Abstract
BACKGROUND
We examined the cost-effectiveness of 2- and 3-dimensional computerized tomography (CT) colonography as a screening test for colorectal neoplasia.
METHODS
We created a Markov model of the natural history of colorectal cancer. Effectiveness of screening was based upon the diagnostic accuracy of tests in detecting polyps and cancer.
RESULTS
CT colonography every 5 or 10 yr was effective and cost-effective relative to no screening. Optical colonoscopy dominates 2-dimensional CT colonography done every 5 or 10 yr. Optical colonoscopy is weakly dominant over 3-dimensional CT colonography done every 10 yr. 3-D CT colonography done every 5 yr is more effective than optical colonoscopy every 10 yr, but costs an incremental $156,000 per life-year gained. Sensitivity analyses show that test costs, accuracy, and adherence are critical determinants of incremental cost-effectiveness. 3-D CT colonography every 5 yr is a dominant strategy if optical colonoscopy costs 1.6 times more than CT colonography. However, optical colonoscopy is a dominant strategy if the sensitivity of CT colonography for 1 cm adenomas is 83% or lower.
CONCLUSIONS
CT colonography is an effective screening test for colorectal neoplasia. However, it is more expensive and generally less effective than optical colonoscopy. CT colonography can be reasonably cost-effective when the diagnostic accuracy of CT colonography is high, as with primary 3-dimensional technology, and if costs are about 60% of those of optical colonoscopy. Overall, CT colonography technology will need to improve its accuracy and reliability to be a cost-effective screening option.
BACKGROUND & AIMS
Colorectal cancer is the second leading cause of cancer death in the United States (1, 2). The majority of cancer arises from adenomatous polyps that can be detected and removed by screening tests (3–7). Colorectal cancer incidence and mortality can clearly be reduced by fecal occult blood testing (FOBT), and indirect evidence supports the effectiveness of sigmoidoscopy and colonoscopy. Despite recommendations from professional organizations and interventions such as media campaigns (8), adherence to screening remains low (9, 10). Among the reasons for nonadherence are the unpleasant nature of the tests, the inaccuracy of non-invasive tests such as FOBT, and the fear of invasive tests (9, 11, 12).
Computed tomography (CT) colonography is a new imaging modality that aims to overcome some of the limitations of existing tests. It is an evolving technique in which CT data are used to generate two- and three-dimensional displays of the colon and rectum. Studies have shown that the sensitivity of CT colonography for 1 cm adenomas ranges from 48–100%; sensitivity for smaller adenomas is lower, ranging from 14–86% for adenomas <6 mm, and 30–95% for adenomas 6–9 mm. These figures are highly variable and meta-analyses have found significant heterogeneity depending on imaging modality used. In contrast, specificity is more homogeneous and pooled estimates range from 91% for <6 mm adenomas to 97% for 1 cm adenomas (13). While CT colonography is an exciting technology, there remain questions about its broad-scale applicability. Foremost among these are projections of its benefit and costs relative to existing tests.
METHODS
We examined the cost-effectiveness of screening with CT colonography using an updated version of a previous model (14). The model is a Markov state-transition model based on the natural history of colorectal cancer. The basic structure is outlined in Figure 1. It tracks the evolution of malignancy from adenoma growth through malignant transformation. Specifically, the simulated cohort is distributed in initial states at age 50 based on adenoma and cancer prevalence studies. The cohort moves through the model states based on progression rates derived from studies of the natural history of colorectal cancer: rates of adenoma incidence and prevalence, general mortality rates, and cancer incidence as detailed below. For the purposes of our analyses, we assumed that screening began at age 50 and continued through age 80, though the cohort was modeled to age 100.
Figure 1.
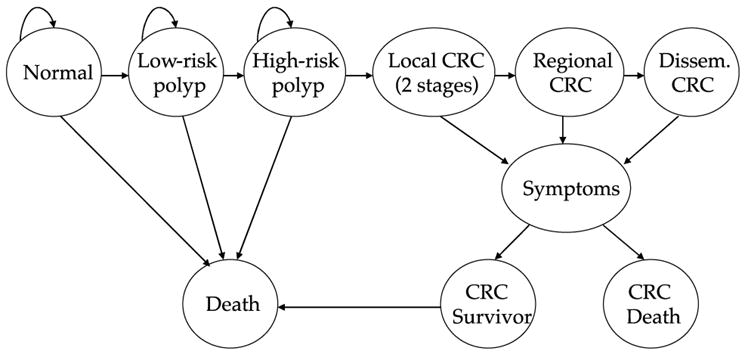
Model structure.
The key assumptions of the model are outlined in Table 1. The primary updates to the model were to use standardized assumptions established by the Institute of Medicine workshop on the economics of colorectal cancer screening (47). We also conducted an updated review of the literature to establish ranges for sensitivity analyses. The initial search for modalities other than CT colonography was conducted in 2000; this was updated to assess the accuracy of CT colonography in February 2004. We did a literature search using the terms “CT colonography” and “virtual colonoscopy,” and supplemented this with review articles and meta-analyses.
Table 1.
Model Assumptions
| Base Case | Sensitivity Analysis Range | References | |
|---|---|---|---|
| Natural History | |||
| Prevalence of adenomatous polyps | 15–19 | ||
| Age 50 yr | 20% | 10–30% | |
| Age 60 yr | 40% | 30–50% | |
| Age 70 yr | 50% | 40–60% | |
| Age 80 yr | 55% | 45–65% | |
| In patients with adenomas: | |||
| Proportion of adenomas that are high-risk | 15% | 5–25% | 15–19 |
| Annual incidence of colorectal cancer | 2 | ||
| Age 50 yr | 0.05% | – | |
| Age 55 yr | 0.09% | – | |
| Age 60 yr | 0.14% | – | |
| Age 65 yr | 0.20% | – | |
| Age 70 yr | 0.27% | – | |
| Age 75 yr | 0.35% | – | |
| Age 80 yr | 0.43% | – | |
| Age 85 yr | 0.45% | – | |
| 5-yr colorectal cancer mortality | 20 | ||
| Localized | 10.5% | – | |
| Regional | 35.1% | – | |
| Disseminated | 91.7% | – | |
| Test Characteristics | |||
| Sensitivity of 2-D VC for adenomas 1–5 mm | 33% | 14–57% | 13 |
| Sensitivity of 2-D VC for adenomas 6–9 mm | 50% | 30–83% | 13 |
| Sensitivity of 2-D VC for adenomas ≥ 10 mm | 82% | 55–95% | 13 |
| Specificity of 2-D VC | 91% | 85–97% | 13 |
| Sensitivity of 3-D VC for adenomas 1–5 mm | 46% | 25–56% | 13 |
| Sensitivity of 3-D VC for adenomas 6–9 mm | 83% | 42–94% | 13 |
| Sensitivity of 3-D VC for adenomas ≥10 mm | 91% | 80–100% | 13 |
| Specificity of 3-D VC | 91% | 85–97% | 13 |
| Sensitivity of FOBT for adenomas | 5% | 2–10% | 21–27 |
| Specificity of FOBT | 97.5% | 90–100% | 21–23;26;27 |
| Sensitivity of FOBT for cancer | 21;23–27 | ||
| Localized | 30% | 20–40% | |
| Regional | 50% | 40–60% | |
| Neoplasia reachable by sigmoidoscopy | 55% | 40–75% | 5;28–30 |
| Sensitivity of optical colonoscopy/sigmoidoscopy for adenomas < 1 cm | 85% | 80–95% | 29;31–36 |
| Sensitivity of optical colonoscopy/sigmoidoscopy for adenomas > 1 cm or cancer | 95% | 90–100% | 29;31–36 |
| Perforation rate—optical colonoscopy | 0.1% | 0.0–0.3% | 37–39 |
| Mortality rate—perforation | 7.5% | 5–10% | 28;40 |
| Costs | |||
| CT colonography | $559 | $100–$1,400 | 41 |
| Fecal occult-blood testing | $18 | $5–$30 | 42 |
| Flexible sigmoidoscopy | $389 | $100–$500 | 41 |
| Flexible sigmoidoscopy with biopsy and pathology | $492 | $100–$550 | 41 |
| Optical colonoscopy | $653 | $150–$1,400 | 41;43 |
| Polypectomy (including pathology) | $178 | $100–$300 | 41 |
| Cancer care | 28;44–52 | ||
| Localized | $34,300 | $20,000–$60,000 | |
| Regional | $47,230 | $30,000–$80,000 | |
| Disseminated | $41,600 | $30,000–$70,000 | |
| Cost of treating colon perforation | $22,269 | $10,000–$30,000 | 40;47 |
Natural History
The main sources of the estimates for natural history were colonoscopic screening studies and autopsy studies for the prevalence of adenomas (15–19) and surveillance, epidemiology and end-results (SEER) registry data for the incidence and mortality rates of colorectal cancer (1, 2). We intentionally used older rates of cancer incidence, primarily from the 1970s through mid-1980s, to minimize biases that might be imparted by increasing CRC screening at later dates. Mortality rates for the general population were derived from National Center for Health Statistics publications (53).
