Abstract
The livers of woodchucks chronically infected with woodchuck hepatitis virus (WHV) contain foci of morphologically altered hepatocytes (FAH) with “basophilic”, “amphophilic” and “clear cell” phenotypes, which are possibly pre-neoplastic in nature. Interestingly, most fail to express detectable levels of WHV proteins and nucleic acids. We studied sections of WHV-infected liver tissue to determine if all foci of hepatocytes that failed to express detectable levels of WHV, as assessed by immunoperoxidase staining for WHV core antigen, could be classified morphologically as FAH. We found that at least half of the foci of WHV core antigen negative hepatocytes did not show clear morphological differences in either H&E or PAS (periodic acid Schiff) stained sections from surrounding hepatocytes, and were therefore not designated as FAH. In the second approach, we assayed core antigen negative foci for the presence of fetuin B, a serum protein produced by normal hepatocytes, but not by neoplastic hepatocytes in hepatocellular carcinomas. Basophilic and amphophilic FAH had reduced levels of fetuin B compared to hepatocytes present in the surrounding liver; fetuin B staining was detected in clear cell FAH but the level could not be accurately assessed because of the displacement of fetuin B to the cell periphery by accumulated glycogen. The foci of morphologically normal WHV core antigen negative hepatocytes had similar levels of fetuin B to that of the surrounding hepatocytes. The co-existence of at least four types of WHV core antigen negative foci, including those with no obvious morphologic changes, raises the possibility that the different foci arise from distinct primary events. We hypothesize that a common event is loss of the ability to express WHV, allowing these hepatocytes to escape immune mediated cell death and to undergo clonal expansion to form distinct foci.
INTRODUCTION
Persistent woodchuck hepatitis virus (WHV) infection leads initially to a quiescent carrier state, with all hepatocytes infected but little liver disease. Nonetheless, in virtually all animals the infection progresses to include chronic liver disease and hepatocellular carcinoma (HCC) (Jacob et al., 2004; Tennant et al., 2004). Prior to the appearance of HCC, tens of thousands of foci of altered hepatocytes (FAH) are found throughout the liver, as is also observed during chemical carcinogenesis (Abe et al., 1988; Bannasch et al., 2003; Jacob et al., 1997; Thorgeirsson and Grisham, 2002; Toshkov et al., 1990; Yang and Rogler, 1991). Various names and phenotypes have been assigned to FAH in different hosts and in response to different agents. Three predominant types of FAH are described in the chronically WHV infected woodchuck. Using the nomenclature of Bannasch and colleagues, these are referred to here as basophilic, amphophilic and clear cell (Radaeva et al., 2000; Yang et al., 1993). These foci are thought to be the pre-neoplastic lesions from which HCCs may arise (Figure 1). In possible agreement with this idea, basophilic and amphophilic FAH have been reported to contain a significantly higher proportion of hepatocytes with Ki67-positive nuclei compared to surrounding liver (Radaeva et al., 2000), suggesting a higher cell proliferation rate in these FAH.
Figure 1. Possible pathways leading to the development of HCC in chronically WHV-infected woodchucks.
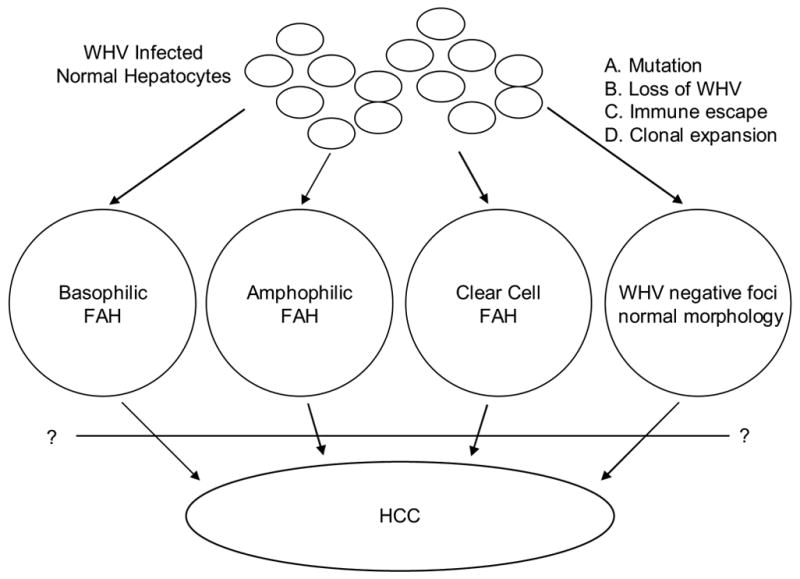
Three major types of FAH, basophilic, amphophilic and clear cell are thought to arise from normal hepatocytes during chronic WHV infection. These FAH generally express undetectable or reduced levels of WHV compared to WHV-infected hepatocytes. A fourth type of focus, which contains morphologically normal hepatocytes but also fails to express WHV, is described in this study.
In general, basophilic FAH contain hepatocytes with elevated ribosome levels, and an atypical appearance, with significant disruption of hepatic plates. Expression of N-myc2 and insulin like growth factor II have been observed in basophilic foci as well as HCC, but not in normal hepatocytes (Yang et al., 1993). Amphophilic FAH contain more normal appearing hepatocytes that have lower levels of glycogen than surrounding hepatocytes (Bannasch et al.,2003; Radaeva et al., 2000). Some disruption of hepatic plate structure may also be observed. Clear cell FAH have elevated glycogen or fat stores detected using Periodic acid-Schiff (PAS) reactions compared to surrounding hepatocytes (Bannasch et al., 2003; Radaeva et al., 2000). Hepatocytes in clear cell FAH may appear larger than in the surrounding liver, and may contain nuclear alterations, but the hepatic plate structure is generally not altered.
In the present study, basophilic FAH were defined on the basis of increased cytoplasmic basophilia in hepatocytes, generally smaller size than adjacent normal hepatocytes, and altered hepatic plate morphology observed by H&E staining. Amphophilic FAH were defined on the basis of reduced glycogen content, while clear cell FAH had elevated levels of glycogen detected by PAS staining, relative to surrounding hepatocytes. These differences in glycogen storage are reflected by enzyme histochemistry (Radaeva et al., 2000). Some FAH contain a mixed cell phenotype, raising the possibility that FAH progress over time from one type to another.
In general, FAH are made up of hepatocytes with low or undetectable levels of WHV (Li et al., 2002; Radaeva et al., 2000; Yang et al., 1993). Apparent clearance of virus from otherwise normal appearing hepatocytes scattered throughout the hepatic lobule is also observed during chronic infections, more so in humans with chronic HBV infection than in chronic WHV-infected woodchucks (Chu et al., 1997; Gowans and Burrell, 1985; Gowans et al., 1985; Hirohashi et al., 1982; Hsu et al., 1988; Kojima et al., 1977; Lamothe et al., 1976; Mason et al., 2005; Nayak et al., 1977; Omata et al., 1978; Ray et al., 1976; Suzuki et al., 1985). This is in contrast to transient WHV infections, where all hepatocytes can produce readily detectable levels of WHV proteins and nucleic acids (Guo et al., 2000; Jilbert et al., 1992; Kajino et al., 1994; Summers et al., 2003), and suggests the occurrence of extensive changes in the hepatocyte population as chronic disease progresses, including but not limited to FAH (Abe et al., 1988; Li et al., 2002; Mason et al., 2005; Popper et al., 1987; Radaeva et al., 2000; Su et al., 1998). The reasons why isolated hepatocytes as well as FAH may fail to express WHV, and the mechanisms leading to formation of FAH during chronic WHV infection are unknown. Elevated hepatocyte proliferation rates per se, if they have any effect on virus replication in vivo, do not appear to explain loss of virus antigen expression in all FAH, since this loss occurs in basophilic, amphophilic and clear cell FAH irrespective of their Ki67 staining index (Radaeva et al., 2000).
