured dendritic cells could present viral antigen following HSV infection (Wilson et al., 2006). Hence the ability of infected, mature DC to directly present viral antigen at a level adequate for naïve T cell activation may provide a potential mechanism to reconcile the seemingly contradictory findings that direct presentation can occur in vivo and that infection of immature DC is cytopathic and fails to induce maturation. While it remains a formal possibility that cross-presentation could contribute to the T cell activation observed in our infected mature BMDC cultures, we believe that this is highly unlikely given the following: 1) previously-matured BMDC make very little viral protein, limiting the amount of antigen available for cross-presentation, 2) the infection is not cytopathic also limiting the antigen source and 3) mature BMDC are not efficient at cross-presentation (Hotta et al., 2006).
While the possibility of previously matured DC becoming infected with VV and directly priming naïve CD8+ T cells is attractive, we realize that additional scenarios are possible following infection with VV in vivo that could also contribute to direct priming of CD8+ T cells. One possibility is that VV can mature DC of a different type, such as plasmacytoid DC. However, although these DC have been shown to produce copious amounts of IFNα/β that may promote maturation of neighboring DC; their ability to prime naïve T cells in vivo remains controversial (Facchetti et al., 1988; Siegal et al., 1999; Nakano et al., 2001; Asselin-Paturel et al., 2001; Krug et al., 2003; Schlecht et al., 2004).
While infection with VV did not induce BMDC maturation, treatment of BMDC with UV-inactivated virus resulted in upregulation of CD40, CD80 and CD86 as well as the production of a number of cytokines. Upregulation of CD86 by UV-inactivated virus is in agreement with results observed for human in vitro generated DC (Drillien et al., 2004). This result opens the door to the possibility that vaccinia virus contains a TLR agonist, as has been reported for a number of other viruses such as measles virus (TLR2), respiratory syncytial virus, and mouse mammary tumor virus (both TLR4) (Bieback et al., 2002; Kurt-Jones et al., 2000; Rassa et al., 2002). Alternatively a non-TLR mediated event may be responsible for the maturation signal. Further studies are required to discriminate among these possibilities.
As previously described, VV encodes for a number of immune evasion proteins, namely cytokine receptor homologues and inhibitors of TLR signaling (Beattie et al., 1991; Chang et al., 1992; Paez and Esteban, 1984; Xiang et al., 2002) (Stack et al., 2005) (Bowie et al., 2000; Harte et al., 2003; Sen and Baltimore, 1986b; Sen and Baltimore, 1986a; Baeuerle and Henkel, 1994) (DiPerna et al., 2004; Bartlett et al., 2002; Shisler and Jin, 2004) (Symons et al., 1995; Colamonici et al., 1995; Smith and Chan, 1991). In VV infected BMDC supernatants, we could not detect significant levels of TNFα, IFNγ, and functional IFNαβ. Certain strains of VV do produce a cytokine receptor homologue for TNFα; however, the WR strain used in our studies is not among them (Upton et al., 1991; Howard et al., 1991). Further, while the WR strain does produce an IFNγ cytokine receptor homologue, studies have shown that it has a very low binding affinity for mouse IFNγ (Alcami and Smith, 1995; Mossman et al., 1995). Hence, we do not believe that the inability to detect these two cytokines is due to a masking effect of any VV-derived cytokine binding protein. The WR strain of VV does produce a cytokine receptor homologue for type I IFN, which may contribute to the inability of VV to induce DC maturation and the inability of supernatants from infected BMDC to protect from VSV-mediated cytolysis in our bioassay (Symons et al., 1995; Colamonici et al., 1995).