We calculated the incidence of adenomas using the age-specific adenoma prevalence observed in screening and autopsy studies (15–19). Adenomas were subdivided into two major risk groups: low-risk (less than 10 mm, without high-risk histology) and high-risk (≥10 mm, or smaller adenomas with villous features or high-grade dysplasia). Transition from low to high-risk adenoma was calibrated so that the distribution of low and high-risk adenomas matched those observed in prevalence studies. The incidence and size of hyperplastic polyps was defined in the same manner; the prevalence ranged from 20% at age 50 yr to 15% at age 80 yr (not shown in table) (15, 16).
We forced rates of adenoma to cancer conversion into the model in order to match observed national cancer incidence rates (1, 2). The proportion of incident cancers arising from adenomas was assumed to be 100%, reflecting a growing consensus that most cancers arise through the adenoma–carcinoma sequence (3, 4, 6, 54). We further assumed that it takes 10 yr for an adenoma to transform from benign to malignant. This 10-yr adenoma “dwell time” is supported by consensus opinion (55), but is based upon indirect evidence, including studies showing that screening sigmoidoscopy provides a 10-yr window of protection from colorectal cancer (56). A way to think of this estimation is that a certain proportion of adenomas, at a rate that matches 10-yr downstream cancer incidence, is tagged to become malignant, then becomes truly invasive in 10 yr.
If a colorectal malignancy developed, we assumed that it took 2 yr to progress through localized cancer and an additional year to progress through regional cancer, as defined in the SEER registry (1, 2). Patients who developed disseminated cancer were diagnosed within 1 yr, whether or not screening was employed. Mortality was varied by stage of colorectal cancer based upon published results from the SEER registry (1, 2).
Test Characteristics
The effectiveness of screening was modeled using the characteristics in Table 1. We identified 39 studies evaluating the diagnostic accuracy of CT colonography; we used data from a recent meta-analysis of these studies to provide primary estimates of diagnostic accuracy (13). CT colonography technology is changing rapidly, and includes 2- and 3-dimensional interpretation, flythrough technology (“virtual colonoscopy”), and a variety of preparatory and imaging techniques; not surprisingly, there is wide discrepancy in the reporting of diagnostic accuracy. The meta-analysis used for primary inputs reported heterogeneity of accuracy by imaging type. However, because only two studies have examined flythrough technology, we modeled the first two technologies: primarily 2-D imaging with 3-D confirmation when necessary (“2-D”), and primary 2-D and 3-D imaging (“3-D”).
The sensitivity, specificity and complication rates of traditional screening tests were derived from observational studies and in the base case were standardized to those used in the Institute of Medicine workshop on the economics of colorectal cancer screening (21–27, 29, 31–36, 47, 57). For flexible sigmoidoscopy, we assumed that a sigmoidoscope could detect 55% of all adenomas and cancers (5, 28–30).
Test adherence was assumed to be 60% in the base case, as recent estimates suggest that about 53% of the U.S. population is current with screening and this is increasing over time (10). Differential adherence between tests seems likely, but there are no studies of the adherence to different tests; we explore this in sensitivity analyses for FOBT in particular given its clear differences in burden and invasiveness. After a positive initial screening test (e.g., FOBT or sigmoidoscopy), a colonoscopy for diagnosis (and polypectomy, if necessary) is performed; adherence to this colonoscopy in the FOBT trials has been about 75%, and we used this figure in the base case (58–60). We did not model dropout from screening because of symptomatic endoscopy.
Those with a negative screening test maintained the average risk of developing new adenomas (61–63). In our base case, we assumed that all adenomas detected on CT colonography, and all adenomas (based on biopsy) detected at sigmoidoscopy are referred for optical colonoscopy. We acknowledge that there are changing perceptions on the need for routine surveillance (7, 64); however, we suspect that at present nearly all adenomas are referred for follow-up.
Those who had polypectomy at optical colonoscopy were referred for surveillance at 3 yr for high-risk adenomas, and at 5 yr for low-risk adenomas. Adenomatous polyp recurrence rates at surveillance optical colonoscopy were taken from the National Polyp Study; patients remained in the surveillance program as long as screening was ongoing (65). Adherence with adenoma surveillance was assumed to be 90% in the base case analysis; this was varied from 50–100% in sensitivity analyses.
Costs
All costs and years of life were discounted at 3%. We took the perspective of a third-party payer; we also conducted analyses from a societal perspective by including lost productivity costs in a sensitivity analyses, but estimates of lost productivity from screening tests are not particularly well defined and did not have major effects on cost-effectiveness, so we focus on the third-party payer analysis. We also briefly examined the possible effect of evaluation of extracolonic findings on CT colonography, based on a recent study (66). However, the benefits and costs of detection of these findings are unclear. In a sensitivity analysis, we assumed an incremental cost of $1,000 for diagnostic workup for each of the 6% of subjects with findings.
The costs of screening tests and interventions were taken from the 2003 Medicare reimbursement schedule (41). Because CT colonography is not yet an approved reimbursable test by Medicare or other insurers, we conducted analyses at various cost levels to help provide benchmarks for payers. In the base case, CT colonography was reimbursed as an abdominal and pelvic computed tomography scan. We included both Medicare physician fees and facility expenses in costs. Polypectomy costs included reimbursement for tissue pathology. Costs of caring for procedural complications have been estimated by Eddy (28, 40) and the OMB (44); these were inflated to 2003 U.S. dollars using the consumer price index. Costs of cancer care range widely in the literature and in prior models; in the base case we used approximate midpoint figures from various sources, including the Institute of Medicine, prior models, and three published analyses of costs (28, 44–52). We then examined a wide range in sensitivity analyses.