In a recent study of chronically WHV-infected woodchuck liver we assayed for clonal expansion of hepatocytes containing integrated WHV DNA, and obtained evidence that 10% or more of the liver may be comprised of clones of at least 1,000 hepatocytes that arose subsequent to WHV infection (Mason et al., 2005). It was suggested in this study that clonal expansion of hepatocytes begins with loss of WHV expression, giving some hepatocytes a survival advantage over hepatocytes that continue to produce WHV and are therefore subjected to immune attack. In support of this suggestion, it was noted, as discussed above, that livers from chronically WHV infected woodchucks contained foci of WHV core antigen and nucleic acid negative hepatocytes. However, it appeared, contrary to expectation, that the WHV core antigen and nucleic acid negative foci in our liver samples did not have the morphological characteristics of FAH (Mason et al., 2005). In the current study the frequency of such foci, in comparison to FAH, was explored further using detection of WHV core antigen (Kajino et al., 1993; Mason et al., 1998; Zhu et al., 2004) as a marker of WHV infection.
We report here that at least half of the WHV core antigen negative foci detected in chronically WHV-infected livers were morphologically normal and were not distinguishable from surrounding hepatocytes by H&E or PAS-staining or by assaying for intracellular accumulation of fetuin B protein, and were therefore not FAH. Fetuin B is a secretory protein, which we detected in woodchuck hepatocytes, but found at reduced levels in amphophilic and basophilic FAH and HCC. Whether the morphologically normal WHV core antigen negative foci contain alterations other than an inability to express WHV is not known.
The absence of detectable WHV core antigen expression from all types of FAH and from the morphologically normal foci suggests that loss of virus antigen, rather than some presumptive neoplastic event, is a crucial first step in the outgrowth of hepatocytes that may eventually give rise to neoplastic hepatocytes (Figure 1). HCC may arise because some rare FAH are derived from hepatocytes that have already acquired a neoplastic change, including integration of WHV DNA in the vicinity of N- or C-myc; indeed, some FAH have been reported to over express N-myc2 (Yang et al., 1993), a characteristic of most woodchuck HCCs (Fourel et al., 1990; Fourel et al., 1994; Jacob et al., 2004).
RESULTS
Assays for WHV expression in HCC, FAH and normal hepatocytes in chronically WHV infected woodchucks
To detect WHV in liver tissue sections from chronically WHV infected woodchucks, we carried out immunoperoxidase assays for viral core antigen, which accumulates in the cytoplasm but not the nucleus of WHV-infected hepatocytes. The results are summarized in Table 1. As illustrated in Figure 2, woodchuck HCC was typically negative for detectable WHV core antigen expression, while adjacent hepatocytes were WHV core antigen positive. Basophilic FAH were also negative for WHV core antigen (Figure 3). HCC and basophilic FAH, which show disruption of hepatic plates, were readily distinguished from surrounding hepatocytes by H&E and PAS staining (Figure 2 and 3).
Table 1.
FAH AND CORE ANTIGEN NEGATIVE FOCI IN CHRONICALLY INFECTED WOODCHUCKS*
| Woodchuck | Age (mo.) | FAH
|
Core neg. (not FAH) | Hepatocytes per section | ||
|---|---|---|---|---|---|---|
| Basophilic | Amphophilic | Clear cell | ||||
| 307 | 15 | 1 | 14,000 | |||
| 19 | 1(f+) | 17,000 | ||||
| 26 | 1 | 1 | 17,000 | |||
| 309 | 12 | 2(f+) | 35,000 | |||
| 15 | 2(f+) | 45,000 | ||||
| 19 | 1(f−) | 34,000 | ||||
| 310 | 9 | 2(f+) | 22,000 | |||
| 15 | 1(f+) | 26,000 | ||||
| 26 | 2(f−) | 1(f+) | 1(f+) | 50,000 | ||
| 313 | 12 | no foci | 24,000 | |||
| 15 | 2(f−) | 19,000 | ||||
| 19 | no foci | 11,000 | ||||
| 314 | 9 | no foci | 20,000 | |||
| 12 | no foci | 18,000 | ||||
| 15 | no foci | 12,000 | ||||
| 19 | 1 | 26,000 | ||||
| 26 | no foci | 11,000 | ||||
| 359 | 14 | 1(f+) | 24,000 | |||
| 366 | 14 | 1(f−) | 32,000 | |||
| 371 | 14 | 1(f+) | 18,000 | |||
| 4961 | 15 | no foci | 53,000 | |||
| 19 | 3(f+) | 64,000 | ||||
| 26 | 1 | 1(f+) | 26,000 | |||
| 501 | 40(au) | 1(f−) | 82,000 | |||
| 5114 | 15 | 1(f−) | 1(f+) | 40,000 | ||
| 5154 | 15 | no foci | 23,000 | |||
| 19 | 1(f+) | 28,000 | ||||
| 28(au) | 15(f+) | 194,000 | ||||
| 3202 | (au) | 1 | 1 | 1 | 549,000 | |
|
| ||||||
| Totals | 4 | 8 | 5 | 31 | ||
The number and types of FAH detected in biopsy and autopsy (au) samples from chronically WHV infected woodchucks. Selected liver samples were tested for fetuin protein expression by immunoperoxidase staining and are characterized as either f+ or f−. The former yielded a similar level of fetuin protein as the hepatocytes in the surrounding liver, while the latter showed reduced or undetectable staining. All FAH were negative for WHV core antigen expression. The right hand column lists the approximate number of hepatocytes in each tissue section scored.
Figure 2. Lack of WHV core antigen expression in HCC.
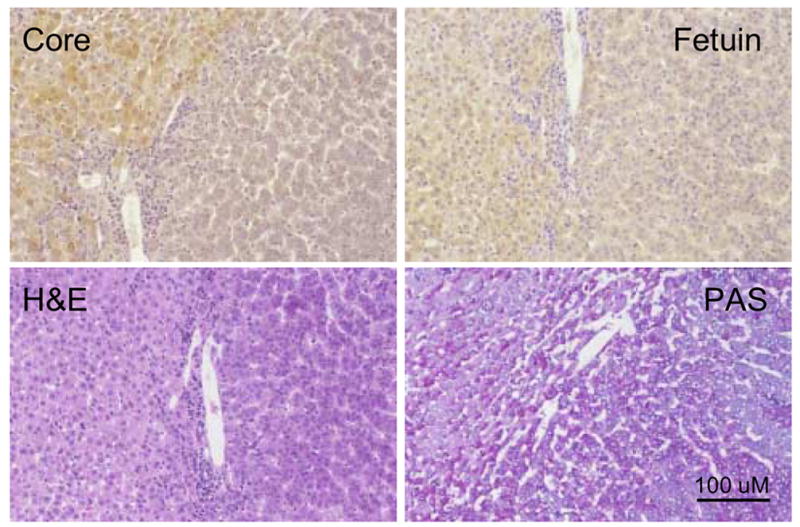
Liver tissue sections showing areas of WHV core antigen positive hepatocytes adjacent to areas of HCC in woodchuck 3202. Adjacent liver sections were stained for detection of WHV core antigen and fetuin B protein or stained by H&E and PAS as described in Materials and Methods. In each panel hepatocytes with normal morphology and detectable WHV core antigen expression were located on the left hand side of the field. The area showing HCC contains characteristic clusters of hepatocytes, 4 cells thick, and disruption of the hepatic plates. Magnification 160x. Bar = 100 uM.
Figure 3. Lack of WHV core antigen expression in basophilic FAH.

Liver sections showing areas of WHV core antigen positive hepatocytes (top of field) and a basophilic FAH (woodchuck 3202). Adjacent sections were stained for detection of WHV core antigen and fetuin B protein or stained by H&E or PAS as described in Materials and Methods. The basophilic FAH has reduced levels of both WHV core antigen and fetuin B protein, as compared to the adjacent WHV core antigen positive hepatocytes, and basophilic appearance by H&E and PAS staining. Magnification 160x. Bar = 100 uM.
Many WHV core antigen negative FAH are not, however, so obviously different from surrounding liver by H&E staining, though a clear distinction due to differences in glycogen storage can be made by PAS staining. A third type of woodchuck FAH, an amphophilic focus deficient in glycogen content when compared to surrounding hepatocytes, is illustrated in Figure 4. The hepatocytes present in the amphophilic focus were also deficient in staining for WHV core antigen. Figure 5 illustrates a WHV core antigen negative focus corresponding to a clear cell (glycogenotic) FAH with increased levels of glycogen seen by PAS staining (Radaeva et al., 2000; Yang et al., 1993) and vacuolated hepatocytes with a centrally placed nucleus distinguishable from surrounding liver by H&E staining.
Figure 4. Lack of WHV core antigen expression in amphophilic FAH.