Previous studies with the human system have shown that cytopathic effects in VV-infected DC are delayed out to 48 hours post-infection. It was then reasoned that the inability of VV to mature human DC was due to immune evasion proteins. In our studies with VV-eGFP, we observed that by 18 hours post-infection with an MOI of 10, most BMDC were infected and undergoing apoptosis as shown by caspase 3 activation and 7AAD staining (Fig. 1 and data not shown). However, at 8 hours post-infection, before the observation of widespread cytopathic effects, we see no increase in the expression of maturation markers in infected or uninfected cells (data not shown). These results leave open the possibility that VV immune evasion proteins can play a role in the failure to induce DC maturation at early times before the initiation of cell death. Alternatively, the ability of vaccinia virus infection to induce maturation may simply be kinetically delayed compared to maturation signals like LPS (as would be suggested by the increase in maturation markers seen at 8 hours post-treatment of BMDC with LPS, but not UV-VV). In support of this, infection of BMDC followed by LPS treatment 8 hours later appeared to “rescue” the BMDC, resulting in maturation marker expression similar to in pattern, but lower in magnitude compared to LPS alone. Not surprisingly, LPS treatment following 24-hour infection did not induce any maturation (data not shown).
Because the mouse is used as a model for the human anti-VV immune response, it is important that its use be validated to the greatest extent possible. One area of importance is the regulation of DC maturation following infection. In our studies we found many parallels in the results of VV infection of murine and human DC. Further these studies provide novel insights into the outcome of infection of CD8+ DC, a subset not previously analyzed. Infection of both human and murine DC results in cytopathic effects. Also in both cases there is a failure to induce upregulation of costimulatory molecules. Together these findings provide support for the use of this model in understanding the generation of protective responses following infection with vaccinia virus.
In summary, our data show that vaccinia virus is incapable of triggering maturation of in vitro differentiated murine BMDC or splenic CD8+ DC following infection and as a result infected DC are inefficient activators of naïve CD8+ T cells. However, VV can initiate infection of mature DC that, even though abortive, allows for the production of a low level of viral protein that is adequate for the activation of naïve CD8+ T cells. In contrast to infectious virus, exposure to UV inactivated virus induced robust DC maturation suggesting that viral components contain a DC maturation signal. Thus one possibility is that viral products released from infected cells or other inflammatory signals produced during vaccinia infection promote DC maturation. Matured DC subsequently abortively infected with VV could then potentially activate virus-specific CD8+ T cells in vivo. These results provide new insights into the regulation of both DC maturation and CD8+ T cell activation as a result of poxvirus infection.
Acknowledgments
We thank Dr. Jack Bennink for provision of VV-Ova and VV-EGFPOva. We would also like to thank Dr. Griffith Parks, Dr. Elizabeth Hiltbold, Dr. Andrew Cawthon, and Dr. Negin Vaghefi for critical review of this manuscript.
Abbreviations used in this paper
- DC
dendritic cell
- VV
vaccinia virus
- BMDC
bone marrow-derived dendritic cell
- UV-VV
UV-inactivated vaccinia virus.
Footnotes
This work was supported by National Institutes of Health grants R21 AI56096 (to M.A. A-M.) and P01 AI 060642 (to S.B.M.)