Screening Strategies
Because there are no current guidelines for screening with CT colonography, we examined CT colonography screening every 5 yr and every 10 yr. We chose these figures because they correspond to existing guidelines for colorectal neoplasia screening using sigmoidoscopy and optical colonoscopy (7).
Sensitivity Analyses
We tested each variable in the model in one-way sensitivity analyses; the ranges of values used for these analyses are presented in Table 1. The ranges were derived from the literature where possible; however, to make the analysis robust, we used broad ranges. The focus of the presented analyses is on assumptions that affected the incremental cost-effectiveness of CT colonography versus other tests, because the choice of optimal test is the primary subject of current debate (7). We then examined the most influential variables in two-way sensitivity analyses.
We conducted multivariable sensitivity analyses using Monte Carlo modeling, sampling from the distributions of parameter estimates, to examine the global impact of uncertainty on incremental cost-effectiveness ratios (67, 68). For continuous variables, we assumed that estimates were distributed normally; the range of the estimates outlined in Table 1 encompassed six standard deviations. This is analogous to a 99% rather than a 95% credible interval, as we used broad ranges for most variables. For cost variables, procedural costs were normally distributed; however, for treatment of cancer and complications, we assumed a log-normal distribution. We conducted 10,000 simulations to establish the distribution of the incremental cost-effectiveness ratios.
Model Calibration
Because model estimates had to be calculated in several instances, we confirmed calibration versus our original data sources for adenoma prevalence and cancer incidence and prevalence (1, 2, 15–19). We also tested the predictive validity of the model by setting up a population similar to that of the Minnesota randomized trial of fecal occult-blood testing, and found that our model predictions for colorectal cancer mortality reduction were similar and within the confidence interval of those seen in the trial (model prediction of mortality reduction = 36%, trial = 33%). This does not verify the accuracy of CT colonography screening, but serves as a more general test that the model reasonably predicts results from the only CRC screening modality that has been examined in randomized trials.
RESULTS
CT Colonography Versus No Screening
The base case analyses are presented in Table 2. The model predicts that no screening leads to a lifetime colorectal cancer risk of 5.6% and mortality of 2.1%. The average discounted life expectancy is 17.1215 yr and the average discounted cost is $1,240 per person. For 2-D imaging, CT colonography done every 10 yr reduces cancer risk to 2.7% and cancer mortality to 0.9%, and costs $17,280 per life yr saved. Done at 5-yr intervals, it reduces cancer risk to 1.6% and cancer mortality to 0.5%, and costs $14,290 per life-year gained.
Table 2.
Effectiveness and Costs of Screening
| Test | Lifetime Cancer Risk | Lifetime Cancer Mortality | Life Expectancy* | Lifetime Costs* | Cost-Effectiveness (vs No Screening) |
|---|---|---|---|---|---|
| No screening | 0.056 | 0.021 | 17.1215 | $1,240 | – |
| 2-D CT colonography every 5 yrs | 0.016 | 0.005 | 17.1738 | $1,990 | $14,290 |
| 2-D CT colonography every 10 yrs | 0.027 | 0.009 | 17.1536 | $1,800 | $17,280 |
| 3-D CT colonography every 5 yr | 0.013 | 0.004 | 17.1766 | $1,980 | $13,460 |
| 3-D CT colonography every 10 yr | 0.023 | 0.008 | 17.1655 | $1,600 | $8,150 |
| FOBT annually | 0.038 | 0.012 | 17.1504 | $1,400 | $5,360 |
| Sigmoidoscopy every 5 yrs | 0.031 | 0.012 | 17.1528 | $1,990 | $23,830 |
| FOBT annually + Sigmoidoscopy every 5 yrs | 0.022 | 0.006 | 17.1719 | $2,140 | $18,000 |
| Optical colonoscopy every 10 yrs | 0.012 | 0.004 | 17.1746 | $1,670 | $8,090 |
Life expectancy and lifetime costs discounted at 3% annually.
For 3-D imaging, CT colonography done every 10 yr reduces cancer risk to 2.3% and mortality to 0.8%; compared with no screening it has a cost-effectiveness of $8,150 per year of life gained. CT colonography done every 5 yr is more effective, decreasing cancer risk to 1.3% and mortality to 0.4%, and, compared with no screening, costs $13,460 per year of life gained.
CT Colonography Versus “Traditional” Screening
Table 2 also shows the costs and outcomes of other screening tests. We found that annual fecal occult-blood testing costs $1,400, and increases discounted life expectancy by an average of 10.5 days. Of the existing strategies, optical colonoscopy every 10 yr is the most effective test, increasing discounted life expectancy by 19.3 days at an incremental cost of $430. Compared with annual FOBT, optical colonoscopy every 10 yr costs about $11,160 per additional life-year gained.
CT colonography, depending on interval and imaging modality, varies widely in terms of costs and effectiveness relative to other strategies, though it is uniformly more effective than FOBT or FS alone. 3-D CT colonography done every 5 yr is the most effective of the strategies in Table 2, with a discounted life expectancy gain of about 20.1 days, about 0.8 days more than optical colonoscopy. 2-D CT colonography is less effective and more expensive than 3-D CT colonography (thus, 3-D imaging is “dominant” relative to 2-D imaging), and we therefore focus our incremental comparisons on 3-D imaging. These results can be seen in Table 3. Done every 5 yr, 3-D CT colonography is dominant over sigmoidoscopy-based strategies, and costs $22,400 per life-year gained compared with annual FOBT. It is relatively expensive, however, compared with optical colonoscopy, costing $156,000 per life-year gained. At 10-yr intervals, 3-D CT colonography is less effective than either optical colonoscopy or combined FOBT and sigmoidoscopy. Optical colonoscopy done every 10 yr costs only $7,840 per life-year gained compared with 3-D colonography done every 10 yr, but after exclusion of dominated strategies, optical colonoscopy is weakly dominant over 3-D CT colonography done every 10 yr.
Table 3.
3-D CT Colonography (CTC) Versus Traditional Tests
| 3-D CTC Every 5 Yr
|
3-D CTC Every 10 Yr
|
|||
|---|---|---|---|---|
| Test | More Effective Test | Incremental Cost-Effectiveness | More Effective Test | Incremental Cost-Effectiveness |
| Annual FOBT | CTC | $22,400 | CTC | $13,480 |
| Sigmoidoscopy every 5 yrs | CTC | CTC dominant | CTC | CTC dominant |
| Annual FOBT + Sigmoidoscopy every 5 yrs | CTC | CTC dominant | FOBT + sigmoidoscopy | $84,160 |
| Optical colonoscopy every 10 yr | CTC | $156,000 | Optical colonoscopy | Optical colonoscopy weakly dominant |
Sensitivity Analyses
The results of one-way sensitivity analyses are found in Table 4. All assumptions were robust for all tests compared with no screening; notably, at the higher end of cost of caring for cancer, most tests were actually cost-saving. However, our focus is on the incremental cost-effectiveness comparing various tests; we chose optical colonoscopy every 10 yr as our major point of reference. Under only extreme scenarios (e.g., very low cost for CT colonography and very high cost for optical colonoscopy) was 2-D CT colonography incrementally cost-effective; therefore, our sensitivity analyses are focused on 3-D CT colonography.