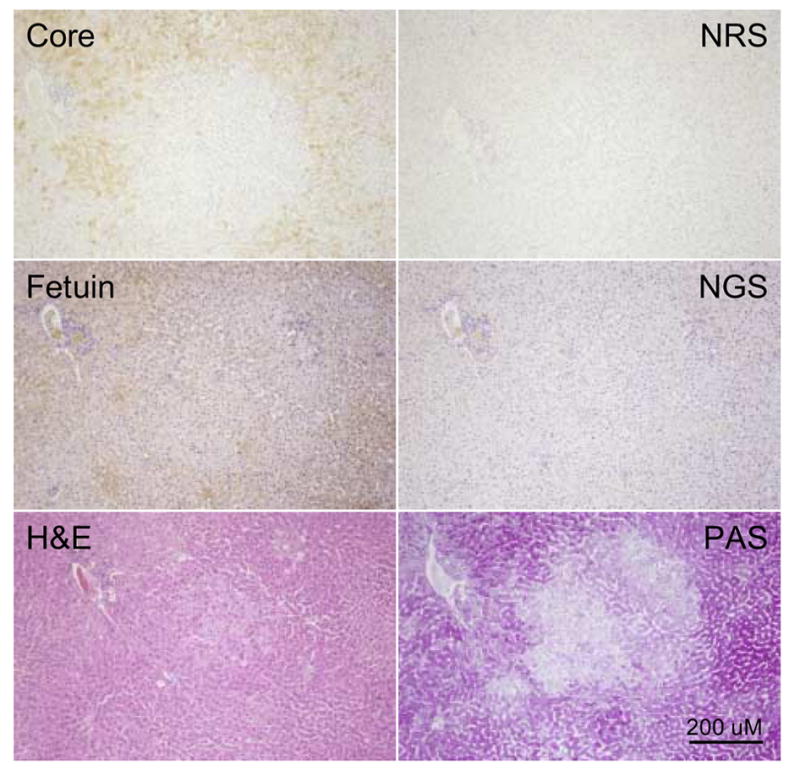
Liver sections showing an amphophilic WHV core antigen negative FAH from woodchuck 307 (26 months of age). Adjacent sections were immuno-stained for detection of WHV core antigen using NRS as a control, or fetuin B protein, using NGS as a control, or stained by H&E or PAS as described in Materials and Methods. The prominent portal tract in the upper left hand corner of each field was used to locate the FAH. The amphophilic FAH, which has some basophilic cells present giving it a mixed cell phenotype, has undetectable levels of WHV core antigen, and reduced but variable levels of fetuin B protein. The FAH has some morphological changes seen on H&E and has lower levels of glycogen leading to lighter staining by PAS. Magnification 80x. Bar = 200 uM.
Figure 5. Lack of WHV core antigen expression in a clear cell (glycogenotic) FAH.
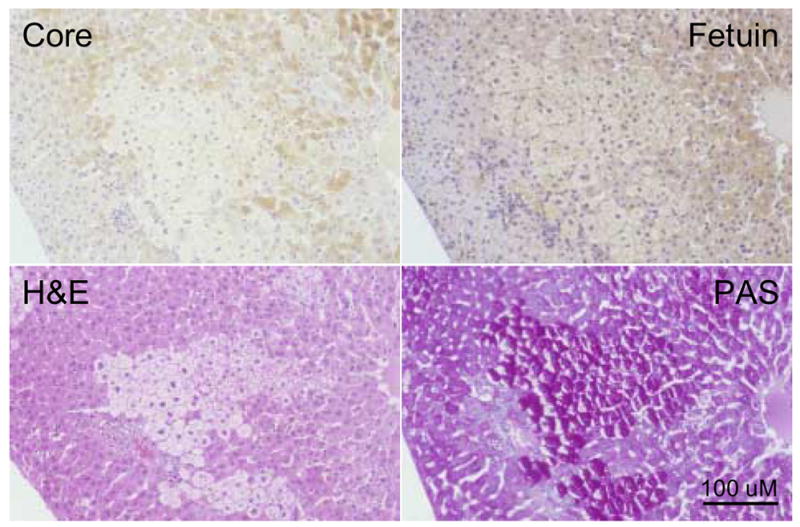
Adjacent tissue sections showing a clear cell WHV core antigen negative FAH from woodchuck 307 (26 months of age). Adjacent sections were immuno-stained for detection of WHV core antigen or fetuin B protein, or stained by H&E and PAS as described in Materials and Methods. The clear cell FAH has reduced or undetectable levels of WHV core antigen. Fetuin B protein is present within the cytoplasm and along the hepatocyte membranes. The clear cell hepatocytes appear larger than surrounding hepatocytes when stained by H&E due to the extensive vacuolization and accumulation of glycogen and have characteristic centrally positioned nuclei and elevated levels of glycogen detected by PAS staining. Magnification 160x. Bar = 100 uM.
In a previous study of chronically WHV infected woodchucks we noted WHV core antigen negative foci, also negative for WHV DNA by in situ hybridization, that did not appear to differ morphologically from surrounding core antigen and in situ hybridization positive hepatocytes (Mason et al., 2005). It was also observed that a small fraction of hepatocytes scattered at random throughout the lobule appeared to be WHV negative, suggesting that loss of virus expression might not be confined to typical FAH. Figure 6 illustrates consecutive liver sections from a focus of WHV core antigen negative hepatocytes that were not morphologically distinguishable from surrounding hepatocytes by either H&E or PAS staining. As summarized in Table 1, these may be one of the most commonly occurring WHV-negative foci in the chronically WHV infected liver.
Figure 6. Foci of WHV core antigen negative hepatocytes not morphologically distinguishable as FAH.
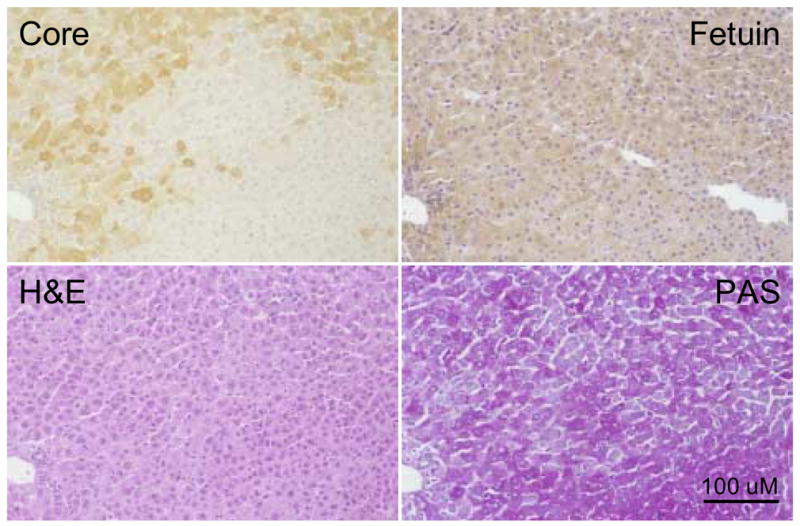
Adjacent liver sections showing a morphologically normal focus of WHV core antigen negative hepatocytes (woodchuck 4961, 19 months of age) subjected to fetuin B protein staining, H&E or PAS. The portal tract in the lower left hand corner has been used to locate the same area in each adjacent liver section. In this case the focus of WHV core antigen negative hepatocytes is not morphologically different from surrounding hepatocytes as judged by fetuin B protein detection or H&E or PAS staining. Magnification 160x. Bar = 100 uM.
In an attempt to further characterize these WHV core antigen negative foci and to differentiate them from FAH that can be identified by H&E and PAS staining, we asked if expression of fetuin B, a highly expressed liver gene which we found to be down-regulated in HCC using a woodchuck specific microarray, was also down regulated at the level of protein expression in HCC and in foci of WHV core antigen negative hepatocytes including those defined as FAH.
Expression and distribution of fetuin B mRNA in peripheral tissues
Fetuin B protein belongs to the cystatin superfamily and most members of this family, including fetuin B, are synthesized in the liver (Brown and Dziegielewska, 1997; Denecke et al., 2003; Hsu et al., 2004; Olivier et al., 2000). Fetuin B, as well as fetuin A, another member of the cystatin superfamily, is known to undergo differential expression during an acute phase response (Olivier et al., 2000). Northern blotting confirmed that woodchuck fetuin B mRNA is preferentially expressed in the woodchuck liver as a 1.5 kb transcript (Figure 7), as compared to other tissues including pancreas, kidney, lung, spleen, heart, brain, tongue and skeletal muscle. As discussed below, this finding was consistent with western blot analyses of tissue samples.