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmed M, McKenzie MO, Puckett S, Hojnacki M, Poliquin L, Lyles DS. Ability of the matrix protein of vesicular stomatitis virus to suppress beta interferon gene expression is genetically correlated with the inhibition of host RNA and protein synthesis. J Virol. 2003;77:4646–4657. doi: 10.1128/JVI.77.8.4646-4657.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akira S, Hoshino K, Kaisho T. The role of Toll-like receptors and MyD88 in innate immune responses. J Endotoxin Res. 2000;6:383–387. [PubMed] [Google Scholar]
- Alcami A, Smith GL. Vaccinia, cowpox, and camelpox viruses encode soluble gamma interferon receptors with novel broad species specificity. J Virol. 1995;69:4633–4639. doi: 10.1128/jvi.69.8.4633-4639.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asselin-Paturel C, Boonstra A, Dalod M, Durand I, Yessaad N, Dezutter-Dambuyant C, Vicari A, O’Garra A, Biron C, Briere F, Trinchieri G. Mouse type I IFN-producing cells are immature APCs with plasmacytoid morphology. Nat Immunol. 2001;2:1144–1150. doi: 10.1038/ni736. [DOI] [PubMed] [Google Scholar]
- Baeuerle PA, Henkel T. Function Amd Activation of Nf-Kappa-B in the Immune-System. Annu Rev Immunol. 1994;12:141–179. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- Bartlett N, Symons JA, Tscharke DC, Smith GL. The vaccinia virus N1L protein is an intracellular homodimer that promotes virulence. J Gen Virol. 2002;83:1965–1976. doi: 10.1099/0022-1317-83-8-1965. [DOI] [PubMed] [Google Scholar]
- Basta S, Chen W, Bennink JR, Yewdell JW. Inhibitory effects of cytomegalovirus proteins US2 and US11 point to contributions from direct priming and cross-priming in induction of vaccinia virus-specific CD8+ T cells. J Immunol. 2002;168:5403–5408. doi: 10.4049/jimmunol.168.11.5403. [DOI] [PubMed] [Google Scholar]
- Beattie E, Tartaglia J, Paoletti E. Vaccinia virus-encoded eIF-2 alpha homolog abrogates the antiviral effect of interferon. Virology. 1991;183:419–422. doi: 10.1016/0042-6822(91)90158-8. [DOI] [PubMed] [Google Scholar]
- Belz GT, Smith CM, Eichner D, Shortman K, Karupiah G, Carbone FR, Heath WR. Cutting edge: conventional CD8 alpha+ dendritic cells are generally involved in priming CTL immunity to viruses. J Immunol. 2004;172:1996–2000. doi: 10.4049/jimmunol.172.4.1996. [DOI] [PubMed] [Google Scholar]
- Bieback K, Lien E, Klagge IM, Avota E, Schneider-Schaulies J, Duprex WP, Wagner H, Kirschning CJ, Ter MV, Schneider-Schaulies S. Hemagglutinin protein of wild-type measles virus activates toll-like receptor 2 signaling. J Virol. 2002;76:8729–8736. doi: 10.1128/JVI.76.17.8729-8736.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowie A, Kiss-Toth E, Symons JA, Smith GL, Dower SK, O’Neill LAJ. A46R and A52R from vaccinia virus are antagonists of host IL-1 and toll-like receptor signaling. Proc Natl Acad Sci USA. 2000;97:10162–10167. doi: 10.1073/pnas.160027697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caux C, Massacrier C, Vanbervliet B, Dubois B, Van Kooten C, Durand I, Banchereau J. Activation of human dendritic cells through CD40 cross-linking. J Exp Med. 1994;180:1263–1272. doi: 10.1084/jem.180.4.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang HW, Watson JC, Jacobs BL. The E3L gene of vaccinia virus encodes an inhibitor of the interferon- induced, double-stranded RNA-dependent protein kinase. Proc Natl Acad Sci U S A. 1992;89:4825–4829. doi: 10.1073/pnas.89.11.4825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colamonici OR, Domanski P, Sweitzer SM, Larner A, Buller RM. Vaccinia virus B18R gene encodes a type I interferon-binding protein that blocks interferon alpha transmembrane signaling. J Biol Chem. 1995;270:15974–15978. doi: 10.1074/jbc.270.27.15974. [DOI] [PubMed] [Google Scholar]