Table 4.
One-way Sensitivity Analyses: Incremental Cost-effectiveness Ratios
| Variable | Cost Per Life-Year Gained: 3-D CT Colonography (CTC) Every 5 yr* | Cost Per Life-Year Gained: 3-D CT Colonography (CTC) Every 10 Yr* |
|---|---|---|
| Base case | $156,000 | $7,840 |
| Cost of CT colonography | ||
| $200 | CTC dominant | $57,410 |
| $400 | CTC dominant | $29,750 |
| $600 | $198,140 | $2,100 |
| $800 | $401,100 | Optical colonoscopy dominant |
| $1,000 | $603,970 | Optical colonoscopy dominant |
| Cost of optical colonoscopy | ||
| $200 | $421,330 | Optical colonoscopy dominant |
| $400 | $304,120 | Optical colonoscopy dominant |
| $600 | $186,920 | Optical colonoscopy dominant |
| $800 | $69,720 | $30,450 |
| $1000 | CTC dominant | $61,160 |
| $1200 | CTC dominant | $91,870 |
| Adherence with initial screening test | ||
| 20% | $20,150 | $1,060 |
| 40% | $48,900 | $3,510 |
| 60% | $156,000 | $7,840 |
| 80% | Optical colonoscopy dominant | $13,640 |
| 100% | Optical colonoscopy dominant | $21,610 |
| Adherence with follow-up optical colonoscopy | ||
| 25% | Optical colonoscopy dominant | Optical colonoscopy dominant |
| 50% | Optical colonoscopy dominant | Optical colonoscopy dominant |
| 75% | $156,000 | $7,840 |
| 95% | $33,210 | $107,530 |
| Sensitivity of CT colonography for 1 cm adenomas | ||
| 60% | Optical colonoscopy dominant | Optical colonoscopy dominant |
| 70% | Optical colonoscopy dominant | Optical colonoscopy dominant |
| 80% | Optical colonoscopy dominant | $1,870 |
| 90% | $175,580 | $7,120 |
| 95% | $102,160 | $10,950 |
| 99% | $73,990 | $14,690 |
Bold print means that CT colonography is the more effective test; italics that optical colonoscopy every 10 yr is the more effective test. Dollar figures are incremental cost per life-year gained of the more versus less effective test. All costs and life expectancy discounted at 3%.
For 3-D CT colonography every 10 yr, only one variable affected conclusions: the rate of adherence with follow-up colonoscopy in those with adenomas on CT colonography. If adherence with follow-up optical colonoscopy can be increased from the base estimate of 75 to 95%, then optical colonoscopy remains more effective, but costs an incremental $107,530 per life-year gained compared with CT colonography every 10 yr.
In contrast, the incremental cost-effectiveness of 3-D CT colonography every 5 yr was highly sensitive to a number of assumptions. The cost of screening tests were the most critical; for example, if the cost of CT colonography is reduced from the base case estimate (about $560) down to $400, then CT colonography every 5 yr is a dominant strategy; it costs less than $50,000 per life-year gained at a test cost of about $450, and less than $100,000 per life-year gained at a test cost of $500. The cost of optical colonoscopy is also important; CT colonography every 5 yr is a dominant strategy at an optical colonoscopy cost of about $950, costs less than $50,000 per life-year gained if optical colonoscopy costs $820, and less than $100,000 per life-year gained if optical colonoscopy costs $750. Variation in other costs, such as cancer care or the workup of extracolonic findings, had little impact on incremental cost-effectiveness ratios.
Test characteristics and adherence were also very important. If the sensitivity of 3-D CT colonography for 1 cm adenomas is below 83% (as it is with 2-D imaging), then optical colonoscopy every 10 yr is a dominant strategy over CT colonography. Alternatively, if sensitivity is as high as 99%, as reported in some flythrough studies, the cost-effectiveness of CT colonography is about $75,220 per life-year saved. Adherence had an impact both in evaluating initial screening and with follow-up testing after CT colonography. For example, if adherence with initial screening is 20%, then CT colonography costs about $20,150 per life-year gained relative to optical colonoscopy; however, if adherence is 80% or higher, then optical colonoscopy dominates. Not surprisingly, modeling differential adherence between tests had large effects on incremental cost-effectiveness. We explored this with FOBT, given its clear distinction from other screening tests. If FOBT has 100% adherence, while 3-D CT colonography every 5 yr has 30% adherence, then FOBT is a dominant strategy; however, at 60% FOBT adherence and 30% CT adherence, CT colonography is more effective and costs about $23,000 per life-year gained, similar to our base case findings. These effects are nonlinear because of frequency of testing, as we found in prior analyses (14).
An uncertain issue in natural history estimates is the dwell time of adenomas. Shorter dwell times improve the cost-effectiveness of 5-yr CT colonography relative to optical colonoscopy every 10 yr. For example, with a 5-yr dwell time, 3-D CT colonography costs an incremental $138,000 per life-year gained relative to optical colonoscopy, compared with $156,000 in the base case.
Two-way sensitivity analyses show that the relative cost of CT colonography to optical colonoscopy is the key factor in establishing the incremental cost-effectiveness of screening with CT colonography (Fig. 2). 3-D CT colonography every 5 yr is a dominant strategy if optical colonoscopy costs more than 1.6 times as much as CT colonography, while it is incrementally cost-effective at the $50,000 per life-year gained threshold at a ratio of about 1.5, and at $100,000 per life-year gained at a ratio of about 1.3. However, for 3-D CT colonography every 10 yr, optical colonoscopy every 10 yr is dominant under most circumstances; only at fairly extreme cost ratios (about 3.5 for $50,000 per life-year gained, or 5.5 for $100,000 per life-year gained) is optical colonoscopy at the threshold of being not cost-effective.
Figure 2.
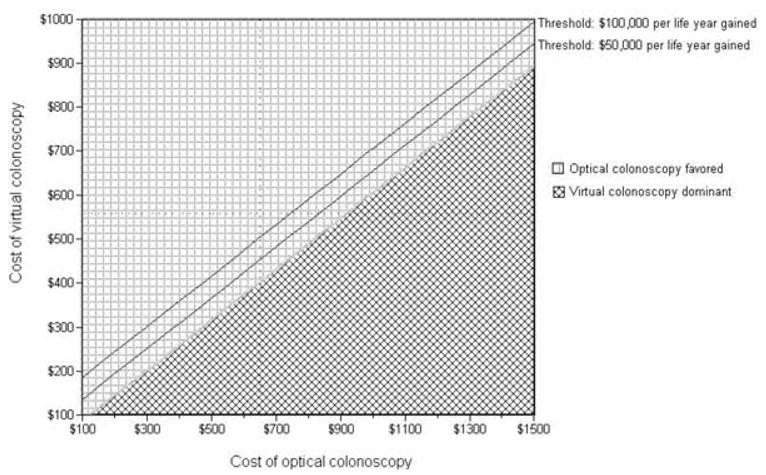
Two-way sensitivity analysis: Cost of virtual versus optical colonoscopy. The graph represents the incremental cost-effectiveness of 3-D CT colonography every 5 yr versus optical colonoscopy every 10 yr. 3-D CT colonography is more effective than optical colonoscopy. The diamond-checked area shows a region where CT colonography is less expensive (and thus dominant, being more effective and less expensive) than optical colonoscopy. The square-checked area shows a region where CT colonography costs more than optical colonoscopy; the solid lines show two commonly cited thresholds of cost-effectiveness ($50,000 and $100,000 per life-year gained). The costs used in the base case analysis are represented by the dotted lines (incremental cost-effectiveness ratio = $156,000 per life-year gained).