Figure 7. Liver specific expression of fetuin B mRNA in woodchuck tissues detected by Northern blot analysis.
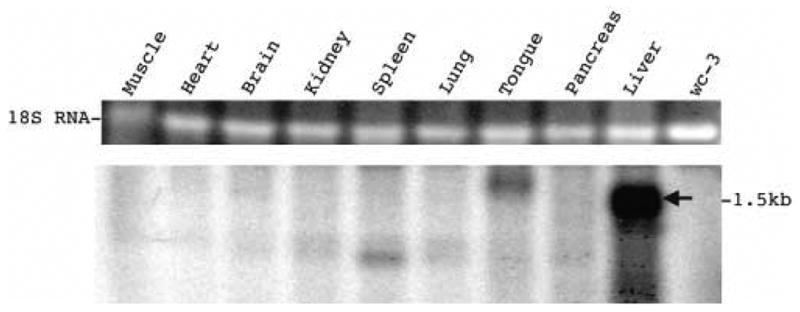
Each sample contained 5 μg of total woodchuck RNA extracted from the respective woodchuck organs as shown and from untransfected wc3 hepatoma cells (Lee et al., 1987). The blot was hybridized with a negative sense, woodchuck fetuin B riboprobe representing the complete ORF. A fetuin B mRNA transcript was detected in the liver. The top panel shows 18S RNA in the gel, stained with ethidium bromide. The minor bands with distinct electrophoretic mobilities, found in tongue and spleen, were not further characterized.
Fetuin B mRNA was not detected in human and woodchuck HCC
Microarray analysis (Xu, Mason, Yamamoto et al, unpublished) suggested that fetuin B mRNA was not expressed in woodchuck HCC. To evaluate the microarray data, fetuin B mRNA expression was assessed by Northern blot analysis using a negative sense fetuin B RNA probe in HCC and non-cancerous liver from 6 WHV-infected woodchucks (Figure 8A), including three (307, 309, 371) listed in Table 1). In each case, fetuin B mRNA levels were at least 50-fold lower in the HCC than the surrounding liver. In contrast, levels of fetuin A mRNA were unchanged. Fetuin B mRNA expression was also lower in HCC than in adjacent liver tissue collected from 3 HBV-infected patients (patients 7, 8 and 9) (Figure 8B) as determined using semi-quantitative RT PCR. Again, levels of fetuin A mRNA did not change.
Figure 8. Analysis of fetuin B and fetuin A mRNA expression in liver and HCC.
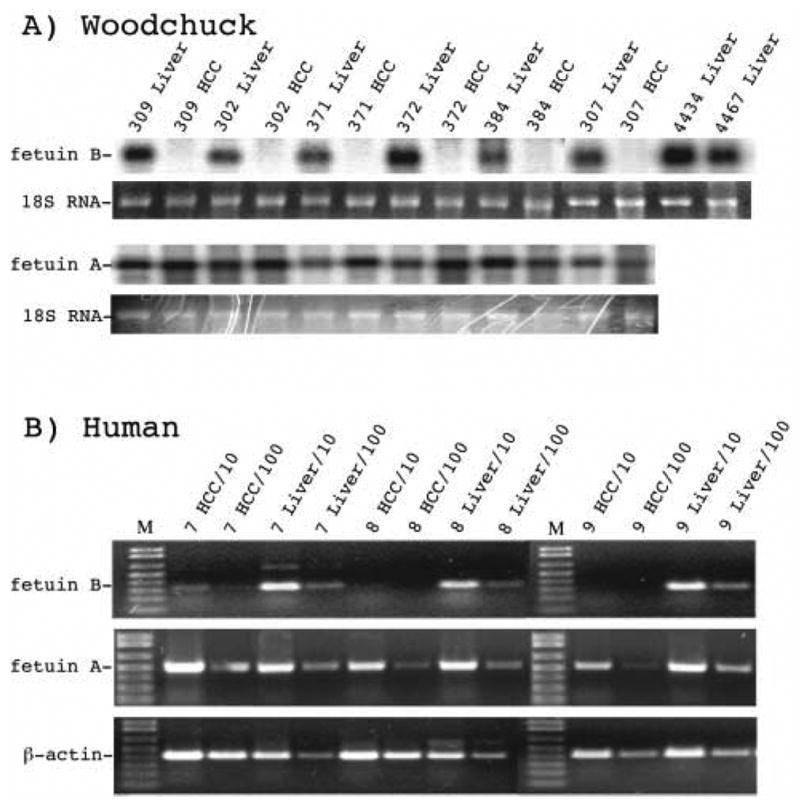
A) Northern blot assay for fetuin B and fetuin A mRNA transcripts in HCC and adjacent non-cancerous liver from woodchucks chronically infected with WHV. Each lane contained 5μg total RNA; 18S ribosomal RNA was visualized as a control. B) Semi-quantitative RT-PCR analysis for fetuin B and fetuin A mRNA expression in human HCC and non-cancerous surrounding liver from 3 individuals (patient 7, patient 8 and patient 9) chronically infected with HBV. After reverse transcription, the cDNA was diluted 10-fold and 100-fold and used as a template for PCR. β-actin was used as an internal control. Human fetuin B mRNA transcripts were at least 10-fold lower in the HCC compared to surrounding liver. Fetuin A mRNA expression was unchanged.
Fetuin B protein expression in liver, serum and transfected cells by western blot
Two forms of the fetuin B protein, α and β (Lee et al., 2002), were detected by western blotting in human and woodchuck liver and in Huh 7 human (Nakabayashi et al., 1982) hepatoma lines transfected with a human fetuin B expression vector (the non-transfected cells did not express fetuin B) (Figure 9A). In wc3 woodchuck hepatoma cells (Lee et al., 1987) transfected with a woodchuck fetuin B expression vector, additional faster moving bands were also seen (Figure 9A), presumably resulting from protein degradation. In human and woodchuck serum, only the β form of fetuin B protein was detected (Figure 9A) with an apparent molecular size of 52kD. The apparent size of the α form from human liver was 48 kD, while in woodchuck liver it was 50kD. The difference in molecular weight between the β and α forms of fetuin B protein may be due to glycosylation occurring during secretion, since serum fetuin B protein, the β form, has been reported to bind to the mannose receptor, which regulates serum levels of glycoproteins (Lee et al., 2002). Western blotting supported the tissue specific expression of fetuin B protein, since both the larger secreted β form and the α form were found in liver, but only the β form was found in serum and other tissues examined, including HCC, suggesting that detection in tissues other than the liver was due to its presence in blood (Figure 9B).
Figure 9. Evidence for liver specific expression of fetuin B protein by western blotting.
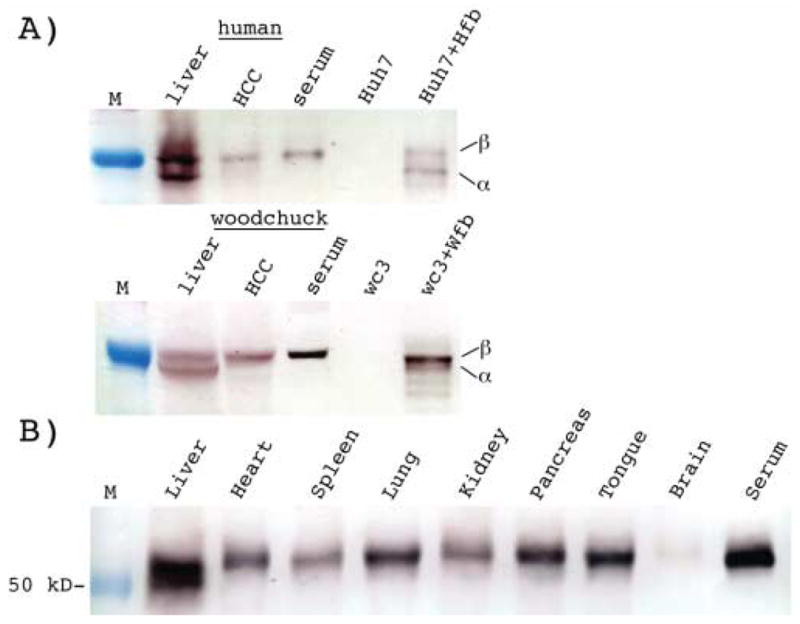
A) Fetuin B expression was determined using human and woodchuck liver, HCC and serum samples, and Huh7 and wc3 hepatoma cell lines transfected with pHuFetuin-B (Huh7+Hfb) and pWcFetuin-B (wc3+Wfb) respectively. Liver and HCC samples were homogenized, then protein concentrations were determined and the equivalent of 50 μg of human and 100 μg of woodchuck tissue was loaded per lane for SDS polyacrylamide gel electrophoresis. 0.2 ul of human and woodchuck serum and 10 μg of Huh7 or wc3 cell lysate was also loaded. Western blots were probed with anti-human fetuin B antibodies, as described in Materials and Methods. Both forms of fetuin B protein (α and β) were detected in normal human and woodchuck liver tissue and transfected Huh7 and wc3 cells. Only the β form was detected in HCC and serum. B) Western blot analysis confirming the presence of the β form of fetuin B protein in each woodchuck tissue analyzed. The α form of fetuin B was only detected in the liver.