- Cumberbatch M, Illingworth I, Kimber I. Antigen-Bearing Dendritic Cells in the Draining Lymph-Nodes of Contact Sensitized Mice - Cluster Formation with Lymphocytes. Immunology. 1991;74:139–145. [PMC free article] [PubMed] [Google Scholar]
- Cumberbatch M, Kimber I. Dermal Tumor-Necrosis-Factor-Alpha Induces Dendritic Cell-Migration to Draining Lymph-Nodes, and Possibly Provides One Stimulus for Langerhans Cell-Migration. Immunology. 1992;75:257–263. [PMC free article] [PubMed] [Google Scholar]
- Curtsinger JM, Lins DC, Mescher MF. Signal 3 determines tolerance versus full activation of naive CD8 T cells: dissociating proliferation and development of effector function. J Exp Med. 2003;197:1141–1151. doi: 10.1084/jem.20021910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtsinger JM, Schmidt CS, Mondino A, Lins DC, Kedl RM, Jenkins MK, Mescher MF. Inflammatory cytokines provide a third signal for activation of naive CD4+ and CD8+ T cells. J Immunol. 1999;162:3256–3262. [PubMed] [Google Scholar]
- Curtsinger JM, Valenzuela JO, Agarwal P, Lins D, Mescher MF. Cutting edge: Type IIFNs provide a third signal to CD8 T cells to stimulate clonal expansion and differentiation. J Immunol. 2005;174:4465–4469. doi: 10.4049/jimmunol.174.8.4465. [DOI] [PubMed] [Google Scholar]
- DiPerna G, Stack J, Bowie AG, Boyd A, Kotwal G, Zhang ZN, Arvikar S, Latz E, Fitzgerald KA, Marshall WL. Poxvirus protein N1L targets the I-kappa B kinase complex, inhibits signaling to NF-kappa B by the tumor necrosis factor superfamily of receptors, and inhibits NF-kappa B and IRF3 signaling by Toll-like receptors. J Biol Chem. 2004;279:36570–36578. doi: 10.1074/jbc.M400567200. [DOI] [PubMed] [Google Scholar]
- Drillien R, Spehner D, Bohbot A, Hanau D. Vaccinia virus-related events and phenotypic changes after infection of dendritic cells derived from human monocytes. Virology. 2000;268:471–481. doi: 10.1006/viro.2000.0203. [DOI] [PubMed] [Google Scholar]
- Drillien R, Spehner D, Hanau D. Modified vaccinia virus Ankara induces moderate activation of human dendritic cells. J Gen Virol. 2004;85:2167–2175. doi: 10.1099/vir.0.79998-0. [DOI] [PubMed] [Google Scholar]
- Earl PL, Koenig S, Moss B. Biological and immunological properties of human immunodeficiency virus type 1 envelope glycoprotein: analysis of proteins with truncations and deletions expressed by recombinant vaccinia viruses. J Virol. 1991;65:31–41. doi: 10.1128/jvi.65.1.31-41.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelmayer J, Larsson M, Subklewe M, Chahroudi A, Cox WI, Steinman RM, Bhardwaj N. Vaccinia virus inhibits the maturation of human dendritic cells: a novel mechanism of immune evasion. J Immunol. 1999;163:6762–6768. [PubMed] [Google Scholar]
- Facchetti F, Dewolfpeeters C, Mason DY, Pulford K, Vandenoord JJ, Desmet VJ. Plasmacytoid T-Cells - Immunohistochemical Evidence for Their Monocyte Macrophage Origin. Am J Pathol. 1988;133:15–21. [PMC free article] [PubMed] [Google Scholar]
- Freeman GJ, Borriello F, Hodes RJ, Reiser H, Hathcock KS, Laszlo G, Mcknight AJ, Kim J, Du LN, Lombard DB, Gray GS, Nadler LM, Sharpe AH. Uncovering of Functional Alternative Ctla-4 Counter-Receptor in B7-Deficient Mice. Science. 1993;262:907–909. doi: 10.1126/science.7694362. [DOI] [PubMed] [Google Scholar]