We also conducted multivariate sensitivity analyses using Monte Carlo simulation with sampling from the ranges of the distributions of variables (Table 5). Optical colonoscopy every 10 yr dominated CT colonography every 5 yr in 17.0% of simulations, while CT colonography dominated in 10.9% of simulations. In the simulations without dominance, the incremental cost-effectiveness ratios were for 3-D CT colonography every 5 yr compared with optical colonoscopy every 10 yr were highly skewed; the median was $137,345 (IQ range $67,112–$292,347). The acceptability at various levels of willingness to pay can also be seen in Table 5; 3-D CT colonography done every 5 yr cost less than $100,000 per life-year gained in about 38% of simulations compared with optical colonoscopy.
Table 5.
Multivariate Sensitivity Analyses: Incremental Cost-effectiveness of CT Colonography Every 5 yr Versus Optical Colonoscopy Every 10 yr
| Statistic | Proportion of Simulations |
|---|---|
| Optical colonoscopy dominant | 17.0% |
| CT colonography dominant | 10.9% |
| Median incremental cost-effectiveness ratio (IQ range)* | $137,345 ($67,112–$292,347) |
| Proportion of simulations where CT colonography is acceptable at willingness to pay of: | |
| $20,000 per life-year gained | 6.4% |
| $40,000 per life-year gained | 14.0% |
| $60,000 per life-year gained | 22.2% |
| $80,000 per life-year gained | 30.2% |
| $100,000 per-life year gained | 37.9% |
Excludes simulations with a dominant strategy.
DISCUSSION
We found that, compared with no screening, either 2-D or 3-D CT colonography is an effective and cost-effective test. Indeed, screening with 3-D CT colonography every 5 yr is more effective than any of our other modeled tests. Screening with 3-D CT colonography every 10 yr costs about $8,150 per life-year gained, and screening every 5 yr costs about $13,460 per life-year gained. Although there is no firmly established threshold for cost-effectiveness, these estimates are both comparable to those of generally accepted medical interventions (69, 70). One other recently published analysis reported similar findings for CT colonography every 10 yr; however, they did not examine the possibility of other intervals for testing, an important consideration given the non-invasive nature of CT colonography, and its status as a first screening step, analogous to sigmoidoscopy (71).
While these findings support the argument that CT colonography be added to our armamentarium, the critical question is how CT colonography fares in comparison to current screening standards, particularly the gold standard of optical colonoscopy. Under base case assumptions, and indeed under most modeled conditions, 2-D CT colonography, and 3-D CT colonography every 10 yr, are not cost-effective relative to optical colonoscopy every 10 yr. There were only two exceptions to this rule. The first is if the costs of CT colonography are substantially (at least three to four times) lower than those of optical colonoscopy. The second is if nearly all of those with adenomas found on CT have a follow-up optical colonoscopy and polypectomy. However, in randomized trials of screening, follow-up happens in about 75% of cases (58–60). Exceeding this would require dedicated resources; an intriguing option, now done at some centers, is same-day optical colonoscopy after CT colonography. Indeed, given the difficulty in bowel preparation for these procedures, such “all-in-one” type screening visits may be the preferable option to optimize adherence and the only way to ensure the cost-effectiveness of CT colonography.
We found that 3-D CT colonography done every 5 yr is the most effective of our primary modeled tests. However, in the base case, it costs $156,000 per life-year gained compared with optical colonoscopy every 10 yr, a figure that is considered expensive relative to most medical interventions (69, 70). Our sensitivity analyses suggest that there is substantial uncertainty around this incremental cost-effectiveness ratio. CT colonography every 5 yr is a dominant strategy if CT colonography costs less than $400 or if optical colonoscopy costs more than $1,000. Similarly, CT colonography every 5 yr is a dominant strategy if the ratio of the cost of optical colonoscopy to CT colonography is greater than 1.6; a ratio of 1.5 leads to an incremental cost-effectiveness ratio for CT colonography of less than $50,000 per life-year gained, and a ratio of 1.3 to an incremental cost-effectiveness ratio of less than $100,000 per life-year gained. Our figures can help to guide a reasonable reimbursement for CT colonography.
Perhaps most important from a clinical perspective is that the incremental cost-effectiveness of 3-D CT colonography done every 5 yr is highly sensitive to test accuracy, particularly test sensitivity for 1 cm adenomas. If test sensitivity is lower than 83%, then CT colonography is almost never a preferred strategy versus optical colonoscopy. There is little or no data on the diagnostic accuracy or reliability of primary 3-D interpretation of CT colonography in non-academic settings. Our analyses suggest that even with primary 3-D interpretation, CT colonography has a fairly small margin of error for adenoma sensitivity; thus, it is critical that issues of training and utilization of optimal technology be considered before CT colonography is disseminated (72). Lower sensitivity levels, such as those found in trials with primary 2-D interpretation, essentially rule out CT colonography as a cost-effective option compared with optical colonoscopy (13, 73, 74).
Another important issue is that of adherence. In our base analyses, we have only compared adherence under the assumption that it is comparable between tests. However, our analyses examining how differential adherence between FOBT and CT colonography impact cost-effectiveness are revealing; FOBT may be dominant if it is highly adhered to and CT colonography is not. We would note that for patients that would refuse screening other than CT colonography, nearly any application of CT colonography is cost-effective, as shown by the cost-effectiveness compared with no screening.
Our study has several limitations. There are no trials showing mortality reduction with CT colonography, and as with most models based on the natural history of a disease process, we relied on multiple data sources, which may bias results. We also have little data to guide us on reimbursement rates; indeed, we would view this analysis as a tool to help establish those rates. Further, there are several issues that are difficult to model accurately. One of these relates to extracolonic findings on CT colonography; the costs and benefits of these findings are completely uncertain. Another issue is the possibility of increased cancer risk related to radiation exposure. Recent analyses have suggested that these risks are very small for colonic imaging in a screening age population (75). There are also resource implications to any shift in screening that may occur; we and others have shown that one limitation of colonoscopic screening is a lack of endoscopist supply (76–78), and CT colonography may help to ameliorate this. This is the reason that we did not examine using more frequent (e.g., every 5 yr) colonoscopy in our scenarios; additionally, guidelines at present endorse 10 yr intervals for optical colonoscopy (7). On the other hand, demands would be placed on radiologists and CT scanners, and it is uncertain whether increased demand for CT colonography can be met given current staffing models, although outsourcing of radiology interpretation may make this a more feasible alternative than increasing endoscopist supply.
Overall, our findings suggest that CT colonography is an effective test, particularly when done with primary 3-D interpretation at 5-yr intervals. Compared with no screening, it is cost-effective, and from this perspective we would argue that it belongs in our screening armamentarium. However, under our base case assumptions, screening with CT colonography is not likely to be cost-effective relative to screening with optical colonoscopy. There are conditions, particularly those related to the relative costs of optical and CT colonography, under which 3-D CT colonography done every 5 yr is a cost-effective or even a dominant strategy. On the other hand, the lower diagnostic test accuracy that is likely to be seen in general practice compared with academic settings, particularly early in the deployment of a new technique, may substantially decrease the effectiveness of CT colonography compared with optical colonoscopy (72–74). CT colonography as a primary screening test should be implemented cautiously, with careful attention to ensuring accurate interpretation in non-academic settings, and a reimbursement rate that is significantly less than that of optical colonoscopy.