Assessment of fetuin B protein expression in WHV core negative foci including FAH
It is believed that HCCs in humans infected with HBV and woodchucks infected with WHV emerge from FAH that appear during a chronic infection (Bannasch et al., 2003; Li et al., 2002; Su and Bannasch, 2003; Thorgeirsson and Grisham, 2002). Because fetuin B mRNA was not detected in HCC but is expressed in normal liver (Figures 8, 9), we speculated that its loss might be a sensitive marker of neoplastic progression (e.g., FAH). Here we have asked if fetuin B protein expression, detected by immunoperoxidase staining, is observed in the different forms of FAH, and in the morphologically normal foci of WHV core negative hepatocytes.
As shown in Figure 2, fetuin B protein was not detected or was present at only low levels in HCC, and basophilic (Figure 3) and in amphophilic FAH (Figure 4). Hepatocytes in clear cell (glycogen storage) FAH expressed fetuin B protein but the assessment of expression levels was hampered by displacement of the cytoplasm by accumulated glycogen (Figure 5). WHV core antigen negative foci that were not identifiable as FAH by H&E or PAS staining expressed fetuin B at a similar level to surrounding WHV core antigen positive hepatocytes (Figure 6). Thus, a reduction in fetuin B protein expression appears to distinguish basophilic and amphophilic FAH and HCC from clusters of morphologically normal WHV core antigen negative hepatocytes (Table 1), and possibly from clear cell (glycogenotic) FAH.
DISCUSSION
Persistent HBV infection in neonatally infected humans initially leads to a largely quiescent carrier state, with all hepatocytes infected but little disease. Typically, however, the infection progresses to include chronic liver disease, cirrhosis, and a high risk of HCC (Beasley, 1988). FAH (Bannasch et al., 2003) which express little or no HBV (Govindarajan et al., 1990) are observed. Partial or complete clearance of virus from hepatocytes scattered throughout the lobule, not necessarily forming recognizable clusters or foci, is also observed (Chu et al., 1997; Gowans and Burrell, 1985; Gowans et al., 1985; Hirohashi et al., 1982; Hsu et al., 1988; Kojima et al., 1977; Lamothe et al., 1976; Nayak et al., 1977; Omata et al., 1978; Ray et al., 1976; Suzuki et al., 1985). There is little evidence bearing on the extent to which these scattered hepatocytes are responding to an external antiviral effecter (e.g., cytokines), or the extent to which the hepatocytes themselves are intrinsically resistant to virus expression and/or infection. While loss of WHV proteins and nucleic acids from isolated hepatocytes may be due to the effects of antiviral cytokines, the histological evidence does not support the possibility that clearance from foci is transient and primarily due to cytokines. It is possible, however, that as a result of genetic or epigenetic changes these cells may be more responsive to the altered cytokine levels that exist in the liver during chronic inflammation, in which case cytokine mediated clearance would be secondary to changes in the target cells.
Elevated rates of hepatocyte proliferation, if they have an effect on virus replication, also appear unlikely to explain the loss of virus expression in all categories of foci. Radaeva et al. (2000) estimated high cell proliferation rates, based on the Ki67 staining, in basophilic and amphophilic FAH, with staining indices in the range of 5–20%, but not in clear cell FAH, which were similar to surrounding liver with a staining index of about 2%. Because of the small number of samples available in the present study, we did not carry out a detailed study of hepatocyte proliferation within foci. WHV core antigen negative foci with normal morphology did not appear to differ from surrounding hepatocytes in staining for PCNA positive nuclei, though small differences can not be ruled out without a larger sample set.
Interestingly, the high Ki67 staining index for amphophilic and basophilic FAH (Radaeva et al., 2000) appear to indicate a very high proliferation and expansion rate. If a Ki67 staining index of 20% equates to 30% of the hepatocytes dividing every day, such FAH would expand exponentially, for example from 1000 cells, a rough minimum estimate for a small spherical FAH with a diameter of ~12 hepatocytes, to 7 x 109 cells in 60 days. With 40% of the hepatocytes dividing every day, this size would be reached by 47 days. In contrast, the entire liver contains ~3 x 1010 hepatocytes. It is unclear if this projection is compatible with observations on tumor development in chronically WHV-infected woodchucks, which typically develop only one or a few HCCs (Jacob et al., 2004, Summers et al., 1978, Tennant, 2001).
Unlike HBV carriers, 90% of woodchuck HCCs are associated with transcriptional activation of N-myc2 due to WHV DNA integration, generally in the 3′ UTR of N-myc2 or in the distal win locus (Fourel et al., 1994; Fourel et al., 1990; Seeger and Mason, 1999). Expression of N-myc2 has also been described in large (>100,000 hepatocytes) FAH as well as HCC (Yang et al., 1993), but not in normal hepatocytes. Indeed, the observation that N-myc2 expression in woodchuck liver is unique to some FAH and most HCC constitutes one of the major pieces of evidence that at least some foci give rise to the HCCs that arise during chronic WHV infection.
The fact that some FAH so closely resemble HCC morphologically has lead to the idea that all FAH are in some sense pre-neoplastic. However, a more unifying concept is that most FAH evolve clonally from hepatocytes, or in some cases, hepatocyte progenitor cells, that are resistant to a stress to which the liver is subjected (Evarts et al., 1996). Thus, progression to HCC would be facilitated by formation of FAH, or WHV negative hepatocytes as a first step, to avoid immune attack by virus antigen specific cytotoxic T lymphocytes (CTL), but other, major changes in cell lineages would be needed for tumor outgrowth. Some of these changes may occur prior to emergence of FAH, explaining the occurrence, in particular, of FAH with elevated N-myc2 expression (Yang et al., 1993), possibly due to nearby integration of WHV DNA. One test of an immune evasion hypothesis for the origin of HCC would be that all FAH are clonal in WHV and HBV carriers. While we have been able to obtain evidence with chronically WHV infected woodchucks that extensive clonality arises in hepatocytes that are, or at one time were, infected with WHV (Mason et al., 2005), it has not yet been demonstrated that WHV negative foci are the histological counterpart of these clones. This is being investigated in ongoing work.
MATERIALS AND METHODS
Tissue samples
Woodchucks were housed in the Laboratory Animal Facility of the Fox Chase Cancer Center. All work was approved by the FCCC Institutional Animal Care and Use Committee. Liver and HCC were collected from woodchucks following euthanasia. Liver samples were also collected by surgical biopsy, as previously described (Mason et al., 1998). Biopsy and autopsy tissue from a total of 13 woodchucks was evaluated histologically (Table 1), including 8 woodchucks described in (Mason et al., 1998) (307, 309, 310, 313, 314, 4961, 5114, 5154), 3 described in (Mason et al., 2005; Zhu et al., 2004) (359, 366, 371), and 2 not previously reported (501; 3202). Autopsy samples from an additional 5 were evaluated biochemically (302, 4434, 4467 (Mason et al., 1998), 372 (Mason et al., 2005; Zhu et al., 2004) and one, 384, not previously reported). Liver tissues were fixed in formalin, dehydrated and embedded in paraffin for histologic assays, or quick frozen and stored at –80°C for subsequent nucleic acid or protein assays. Frozen autopsy tissue samples of woodchuck muscle, heart, brain, kidney, spleen, lung, tongue, and pancreas were also collected, quick frozen and stored at –80 °C for total RNA extraction.