- Harding FA, Allison JP. CD28-B7 interactions allow the induction of CD8+ cytotoxic T lymphocytes in the absence of exogenous help. J Exp Med. 1993;177:1791–1796. doi: 10.1084/jem.177.6.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding FA, McArthur JG, Gross JA, Raulet DH, Allison JP. CD28-mediated signalling co-stimulates murine T cells and prevents induction of anergy in T-cell clones. Nature. 1992;356:607–609. doi: 10.1038/356607a0. [DOI] [PubMed] [Google Scholar]
- Harte MT, Haga IR, Maloney G, Gray P, Reading PC, Bartlett NW, Smith GL, Bowie A, O’Neill LAJ. The poxvirus protein A52R targets toll-like receptor signaling complexes to suppress host defense. J Exp Med. 2003;197:343–351. doi: 10.1084/jem.20021652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson DA. Eradication of Smallpox. Sci Am. 1976;235:25–33. doi: 10.1038/scientificamerican1076-25. [DOI] [PubMed] [Google Scholar]
- Henderson DA, Inglesby TV, Bartlett JG, Ascher MS, Eitzen E, Jahrling PB, Hauer J, Layton M, McDade J, Osterholm MT, O’Toole T, Parker G, Perl T, Russell PK, Tonat K. Smallpox as a biological weapon: medical and public health management. Working Group on Civilian Biodefense. JAMA. 1999;281:2127–2137. doi: 10.1001/jama.281.22.2127. [DOI] [PubMed] [Google Scholar]
- Hotta C, Fujimaki H, Yoshinari M, Nakazawa M, Minami M. The delivery of an antigen from the endocytic compartment into the cytosol for cross-presentation is restricted to early immature dendritic cells. Immunology. 2006;117:97–107. doi: 10.1111/j.1365-2567.2005.02270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard ST, Chan YS, Smith GL. Vaccinia virus homologues of the Shope fibroma virus inverted terminal repeat proteins and a discontinuous ORF related to the tumor necrosis factor receptor family. Virology. 1991;180:633–647. doi: 10.1016/0042-6822(91)90077-o. [DOI] [PubMed] [Google Scholar]
- Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinman RM. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176:1693–1702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieny MP, Lathe R, Drillien R, Spehner D, Skory S, Schmitt D, Wiktor T, Koprowski H, Lecocq JP. Expression of rabies virus glycoprotein from a recombinant vaccinia virus. Nature. 1984;312:163–166. doi: 10.1038/312163a0. [DOI] [PubMed] [Google Scholar]
- Koszinowski U, Thomssen R. Target Cell-Dependent T-Cell-Mediated Lysis of Vaccinia Virus-Infected Cells. Eur J Immunol. 1975;5:245–251. doi: 10.1002/eji.1830050405. [DOI] [PubMed] [Google Scholar]
- Krug A, Veeraswamy R, Pekosz A, Kanagawa O, Unanue ER, Colonna M, Cella M. Interferon-producing cells fail to induce proliferation of naive T cells but can promote expansion and T helper 1 differentiation of antigen-experienced unpolarized T cells. J Exp Med. 2003;197:899–906. doi: 10.1084/jem.20021091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukutsch NA, Rossner S, Austyn JM, Schuler G, Lutz MB. Formation and kinetics of MHC class I-ovalbumin peptide complexes on immature and mature murine dendritic cells. J Invest Dermatol. 2000;115:449–453. doi: 10.1046/j.1523-1747.2000.00084.x. [DOI] [PubMed] [Google Scholar]
- Kurt-Jones EA, Popova L, Kwinn L, Haynes LM, Jones LP, Tripp RA, Walsh EE, Freeman MW, Golenbock DT, Anderson LJ, Finberg RW. Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus. Nat Immunol. 2000;1:398–401. doi: 10.1038/80833. [DOI] [PubMed] [Google Scholar]
- Li J, Schuler-Thurner B, Schuler G, Huber C, Seliger B. Bipartite regulation of different components of the MHC class I antigen-processing machinery during dendritic cell maturation. Int Immunol. 2001;13:1515–1523. doi: 10.1093/intimm/13.12.1515. [DOI] [PubMed] [Google Scholar]
- Luft T, Pang KC, Thomas E, Hertzog P, Hart DN, Trapani J, Cebon J. Type I IFNs enhance the terminal differentiation of dendritic cells. J Immunol. 1998;161:1947–1953. [PubMed] [Google Scholar]