STUDY HIGHLIGHTS
What Is Current Knowledge
Computerized tomography (CT) colonography is a technology for colorectal cancer screening.
The costs and benefits of computerized tomography colonography are uncertain.
What Is New Here
CT colonography is expensive compared with colonoscopy.
CT colonography can be cost-effective if low-priced and accurate.
Three-dimensional imaging at 5-yr intervals is necessary for cost-effectiveness.
Acknowledgments
The study was supported in part by NCI R01 CA106782. Dr. Vijan was supported by a Veterans Affairs Advanced Career Development Award during part of the study. The funding agencies played no role in the design, conduct, or reporting of this study.
Footnotes
CONFLICT OF INTEREST
Dr. Perry J. Pickhardt is a consultant for Viatronix, Medic-sight, and Fleet.
References
- 1.Parker SL, Tong T, Bolden S, et al. Cancer statistics, 1996. CA Cancer J Clin. 1996;46:5–27. doi: 10.3322/canjclin.46.1.5. [DOI] [PubMed] [Google Scholar]
- 2.Ries LAG, Kosary CL, Hankey BF, et al. SEER Cancer Statistics Review, 1973–1996. 99. Bethesda, MD: National Cancer Institute; [Google Scholar]
- 3.Morson BC. The evolution of colorectal carcinoma. Clin Radiol. 1984;35:425–31. doi: 10.1016/s0009-9260(84)80033-1. [DOI] [PubMed] [Google Scholar]
- 4.Muto T, Bussey HJR, Morson BC. The evolution of cancer of the colon and rectum. Cancer. 1975;36:2251–70. doi: 10.1002/cncr.2820360944. [DOI] [PubMed] [Google Scholar]
- 5.Atkin WS, Morson BC, Cuzick J. Long-term risk of colorectal cancer after excision of rectosigmoid adenomas. N Engl J Med. 1992;326:658–62. doi: 10.1056/NEJM199203053261002. [DOI] [PubMed] [Google Scholar]
- 6.Winawer SJ, Zauber AG, Ho MN, et al. Prevention of colorectal cancer by colonoscopic polypectomy. N Engl J Med. 1993;329:1977–81. doi: 10.1056/NEJM199312303292701. [DOI] [PubMed] [Google Scholar]
- 7.Winawer S, Fletcher R, Rex D, et al. Colorectal cancer screening and surveillance: Clinical guidelines and rationale—Update based on new evidence. Gastroenterology. 2003;124:544–60. doi: 10.1053/gast.2003.50044. [DOI] [PubMed] [Google Scholar]
- 8.Cram P, Inadomi J, Cowen ME, et al. The impact of a healthy celebrity spokesperson on preventive health behavior: The Katie couric effect. Arch Int Med. 2003;163:1601–5. doi: 10.1001/archinte.163.13.1601. [DOI] [PubMed] [Google Scholar]
- 9.Vernon SW. Participation in colorectal cancer screening: A review. J Natl Cancer Inst. 1997;89:1406–22. doi: 10.1093/jnci/89.19.1406. [DOI] [PubMed] [Google Scholar]
- 10.Seeff L, Nadel M, Blackman D, Pollack LA. Colorectal cancer test use among persons aged 50 years and older–United States, 2001. MMWR. 2003;52:193–6. [PubMed] [Google Scholar]
- 11.Hynam KA, Hart AR, Gay SP, et al. Screening for colorectal cancer: reasons for refusal of faecal occult blood testing in a general practice in England. J Epidemiol Community Health. 1995;49:84–6. doi: 10.1136/jech.49.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harewood GC, Wiersema MJ, Melton LJ., 3rd A prospective, controlled assessment of factors influencing acceptance of screening colonoscopy. Am J Gastroenterol. 2002;97:3186–94. doi: 10.1111/j.1572-0241.2002.07129.x. [DOI] [PubMed] [Google Scholar]
- 13.Mulhall BP, Veerappan GR, Jackson JL. Meta-analysis: Computed tomographic colonography. Ann Intern Med. 2005;142:635–50. doi: 10.7326/0003-4819-142-8-200504190-00013. [DOI] [PubMed] [Google Scholar]
- 14.Vijan S, Hwang EW, Hofer TP, Hayward RA. Which colon cancer screening test? A comparison of costs, effectiveness, and compliance. Am J Med. 2001;111:593–601. doi: 10.1016/s0002-9343(01)00977-9. [DOI] [PubMed] [Google Scholar]
- 15.Johnson DA, Gurney MS, Volpe RJ, et al. A prospective study of the prevalence of colonic neoplasms in asymptomatic patients with an age-related risk. Am J Gastroenterol. 1990;85:969–74. [PubMed] [Google Scholar]
- 16.DiSario JA, Foutch PG, Mai HD, et al. Prevalence and malignant potential of colorectal polyps in asymptomatic, average-risk men. Am J Gastroenterol. 1991;86:941–45. [PubMed] [Google Scholar]
- 17.Rickert RR, Auerback O, Garfinkel L, et al. Adenomatous lesions of the large bowel: An autopsy survey. Cancer. 1979;43:1847–57. doi: 10.1002/1097-0142(197905)43:5<1847::aid-cncr2820430538>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 18.Blatt LJ. Polyps of the colon and rectum: Incidence and distribution. Dis Colon Rectum. 1961;4:277–82. [Google Scholar]
- 19.Arminski TC, McLean W. Incidence and distribution of adenomatous polyps of the colon and rectum based on 1,000 autopsy examinations. Dis Colon Rectum. 1964;7:249–61. doi: 10.1007/BF02630528. [DOI] [PubMed] [Google Scholar]
- 20.Myers M, Ries LAG. Cancer patient survival rates: SEER program results for 10 years of follow-up. CA Cancer J Clin. 1989;39:21–32. doi: 10.3322/canjclin.39.1.21. [DOI] [PubMed] [Google Scholar]
- 21.Ahlquist DA, Wieand HS, Moertell CG, et al. Accuracy of fecal occult blood screening for colorectal neoplasia. JAMA. 1993;269:1262–67. [PubMed] [Google Scholar]
- 22.Mandel JS, Bond JH, Bradley M, et al. Sensitivity, specificity, and positive predictivity of the hemoccult test in screening for colorectal cancers. Gastroenterology. 1989;97:597–600. doi: 10.1016/0016-5085(89)90629-x. [DOI] [PubMed] [Google Scholar]
- 23.Macrae FA, St John DJB. Relationship between patterns of bleeding with hemoccult sensitivity in patients with colorectal cancers or adenomas. Gastroenterology. 1982;82:891–8. [PubMed] [Google Scholar]
- 24.Crowley ML, Feeman LD, Mottet MD, et al. Sensitivity of guaiac-impregnated cards for the detection of colorectal neoplasia. J Clin Gastroenterol. 1983;5:127–30. doi: 10.1097/00004836-198304000-00007. [DOI] [PubMed] [Google Scholar]
- 25.Rex DK, Lehman GA, Hawes RH, et al. Screening colonoscopy in asymptomatic average-risk persons with negative fecal occult blood tests. Gastroenterology. 1991;100:64–7. doi: 10.1016/0016-5085(91)90583-7. [DOI] [PubMed] [Google Scholar]