Primary human HCC, adjoining non-cancerous liver, and human serum were obtained from the Liver Cancer Prevention Program Clinic and under the control of the FCCC Human Subjects Protection Program. Tissues were quick frozen in liquid nitrogen and stored at −80º C for nucleic acid extractions.
RNA extraction
Total RNA was extracted with TRIZOL reagent (Invitrogen). Briefly, 30~50 mg of tissue was homogenized in 1 ml of Trizol reagent and 0.2 ml of chloroform. RNA was precipitated from the aqueous phase by addition of 0.5 ml of isopropanol and collected by centrifugation at 12,000 g for 10 min at 4° C. The RNA pellet was dissolved in 20 μl of DEPC-treated H2O.
Cloning of human and woodchuck fetuin B cDNA
The first strand of human fetuin B mRNA was reverse transcribed, from human liver RNA, with an oligo(dT)12–18 primer using Superscript II (Invitrogen). Upstream and downstream primers for amplification of human fetuin B coding sequences were designed based on a cDNA sequence reported in GenBank (NM_014375): upstream primer: 5′-GAA TTC AAG ATG GGT CTG CTC CTT CC-3′ (containing an EcoR I site, underlined); downstream primer: 5′-GGA TCC TCA TGG CGG AAG GAC AAG-3′ (containing a BamH I site, underlined). The sequences in bold are located at the 5′ and 3′ ends, respectively, of the ORF for human fetuin B.
The sequence of the cDNAs generated from the 3′ end of woodchuck fetuin B mRNA including most of the ORF was determined with Sequencher software using sequences in a woodchuck cDNA library (Xu, Mason, Yamamoto, unpublished). The 5′ end of the transcript was acquired by rapid amplification of the cDNA ends (RACE) using the SMART RACE cDNA Amplification Kit, following the manufacturer’s instructions (Clontech). Briefly, the first woodchuck cDNA strand was reverse transcribed from RNA, extracted from woodchuck liver, with the 5′-RACE CDS Primer (5′–(T)25N−1N–3′ (N = A, C, G, or T; N−1 = A, G, or C)), PowerScript Reverse Transcriptase and Smart II A Oligonucleotide. The 5′ end of the fetuin B mRNA was then amplified with the antisense primer 5′-AGC TAC TGG CCT GGG ATC TGG TAC A-3 (nucleotides (nt) 670–646 of the ORF for woodchuck fetuin B), and Universal Primer Mix, then nested with 5′-TCT CCG AGA AAC TGG GCG AAG AGT A-3′ (nt 435–411 of the woodchuck fetuin B ORF) and Nested Universal Primer A. The full length fetuin B ORF sequence was then amplified from the original first strand cDNAs with the confirmed upstream primer (5′-GAC TCG AGA TGG GTC TGC TCC AAC TG-3′, containing an Xhol I site, underlined) and down-stream primer (5′-GCA AGC TTT CAG GGT GGG AGG AGA AG -3′, containing a Hind III, underlined). Start and stop codons for the ORF are in bold type.
Human and woodchuck fetuin B ORF sequences were cloned into pGEM-T (Promega), and sequenced. Identical sequences for woodchuck fetuin B were amplified from six different woodchucks (accession no. DQ417362). The predicted amino acid sequence of the woodchuck fetuin B gene product was 63% identical to that of human fetuin B. In 4 animals, a variant containing a single nucleotide substitution was also found (accession no. DQ417361).
A cDNA fragment of woodchuck fetuin A was amplified from woodchuck RNA, essentially as described above. Woodchuck fetuin A primers were designed based on conserved regions of the respective human (accession no. NM_001622), mouse (accession no. NM_013465), Pan troglodytes (accession no. NM_001009098) and Cercopithecus aethiops (accession no. AB004046) mRNAs. The upstream primer was 5′-CCT TTG TCT TGC TCA GCT CT-3′ (nt 21–40 of the human fetuin A ORF) and the downstream primer, 5′-TTA CAA AAG CCATAT TGC TT-3′ (nt 673–692 of the human fetuin A ORF). The amplified fragment was inserted into pGEM-T (Promega) and sequenced (accession no. DQ417363). The woodchuck fetuin A fragment encoded a polypeptide that was 78% identical to the corresponding fragment of human fetuin A (accession no. NM_001622).
RNA analyses
For Northern blotting, 5 μg of total RNA extracted from HCC and adjacent non- cancerous liver, or from transfected cells, was electrophoresed into a 1.5% agarose gel containing 0.02 M MOPS and 18% formaldehyde. Samples were transferred to a nylon membrane (Hybond-N, Amersham Biosciences) in 20X SSC. RNA probes were labeled with alpha-32P-UTP by in vitro transcription with T7 or SP6 RNA polymerase (Promega) of cDNAs cloned in pGEM-T. Hybridization was performed in EKONO hybridization buffer (Research Products International Corp.) at 65ºC overnight.
End-point dilution RT-PCR was used to detect human fetuin B mRNA levels in human HCC and adjacent liver. The first strand of human cDNA was reverse transcribed from 5 μg of RNA with 200 units of SuperScript II and Oligo(dT)12–18 (Invitrogen) at 42°C for 2 hr in a reaction volume of 20 μl. 0.5 ul of the cDNA was used, either undiluted or at a 1:10 dilution, as a template for PCR. PCR primers for human fetuin B amplification included an upstream sense primer 5′-TCC GAC TCT GTG CCT GTT-3′ (nt 786–803) and a downstream anti-sense primer 5′-GGG ATT CGT GGT TAG GTC-3′ (nt 1103–1086). Human beta actin (accession no. BC013835) was used an as internal control, with 5′-AGC TAC GAG CTG CCT GAC G-3′ (nt 787–805) as an upstream sense primer and 5′-TAG AAG CAT TTG CGG TGG-3′ (nt 1199–1182) as a downstream anti-sense primer. Human fetuin A (accession no. NM_001622) cDNA was amplified with an upstream sense primer, 5′-CCC TCC CAC CTT CTA CCT-3′ (nt 623–640) and a downstream antisense primer, 5′-GCA GCA GCA CCAACA CTA-3′ (nt 1100–1083). PCR amplified cDNAs were resolved by electrophoresis on a 1.0% agarose gel in E-buffer (40 mM Tris-acetate, 20 mM Na Acetate, 1 mM EDTA) containing 0.1 μg of ethidium bromide per ml.
Transient expression of fetuin B protein in hepatoma cell lines
Human fetuin B cDNA was released with EcoR I and BamH I from the pGEM-T cloning vector; woodchuck fetuin B cDNA was released with Xhol I and Hind III. These fragments were subcloned in the sense orientation into the corresponding restriction endonuclease sites of phCMV1 (Gene Therapy Systems Inc.). The recombinant expression vectors were named pHuFetuin-B and pWcFetuin-B. Huh7 human (Nakabayashi et al., 1982) and wc3 woodchuck hepatoma cells (Lee et al., 1987), which do not express fetuin B (data not shown), were grown in DMEM/F12 medium supplemented with 10% (v/v) fetal bovine serum at 37°C with 5% CO2. The cells (106 per 35 mm tissue culture dish) were transfected with 4 μg of the fetuin B expression vectors, or an empty vector as a control, using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. Forty eight hours after transfection, the cells were washed twice with ice-cold PBS and lysed with CytoBuster Protein Extraction Reagent (Novagen) containing 25 units per ml of the nuclease, Benzonase (BD Biosciences).
Western blot assays for fetuin B protein
Homogenates of woodchuck liver and HCC, and lysates of Huh7 and wc3 hepatoma cells transfected with fetuin B expression vectors, were prepared in SDS gel-loading buffer containing 100 mM DTT, and separated by electrophoresis in 12% SDS-polyacrylamide gels, essentially as described (Laemmli, 1970) except that the separation gel contained 7 M urea. Proteins were then electro-transferred to SS BA85 nitrocellulose filters (Burnette, 1981). The filters were blocked with 3% gelatin and incubated with mouse anti-human fetuin B monoclonal antibodies (for Huh7 cells transfected with human fetuin B cDNA) or goat anti-human fetuin B polyclonal antibodies (affinity purified) (R&D Systems) (for woodchuck tissues and cell lines transfected with woodchuck fetuin B cDNA). The secondary antibodies were alkaline phosphatase (AP) conjugated goat anti-mouse or donkey anti-goat polyclonal antibody (Jackson ImmunoResearch Laboratories, Inc.). The blots were developed with the Alkaline Phosphatase Conjugate Substrate Kit (Bio-Rad). Total protein was stained using coomassie brilliant blue.