- Lyles DS, McKenzie MO, Ahmed M, Woolwine SC. Potency of wild-type and temperature-sensitive vesicular stomatitis virus matrix protein in the inhibition of host-directed gene expression. Virology. 1996;225:172–180. doi: 10.1006/viro.1996.0585. [DOI] [PubMed] [Google Scholar]
- MacAry PA, Lindsay M, Scott MA, Craig JI, Luzio JP, Lehner PJ. Mobilization of MHC class I molecules from late endosomes to the cell surface following activation of CD34-derived human Langerhans cells. Proc Natl Acad Sci U S A. 2001;98:3982–3987. doi: 10.1073/pnas.071477498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medzhitov R. Toll-like receptors and innate immunity. Nat Rev Immunol. 2001;1:135–145. doi: 10.1038/35100529. [DOI] [PubMed] [Google Scholar]
- Montoya M, Schiavoni G, Mattei F, Gresser I, Belardelli F, Borrow P, Tough DF. Type I interferons produced by dendritic cells promote their phenotypic and functional activation. Blood. 2002;99:3263–3271. doi: 10.1182/blood.v99.9.3263. [DOI] [PubMed] [Google Scholar]
- Mossman K, Upton C, Buller RM, McFadden G. Species specificity of ectromelia virus and vaccinia virus interferon-gamma binding proteins. Virology. 1995;208:762–769. doi: 10.1006/viro.1995.1208. [DOI] [PubMed] [Google Scholar]
- Nakano H, Yanagita M, Gunn MD. CD11c+B220+Gr-1+ cells in mouse lymph nodes and spleen display characteristics of plasmacytoid dendritic cells. J Exp Med. 2001;194:1171–1178. doi: 10.1084/jem.194.8.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norbury CC, Malide D, Gibbs JS, Bennink JR, Yewdell JW. Visualizing priming of virus-specific CD8+ T cells by infected dendritic cells in vivo. Nat Immunol. 2002;3:265–271. doi: 10.1038/ni762. [DOI] [PubMed] [Google Scholar]
- Paez E, Esteban M. Nature and Mode of Action of Vaccinia Virus Products That Block Activation of the Interferon-Mediated Ppp(A2’P)Na-Synthetase. Virology. 1984;134:29–39. doi: 10.1016/0042-6822(84)90269-1. [DOI] [PubMed] [Google Scholar]
- Paoletti E. Applications of pox virus vectors to vaccination: an update. Proc Natl Acad Sci U S A. 1996;93:11349–11353. doi: 10.1073/pnas.93.21.11349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassa JC, Meyers JL, Zhang YM, Kudaravalli R, Ross SR. Murine retroviruses activate B cells via interaction with toll-like receptor 4. Proc Natl Acad Sci USA. 2002;99:2281–2286. doi: 10.1073/pnas.042355399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzitelli A, Vremec D, Villadangos JA, Mavaddat N, Wright MD, Shortman K. Switching from a restricted to an effective CD4 T cell response by activating CD8+ murine dendritic cells with a Toll-like receptor 9 ligand. Eur J Immunol. 2005;35:3209–3220. doi: 10.1002/eji.200526231. [DOI] [PubMed] [Google Scholar]
- Sallusto F, Lanzavecchia A. Efficient Presentation of Soluble-Antigen by Cultured Human Dendritic Cells Is Maintained by Granulocyte-Macrophage Colony-Stimulating Factor Plus Interleukin-4 and Down-Regulated by Tumor-Necrosis-Factor-Alpha. J Exp Med. 1994;179:1109–1118. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlecht G, Garcia S, Escriou N, Freitas AA, Leclerc C, Dadaglio G. Murine plasmacytoid dendritic cells induce effector/memory CD8+ T-cell responses in vivo after viral stimulation. Blood. 2004;104:1808–1815. doi: 10.1182/blood-2004-02-0426. [DOI] [PubMed] [Google Scholar]
- Schmidt CS, Mescher MF. Adjuvant effect of IL-12: Conversion of peptide antigen administration from tolerizing to immunizing for CD8+ T cells in vivo. J Immunol. 1999;163:2561–2567. [PubMed] [Google Scholar]
- Schmidt CS, Mescher MF. Peptide antigen priming of naive, but not memory, CD8+ T cells requires a third signal that can be provided by IL-12. J Immunol. 2002;168:5521–5529. doi: 10.4049/jimmunol.168.11.5521. [DOI] [PubMed] [Google Scholar]