- 26.Allison JE, Feldman R, Tekawa IS. Hemoccult screening in detecting colorectal neoplasm: Sensitivity, specificity, and predictive value. Ann Intern Med. 1990;112:328–33. doi: 10.7326/0003-4819-112-5-328. [DOI] [PubMed] [Google Scholar]
- 27.Niv Y, Sperber AD. Sensitivity, specificity, and predictive value of fecal occult blood testing (hemoccult II) for colorectal neoplasia in symptomatic patients: a prospective study with total colonoscopy. Am J Gastroenterol. 1995;90:1974–7. [PubMed] [Google Scholar]
- 28.Eddy DM. Screening for colorectal cancer. Ann Intern Med. 1990;113:373–84. doi: 10.7326/0003-4819-113-5-373. [DOI] [PubMed] [Google Scholar]
- 29.Foutch PG, Mai H, Pardy K, et al. Flexible sigmoidoscopy may be ineffective for secondary prevention of colorectal cancer in asymptomatic, average-risk men. Dig Dis Sci. 1991;36:924–8. doi: 10.1007/BF01297142. [DOI] [PubMed] [Google Scholar]
- 30.Lieberman DA, Smith FW. Screening for colon malignancy with colonoscopy. Am J Gastroenterol. 1991;86:946–51. [PubMed] [Google Scholar]
- 31.Rex DK, Cutler CS, Lemmel GT, et al. Colonoscopic miss rates of adenomas determined by back-to-back colonoscopies. Gastroenterology. 1997;112:24–8. doi: 10.1016/s0016-5085(97)70214-2. [DOI] [PubMed] [Google Scholar]
- 32.Hixson LJ, Fennerty MB, Sampliner RE, et al. Prospective blinded trial of the colonoscopic miss-rate of large colorectal polyps. Gastrointest Endosc. 1991;37:125–7. doi: 10.1016/s0016-5107(91)70668-8. [DOI] [PubMed] [Google Scholar]
- 33.Rex DK. Colonoscopy. a review of its yield for cancers and adenomas by indication. Am J Gastroenterol. 1995;90:353–65. [PubMed] [Google Scholar]
- 34.Castiglione G, Mazzotta A, Grazzini G. Sensitivity of screening sigmoidoscopy for proximal colorectal tumours (letter) Lancet. 1995;345:726–7. doi: 10.1016/s0140-6736(95)90899-4. [DOI] [PubMed] [Google Scholar]
- 35.Zarchy TM, Ershoff D. Do characteristics of adenomas on flexible sigmoidoscopy predict advanced lesions on baseline colonoscopy? Gastroenterology. 1994;106:1501–4. doi: 10.1016/0016-5085(94)90403-0. [DOI] [PubMed] [Google Scholar]
- 36.Achkar E, Carey W. Small polyps found during fiberoptic sigmoidoscopy in asymptomatic patients. Ann Intern Med. 1988;109:880–3. doi: 10.7326/0003-4819-109-11-880. [DOI] [PubMed] [Google Scholar]
- 37.Habr-Gama A, Waye JD. Complications and hazards of gastrointestinal endoscopy. World J Surg. 1989;13:193–201. doi: 10.1007/BF01658399. [DOI] [PubMed] [Google Scholar]
- 38.Macrae FA, Tan KG, Williams CB. Towards safer colonoscopy: A report on the complications of 5000 diagnostic or therapeutic colonoscopies. Gut. 1983;24:376–83. doi: 10.1136/gut.24.5.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Waye JD, Lewis BS, Yessayan S. Colonoscopy: A prospective report of complications. J Clin Gastroenterol. 1992;15:347–51. [PubMed] [Google Scholar]
- 40.Eddy DM, Nugent FW, Eddy JF, et al. Screening for colorectal cancer in a high-risk population. Gastroenterology. 1987;92:682–92. doi: 10.1016/0016-5085(87)90018-7. [DOI] [PubMed] [Google Scholar]
- 41.Department of Health and Human Services, Centers for Medicare and Medicaid Services. Medicare Program: Physician Fee Schedule Update for Calendar Year 2003. Federal Register. 2003;68:9567–80. [PubMed] [Google Scholar]
- 42.Department of Health and Human Services HCFA. Medicare program; revisions to payment policies and five-year review of and adjustments to the relative value units under the physician fee schedule for calendar year 2000 and physician volume performance standard rates of increase for federal fiscal year 1999, notice. Federal Register. 1999;64:59379–428. [Google Scholar]
- 43.Rogge JD, Elmore MF, Mahoney SJ, et al. Low-cost, office-based, screening colonoscopy. Am J Gastroenterol. 1994;89:1775–80. [PubMed] [Google Scholar]
- 44.Wagner JL, Tunis S, Brown M, et al. Cost-effectiveness of colorectal cancer screening in average-risk adults. In: Young GP, Rozen P, Levin B, editors. Prevention and early detection of colorectal cancer. Philadelphia: WB Saunders; 1996. pp. 321–41. [Google Scholar]
- 45.Fireman BH, Quesenberry CP, Somkin CP, et al. Cost of care for cancer in a health maintenance organization. Helath Care Financing Rev. 1997;18:51–76. [PMC free article] [PubMed] [Google Scholar]
- 46.Baker MS, Kessler LG, Smucker RC. Site-Specific Treatment Costs for Cancer: An Analysis of the Medicare Continuous History Sample File. In: Scheffler RM, Andrews NC, editors. Cancer Care and Costs: DRGs and Beyond. Ann Arbor, Michigan: Health Administration Press; 1989. pp. 127–38. [Google Scholar]
- 47.Pignone M, Russell L, Wagner J. Economic models of colorectal cancer screening in average risk adults. Washington, DC: The National Academies Press.; 2005. [PubMed] [Google Scholar]
- 48.Taplin SH, Barlow W, Urban N, et al. Stage, age, comorbidity, and direct costs of colon, prostate, and breast cancer care. J Natl Cancer Inst. 1995;87:417–26. doi: 10.1093/jnci/87.6.417. [DOI] [PubMed] [Google Scholar]
- 49.Frazier AL, Colditz GA, Fuchs CS, et al. Cost-effectiveness of screening for colorectal cancer in the general population. JAMA. 2000;284:1954–61. doi: 10.1001/jama.284.15.1954. [DOI] [PubMed] [Google Scholar]
- 50.Loeve F, Brown ML, Boer R, et al. Endoscopic colorectal cancer screening: a cost-saving analysis. J Natl Cancer Inst. 2000;92:557–63. doi: 10.1093/jnci/92.7.557. [DOI] [PubMed] [Google Scholar]
- 51.Khandker RK, Dulski JD, Kilpatrick JB, et al. A decision model and cost-effectiveness analysis of colorectal cancer screening and surveillance guidelines for average-risk adults. Int J Technol Assess Health Care. 2000;16:799–810. doi: 10.1017/s0266462300102077. [DOI] [PubMed] [Google Scholar]
- 52.Sonnenberg A, Delco F, Inadomi JM. Cost-effectiveness of colonoscopy in screening for colorectal cancer. Ann Intern Med. 2000;133:573–84. doi: 10.7326/0003-4819-133-8-200010170-00007. [DOI] [PubMed] [Google Scholar]