Histological analysis of liver sections
After deparaffinization and rehydration with PBS, formalin fixed liver sections were incubated with 3% H2O2 for 10 min to deplete endogenous peroxidase activity. For fetuin B protein detection, sections were treated with Antigen Retrieval Reagent (R&D Systems) at 92–95°C in a microwave oven for 10 min. Immunoperoxidase assays were performed using Dako LSAB-2 kits. Briefly, after application of the blocking reagent, the tissues were sequentially incubated with affinity purified goat anti-fetuin B primary antibody (R&D Systems) at a dilution of 1:200, biotinylated donkey anti-goat immunoglobulins (Jackson Immunoresearch Laboratories, Inc.) and streptavidin-conjugated peroxidase, and reactions were developed using diaminobenzidine tetrahydrochloride with counterstaining with Mayer’s hematoxylin. Non-immunized-goat serum (NGS) was used as a negative control. Immunoperoxidase assays for detection of WHV core antigen were carried out without antigen retrieval using normal rabbit serum (NRS) as a negative control as previously described (Kajino et al., 1994). Formalin fixed tissues were also deparaffinized and re-hydrated and used for staining by H&E and, before and after diastase treatment to remove glycogen, for PAS staining, carried out by incubation with 1% periodic acid Schiff reagent followed by counterstaining with Mayer’s hematoxylin.
Acknowledgments
We are grateful to Christoph Seeger, Ju-Tao Guo, and Jesse Summers (University of New Mexico) for helpful suggestions during the course of these studies. WSM was supported by NIH grants from the Institute of Allergies and Infectious Diseases and the National Cancer Institute, and by an Appropriation from the Commonwealth of Pennsylvania. ARJ was supported as a subcontractor on the NIH grant and by the research committee of the Royal Adelaide Hospital and Institute of Medical and Veterinary Science.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
BIBLIOGRAPHY
- Abe K, Kurata T, Shikata T, Tennant BC. Enzyme-altered liver cell foci in woodchucks infected with woodchuck hepatitis virus. Japanese J Canc Res. 1988;79:466–472. doi: 10.1111/j.1349-7006.1988.tb01615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannasch P, Haertel T, Su Q. Significance of hepatic preneoplasia in risk identification and early detection of neoplasia. Toxicol Pathol. 2003;31:134–139. doi: 10.1080/01926230390173923. [DOI] [PubMed] [Google Scholar]
- Beasley RP. Hepatitis B virus. The major etiology of hepatocellular carcinoma. Cancer. 1988;61:1942–1956. doi: 10.1002/1097-0142(19880515)61:10<1942::aid-cncr2820611003>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Brown WM, Dziegielewska KM. Friends and relations of the cystatin superfamily--new members and their evolution. Protein Sci. 1997;6:5–12. doi: 10.1002/pro.5560060102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnette WN. Western blotting”: electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981;112:195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Chu CM, Yeh CT, Chien RN, Sheen IS, Liaw YF. The degrees of hepatocyte nuclear but not cytoplasmic expression of hepatitis B core antigen reflect the level of viral replication in chronic hepatitis B virus infection. J Clin Microbiol. 1997;35:102–105. doi: 10.1128/jcm.35.1.102-105.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denecke B, Graber S, Schafer C, Heiss A, Woltje M, Jahnen-Dechent W. Tissue distribution and activity testing suggest a similar but not identical function of fetuin-B and fetuin-A. Biochem J. 2003;376:135–145. doi: 10.1042/BJ20030676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evarts RP, Hu Z, Omori N, Omori M, Marsden ER, Thorgeirsson SS. Precursor-product relationship between oval cells and hepatocytes: comparison between tritiated thymidine and bromodeoxyuridine as tracers. Carcinogenesis. 1996;17:2143–2151. doi: 10.1093/carcin/17.10.2143. [DOI] [PubMed] [Google Scholar]
- Fourel G, Couturier J, Wei Y, Apiou F, Tiollais P, Buendia MA. Evidence for long-range oncogene activation by hepadnavirus insertion. EMBO J. 1994;13:2526–2534. doi: 10.1002/j.1460-2075.1994.tb06542.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fourel G, Trepo C, Bougueleret L, Henglein B, Ponzetto A, Tiollais P, Buendia MA. Frequent activation of N-myc genes by hepadnavirus insertion in woodchuck liver tumours. Nature. 1990;347:294–298. doi: 10.1038/347294a0. [DOI] [PubMed] [Google Scholar]
- Fu XX, Su CY, Lee Y, Hintz R, Biempica L, Synder R, Rogler C. Insulin like growth factor II expression and oval cell proliferation associated with hepatocarcinogenesis in woodchuck hepatitis virus carriers. J Virol. 1988;62:3422–3430. doi: 10.1128/jvi.62.9.3422-3430.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindarajan S, Conrad A, Lim B, Valinluck B, Kim AM, P S. Study of preneoplastic changes in liver cells by immunohistochemical and molecular hybridization techniques. Arch Path Lab Med. 1990;114:1042–1045. [PubMed] [Google Scholar]
- Gowans EJ, Burrell CJ. Widespread presence of cytoplasmic HBcAg in hepatitis B infected liver detected by improved immunochemical methods. J Clin Pathol. 1985;38:393–398. doi: 10.1136/jcp.38.4.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowans EJ, Burrell CJ, Jilbert AR, Marmion BP. Cytoplasmic (but not nuclear) hepatitis B virus (HBV) core antigen reflects HBV DNA synthesis at the level of the infected hepatocyte. Intervirology. 1985;24:220–225. doi: 10.1159/000149646. [DOI] [PubMed] [Google Scholar]
- Guo JT, Zhou H, Liu C, Aldrich C, Saputelli J, Whitaker T, Barrasa MI, Mason WS, Seeger C. Apoptosis and regeneration of hepatocytes during recovery from transient hepadnavirus infections. J Virol. 2000;74:1495–1505. doi: 10.1128/jvi.74.3.1495-1505.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirohashi S, Shimosato Y, Ino Y, Kishi K. Distribution of hepatitis B surface and core antigens in human liver cell carcinoma and surrounding nontumorous liver. JNCI. 1982;69:565–568. [PubMed] [Google Scholar]
- Hsu HY, Lin YH, Chang MH, Su IJ, Chen DS. Pathology of chronic hepatitis B infection in children: With special reference to the intrahepatic expression of hepatitis B virus antigens. Hepatology. 1988;8:378–382. doi: 10.1002/hep.1840080232. [DOI] [PubMed] [Google Scholar]
- Hsu SJ, Nagase H, Balmain A. Identification of Fetuin-B as a member of a cystatin-like gene family on mouse chromosome 16 with tumor suppressor activity. Genome. 2004;47:931–946. doi: 10.1139/g04-043. [DOI] [PubMed] [Google Scholar]
- Jacob JR, Ascenzi MA, Roneker CA, Toshkov IA, Cote PJ, Gerin JL, Tennant BC. Hepatic expression of the woodchuck hepatitis virus X-antigen during acute and chronic infection and detection of a woodchuck hepatitis virus X-antigen antibody response. Hepatology. 1997;26:1607–1615. doi: 10.1002/hep.510260632. [DOI] [PubMed] [Google Scholar]
- Jacob JR, Sterczer A, Toshkov IA, Yeager AE, Korba BE, Cote PJ, Buendia MA, Gerin JL, Tennant BC. Integration of woodchuck hepatitis and N-myc rearrangement determine size and histologic grade of hepatic tumors. Hepatology. 2004;39:1008–1016. doi: 10.1002/hep.20106. [DOI] [PubMed] [Google Scholar]