- Sen R, Baltimore D. Inducibility of Kappa-Immunoglobulin Enhancer-Binding Protein Nf-Kappa-B by A Posttranslational Mechanism. Cell. 1986a;47:921–928. doi: 10.1016/0092-8674(86)90807-x. [DOI] [PubMed] [Google Scholar]
- Sen R, Baltimore D. Multiple Nuclear Factors Interact with the Immunoglobulin Enhancer Sequences. Cell. 1986b;46:705–716. doi: 10.1016/0092-8674(86)90346-6. [DOI] [PubMed] [Google Scholar]
- Shen XF, Wong SBJ, Buck CB, Zhang JW, Siliciano RF. Direct priming and cross-priming contribute differentially to the induction of CD8+ CTL following exposure to vaccinia virus via different routes. J Immunol. 2002;169:4222–4229. doi: 10.4049/jimmunol.169.8.4222. [DOI] [PubMed] [Google Scholar]
- Shisler JL, Jin XL. The vaccinia virus K1L gene product inhibits host NF-kappa B activation by preventing I kappa B alpha degradation. J Virol. 2004;78:3553–3560. doi: 10.1128/JVI.78.7.3553-3560.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegal FP, Kadowaki N, Shodell M, Fitzgerald-Bocarsly PA, Shah K, Ho S, Antonenko S, Liu YJ. The nature of the principal type 1 interferon-producing cells in human blood. Science. 1999;284:1835–1837. doi: 10.1126/science.284.5421.1835. [DOI] [PubMed] [Google Scholar]
- Smith GL, Chan YS. 2 Vaccinia Virus Proteins Structurally Related to the Interleukin-1 Receptor and the Immunoglobulin Superfamily. J Gen Virol. 1991;72:511–518. doi: 10.1099/0022-1317-72-3-511. [DOI] [PubMed] [Google Scholar]
- Smith SA, Kotwal GJ. Immune response to poxvirus infections in various animals. Crit Rev Micro. 2002;28:149–185. doi: 10.1080/1040-840291046722. [DOI] [PubMed] [Google Scholar]
- Stack J, Haga IR, Schroder M, Bartlett NW, Maloney G, Reading PC, Fitzgerald KA, Smith GL, Bowie AG. Vaccinia virus protein Toll-like-interleukin-1 A46R targets multiple receptor adaptors and contributes to virulence. J Exp Med. 2005;201:1007–1018. doi: 10.1084/jem.20041442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symons JA, Alcami A, Smith GL. Vaccinia Virus Encodes A Soluble Type-I Interferon Receptor of Novel Structure and Broad Species-Specificity. Cell. 1995;81:551–560. doi: 10.1016/0092-8674(95)90076-4. [DOI] [PubMed] [Google Scholar]
- Upton C, Macen JL, Schreiber M, McFadden G. Myxoma virus expresses a secreted protein with homology to the tumor necrosis factor receptor gene family that contributes to viral virulence. Virology. 1991;184:370–382. doi: 10.1016/0042-6822(91)90853-4. [DOI] [PubMed] [Google Scholar]
- Wesa AK, Galy A. IL-1 beta induces dendritic cells to produce IL-12. Int Immunol. 2001;13:1053–1061. doi: 10.1093/intimm/13.8.1053. [DOI] [PubMed] [Google Scholar]
- Whitton JL, Sheng N, Oldstone MB, McKee TA. A “string-of-beads” vaccine, comprising linked minigenes, confers protection from lethal-dose virus challenge. J Virol. 1993;67:348–352. doi: 10.1128/jvi.67.1.348-352.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson NS, Behrens GM, Lundie RJ, Smith CM, Waithman J, Young L, Forehan SP, Mount A, Steptoe RJ, Shortman KD, Koning-Ward TF, Belz GT, Carbone FR, Crabb BS, Heath WR, Villadangos JA. Systemic activation of dendritic cells by Toll-like receptor ligands or malaria infection impairs cross-presentation and antiviral immunity. Nat Immunol. 2006;7:165–172. doi: 10.1038/ni1300. [DOI] [PubMed] [Google Scholar]
- Xiang Y, Condit RC, Vijaysri S, Jacobs B, Williams BRG, Silverman RH. Blockade of interferon induction and action by the E3L double-stranded RNA binding proteins of vaccinia virus. J Virol. 2002;76:5251–5259. doi: 10.1128/JVI.76.10.5251-5259.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]