- 53.National Center for Health Statistics. Vital statistics of the United States, 1991, vol II, mortality, part B. Washington, DC: Public Health Service; 1995. [Google Scholar]
- 54.Eide TJ. Remnants of adenomas in colorectal carcinomas. Cancer. 1983;51:1866–72. doi: 10.1002/1097-0142(19830515)51:10<1866::aid-cncr2820511019>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 55.Winawer SJ, Fletcher RH, Miller L, et al. Colorectal cancer screening: clinical guidelines and rationale. Gastroenterology. 1997;112:594–642. doi: 10.1053/gast.1997.v112.agast970594. [DOI] [PubMed] [Google Scholar]
- 56.Selby JV, Friedman GD, Quesenberry CP, et al. A case-control study of screening sigmoidoscopy and mortality from colorectal cancer. N Engl J Med. 1992;326:653–57. doi: 10.1056/NEJM199203053261001. [DOI] [PubMed] [Google Scholar]
- 57.Cauffman JG, Hara JH, Rasgon IM, et al. Flexible sigmoidoscopy in asymptomatic patients with negative fecal occult blood tests. J Fam Pract. 1992;34:281–6. [PubMed] [Google Scholar]
- 58.Mandel JS, Bond JH, Church TR, et al. Reducing mortality from colorectal cancer by screening for fecal occult blood. N Engl J Med. 1993;328:1365–71. doi: 10.1056/NEJM199305133281901. [DOI] [PubMed] [Google Scholar]
- 59.Hardcastle JD, Chamberlain JO, Robinson MHE, et al. Randomised controlled trial of faecal-occult-blood screening for colorectal cancer. Lancet. 1996;348:1472–7. doi: 10.1016/S0140-6736(96)03386-7. [DOI] [PubMed] [Google Scholar]
- 60.Kronborg O, Fenger C, Olsen J, et al. Randomised study of screening for colorectal cancer with faecal-occult-blood test. Lancet. 1996;348:1467–71. doi: 10.1016/S0140-6736(96)03430-7. [DOI] [PubMed] [Google Scholar]
- 61.Squillace S, Berggreen P, Jaffe P, et al. A normal initial colonoscopy after age 50 does not predict a polyp-free status for life. Am J Gastroenterol. 1994;89:1156–9. [PubMed] [Google Scholar]
- 62.Rex DK, Cummings OW, Helper DJ, et al. 5-year incidence of adenomas after negative colonoscopy in asymptomatic average-risk persons. Gastroenterology. 1996;111:1178–81. doi: 10.1053/gast.1996.v111.pm8898630. [DOI] [PubMed] [Google Scholar]
- 63.Rex DK, Lehman GA, Ulbright TM, et al. The yield of a second screening flexible sigmoidoscopy in average- risk persons after one negative examination. Gastroenterology. 1994;106:593–5. doi: 10.1016/0016-5085(94)90690-4. [DOI] [PubMed] [Google Scholar]
- 64.Noshirwani KC, van Stolk RU, Rybicki LA, et al. Adenoma size and number are predictive of adenoma recurrence: Implications for surveillance endoscopy. Gastrointest Endosc. 2000;51:433–7. doi: 10.1016/s0016-5107(00)70444-5. [DOI] [PubMed] [Google Scholar]
- 65.Winawer SJ, Zauber AG, O’Brien MJ, et al. Randomized comparison of surveillance intervals after colonoscopic removal of newly diagnosed adenomatous polyps. N Engl J Med. 1993;328:901–5. doi: 10.1056/NEJM199304013281301. [DOI] [PubMed] [Google Scholar]
- 66.Pickhardt PJ, Choi JR, Hwang I, et al. Computed tomographic virtual colonoscopy to screen for colorectal neoplasia in asymptomatic adults. N Engl J Med. 2003;349:2191–200. doi: 10.1056/NEJMoa031618. [DOI] [PubMed] [Google Scholar]
- 67.Critchfield GC, Willard KE. Probabilistic analysis of decision trees using Monte Carlo simulation. Med Decis Making. 1986;6:85–92. doi: 10.1177/0272989X8600600205. [DOI] [PubMed] [Google Scholar]
- 68.Manning WG, Fryback DG, Weinstein MC. Reflecting uncertainty in Cost-effectiveness analysis. In: Gold MR, Siegel JE, Russell LB, Weinstein MC, editors. Cost-effectiveness in health and medicine. New York: Oxford University Press; 1996. pp. 247–75. [Google Scholar]
- 69.Laupacis A, Feeny D, Detsky AS, et al. How attractive does a new technology have to be to warrant adoption and utilization? Tentative guidelines for using clinical and economic evaluations. CMAJ. 1992;146:473–81. [PMC free article] [PubMed] [Google Scholar]
- 70.Hirth RA, Chernew ME, Miller E, et al. Willingness to pay for a quality-adjusted life year: in search of a standard. Med Decis Making. 2000;20:332–42. doi: 10.1177/0272989X0002000310. [DOI] [PubMed] [Google Scholar]
- 71.Ladabaum U, Song K, Fendrick AM. Colorectal neoplasia screening with virtual colonoscopy: when, at what cost, and with what national impact? Gastroenterol Hepatol. 2004;2:554–63. doi: 10.1016/s1542-3565(04)00247-2. [DOI] [PubMed] [Google Scholar]
- 72.Ransohoff DF. Virtual colonoscopy–What it can do vs. what it will do. JAMA. 2004;291:1772–4. doi: 10.1001/jama.291.14.1772. [DOI] [PubMed] [Google Scholar]
- 73.Cotton PB, Durkalski VL, Pineau BC, et al. Computed tomographic colonography (virtual colonoscopy): A multicenter comparison with standard colonoscopy for detection of colorectal neoplasia. JAMA. 2004;291:1713–9. doi: 10.1001/jama.291.14.1713. [DOI] [PubMed] [Google Scholar]
- 74.Rockey DC, Paulson E, Niedzwiecki D, et al. Analysis of air contrast barium enema, computed tomographic colonography, and colonoscopy: prospective comparison. Lancet. 2005;365:305–11. doi: 10.1016/S0140-6736(05)17784-8. [DOI] [PubMed] [Google Scholar]
- 75.Brenner DJ, Elliston CD. Estimated radiation risks potentially associated with full-body CT screening. Radiology. 2004;232:735–8. doi: 10.1148/radiol.2323031095. [DOI] [PubMed] [Google Scholar]
- 76.Vijan S, Inadomi J, Hayward RA, et al. Projections of demand and capacity for colonoscopy related to increasing rates of colorectal cancer screening in the United States. Aliment Pharmacol Ther. 2004;20:507–15. doi: 10.1111/j.1365-2036.2004.01960.x. [DOI] [PubMed] [Google Scholar]
- 77.Kolata G. 50 and ready for Colonoscopy? doctors say wait is often long. 1. A. New York, NY: New York Times; 2003. p. 1. [Google Scholar]
- 78.Brown ML, Klabunde CN, Mysliwiec P. Current capacity for endoscopic colorectal cancer screening in the United States: Data from the National Cancer Institute survey of colorectal cancer screening practices. Am J Med. 2003;115:129–33. doi: 10.1016/s0002-9343(03)00297-3. [DOI] [PubMed] [Google Scholar]