- Jilbert AR, Wu TT, England JM, de la M, Hall P, Carp NZ, O’Connell AP, Mason WS. Rapid resolution of duck hepatitis B virus infections occurs after massive hepatocellular involvement. J Virol. 1992;66:1377–1388. doi: 10.1128/jvi.66.3.1377-1388.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajino K, Jilbert AR, Saputelli J, Aldrich CE, Cullen J, Mason WS. Woodchuck hepatitis virus infections: very rapid recovery after a prolonged viremia and infection of virtually every hepatocyte. J Virol. 1994;68:5792–5803. doi: 10.1128/jvi.68.9.5792-5803.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima M, Udo K, Takahashi Y, Yoshizawa H, Tsuda F, Itoh Y, Miyakawa Y, Mayumi M. Correlation between the titer of antibody to hepatitis B core antigen and presence of viral antigens in the liver. Gastroenterology. 1977;73:664–667. [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature. 1970;227:680–682. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lamothe F, Laurencin-Piche J, Cote J, Guevin R, Viallet A, Richer G. Detection of surface and core antigens of hepatitis B virus in the liver of 164 human subjects. Gastroenterology. 1976;71:102–108. [PubMed] [Google Scholar]
- Lee H, Kawaguchi T, Nomura K, Kitagawa T. Establishment and characterization of a diethylnitrosamine-initiated woodchuck hepatocyte cell line. Hepatology. 1987;7:937–940. doi: 10.1002/hep.1840070524. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Evers S, Roeder D, Parlow AF, Risteli J, Risteli L, Lee YC, Feizi T, Langen H, Nussenzweig MC. Mannose receptor-mediated regulation of serum glycoprotein homeostasis. Science. 2002;295:1898–1901. doi: 10.1126/science.1069540. [DOI] [PubMed] [Google Scholar]
- Li Y, Hacker H, Kopp-Schneider A, Protzer U, Bannasch P. Woodchuck hepatitis virus replication and antigen expression gradually decrease in preneoplastic hepatocellular lineages. J Hepatol. 2002;37:478–485. doi: 10.1016/s0168-8278(02)00233-7. [DOI] [PubMed] [Google Scholar]
- Mason WS, Cullen J, Moraleda G, Saputelli J, Aldrich CE, Miller DS, Tennant B, Frick L, Averett D, Condreay LD, Jilbert AR. Lamivudine therapy of WHV-infected woodchucks. Virology. 1998;245:18–32. doi: 10.1006/viro.1998.9150. [DOI] [PubMed] [Google Scholar]
- Mason WS, Jilbert AR, Summers J. Clonal expansion of hepatocytes during chronic woodchuck hepatitis virus infection. Proc Natl Acad Sci USA. 2005;102:1139–1144. doi: 10.1073/pnas.0409332102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakabayashi H, Taketa K, Miyano K, Yamane T, Sato J. Growth of human hepatoma cell lines with differentiated functions in chemically defined medium. Cancer Res. 1982;42:3858–3863. [PubMed] [Google Scholar]
- Nayak NC, Dhar A, Sachdeva R, Mittal A, Seth HN, Sudarsanam D, Reddy B, Wagholikar UL, Reddy CRRM. Association of human hepatocellular carcinoma and cirrhosis with hepatitis B virus surface and core antigens in the liver. Int J Cancer. 1977;20:643–654. doi: 10.1002/ijc.2910200502. [DOI] [PubMed] [Google Scholar]
- Olivier E, Soury E, Ruminy P, Husson A, Parmentier F, Daveau M, Salier JP. Fetuin-B, a second member of the fetuin family in mammals. Biochem J 350 Pt. 2000;2:589–97. [PMC free article] [PubMed] [Google Scholar]
- Omata M, Afroudakis A, Liew CT, Ashcavai M, Peters RL. Comparison of serum hepatitis B surface antigen (HBsAg) and serum anticore with tissue HBsAg and hepatitis B core antigen (HBcAg) Gastroenterology. 1978;75:1003–1009. [PubMed] [Google Scholar]
- Popper H, Roth L, Purcell R, Tennant BC, Gerin JL. Hepatocarcinogenicity of the woodchuck hepatitis virus. Proc Natl Acad Sci USA. 1987;84:866–870. doi: 10.1073/pnas.84.3.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radaeva S, Li Y, Hacker HJ, Burger V, Kopp-Schneider A, Bannasch P. Hepadnaviral hepatocarcinogenesis: in situ visualization of viral antigens, cytoplasmic compartmentation, enzymic patterns, and cellular proliferation in preneoplastic hepatocellular lineages in woodchucks. J Hepatol. 2000;33:580–600. doi: 10.1034/j.1600-0641.2000.033004580.x. [DOI] [PubMed] [Google Scholar]
- Ray MB, Desmet VJ, Bradburne AF, Desmyter J, Fevery J, De Groote J. Differential distribution of hepatitis B surface antigen and hepatitis B core antigen in the liver of hepatitis B patients. Gastroenterology. 1976;71:462–467. [PubMed] [Google Scholar]
- Seeger C, Mason WS. Woodchuck and duck hepatitis B viruses. In: Ahmed R, Chen I, editors. Persistent Viral Infections. John Wiley and Sons Ltd; 1999. pp. 607–621. [Google Scholar]
- Su Q, Bannasch P. Relevance of hepatic preneoplasia for human hepatocarcinogenesis. Toxicol Pathol. 2003;31:126–133. doi: 10.1080/01926230390173905. [DOI] [PubMed] [Google Scholar]
- Su Q, Zerban H, Otto G, Bannasch P. Cytokeratin expression is reduced in glycogenotic clear hepatocytes but increased in ground-glass cells in chronic human and woodchuck hepadnaviral infection. Hepatology. 1998;28:347–359. doi: 10.1002/hep.510280209. [DOI] [PubMed] [Google Scholar]
- Summers J, Jilbert AR, Yang W, Aldrich CE, Saputelli J, Litwin S, Toll E, Mason WS. Hepatocyte turnover during resolution of a transient hepadnaviral infection. Proc Natl Acad Sci USA. 2003;100:11652–11659. doi: 10.1073/pnas.1635109100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers J, Smolec JM, Snyder R. A virus similar to human hepatitis B virus associated with hepatitis and hepatoma in woodchucks. Proc Natl Acad Sci USA. 1978;75:4533–4537. doi: 10.1073/pnas.75.9.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, Uchida T, Horiuchi R, Shikata T. Localization of hepatitis B surface and core antigens in human hepatocellular carcinoma by immunoperoxidase methods. Replication of complete virions of carcinoma cells. Cancer. 1985;56:321–327. doi: 10.1002/1097-0142(19850715)56:2<321::aid-cncr2820560220>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Tennant BC. Animal models of hepadnavirus-associated hepatocellular carcinoma. Clinics in Liver Disease. 2001;5:43–68. doi: 10.1016/s1089-3261(05)70153-7. [DOI] [PubMed] [Google Scholar]
- Tennant BC, Toshkov IA, Peek SF, Jacob JR, Menne S, Hornbuckle WE, Schinazi RD, Korba BE, Cote PJ, Gerin JL. Hepatocellular carcinoma in the woodchuck model of hepatitis B virus infection. Gastroenterology. 2004;127:S283–293. doi: 10.1053/j.gastro.2004.09.043. [DOI] [PubMed] [Google Scholar]
- Thorgeirsson SS, Grisham JW. Molecular pathogenesis of human hepatocellular carcinoma. Nature Genetics. 2002;31:339–346. doi: 10.1038/ng0802-339. [DOI] [PubMed] [Google Scholar]
- Toshkov I, Hacker HJ, Roggendorf M, Bannasch P. Phenotypic patterns of preneoplastic and neoplastic hepatic lesions in woodchucks infected with woodchuck hepatitis virus. J Canc Res Clin Oncol. 1990;116:581–590. doi: 10.1007/BF01637078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, Alt E, Rogler CE. Coordinate expression of N-myc 2 and insulin-like growth factor II in pre-cancerous altered hepatic foci in woodchuck hepatitis virus carriers. Cancer Res. 1993;53:2020–2027. [PubMed] [Google Scholar]
- Yang D, Rogler CE. Analysis of insulin-like growth factor II (IGF-II) expression in neoplastic nodules and hepatocellular carcinomas of woodchucks utilizing in situ hybridization and immunochemistry. Carcinogenesis. 1991;12:1893–1901. doi: 10.1093/carcin/12.10.1893. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Cullen JM, Aldrich CE, Saputelli J, Miller D, Seeger C, Mason WS, Jilbert AR. Adenovirus based gene therapy during clevudine treatment of woodchucks chronically infected with woodchuck hepatitis virus. Virology. 2004;327:26–40. doi: 10.1016/j.virol.2004.06.017. [DOI] [PubMed] [Google Scholar]


