Abstract
The masseter muscle participates in a wide variety of activities including mastication, swallowing and speech. The functional demands for accurate mandibular positioning and generation of forces during incising or a power stroke require a diverse set of forces that are determined by the innate muscle form. The complex internal tendon architecture subdivides the masseter into multiple partitions that can be further subdivided into neuromuscular compartments representing small motor unit territories. Individual masseter compartments have unique biomechanical properties that, when activated individually or in groups, can generate a wide range of sagittal and off-sagittal torques about the temporomandibular joint. The myosin heavy chain (MyHC) fiber-type distribution in the adult masseter is sexually dimorphic and is influenced by hormones such as testosterone. These testosterone-dependent changes cause a phenotype switch from slower to faster fiber-types in the male. The development of the complex organization of the masseter muscle, the MyHC fiber-type message and protein expression, and the formation of endplates appear to be pre-programmed and not under control of the muscle nerve. However, secondary myotube generation and endplate maturation are nerve dependent. The delayed development of the masseter muscle compared to the facial, tongue and jaw opening muscles may be related to the delayed functional requirements for chewing. In summary, masseter muscle form is pre-programmed prior to birth while muscle fiber contractile characteristics are refined postnatally in response to functional requirements. The motor control mechanisms that are required to coordinate the activation of discrete functional elements of this muscle remain to be determined.
Keywords: masseter, masticatory muscle, tongue, compartments, development, myosin, endplate
Introduction
The masseter muscle, one of four muscles of mastication, participates in a wide variety of activities including mastication, swallowing and speech. This diversity of function requires coordination of motor output elements of masticatory muscles (i.e., compartments) along with appropriate activation of tongue, facial and oropharyngeal muscles. Mastication involves diverse and accurate mandibular movements to incise and grind food suitable for swallowing. Accurate mandibular positioning in the medial-lateral or anterior-posterior positions combined with the generation of high forces during a protruded jaw position for incision or a lateral to medial power stroke requires the ability to activate distinct combinations of muscle compartments to accomplish the required task. This overview will focus on one jaw closing masticatory muscle, the masseter, and the behavior of this muscle along with other masticatory muscles to produce a variety of torques about the temporomandibular joint. In addition, the temporospatial development of the form and function of the masseter muscle will be examined relative to other jaw and tongue muscles.
Masseter Muscle Architecture and Function
The masseter muscle has been viewed for a long time as a complex muscle that is composed of three layers: superficial, intermediate and deep1. The functional elements of the masseter anatomy have been examined in an attempt to understand their fundamental properties. The complex internal tendon architecture subdivides the muscle into multiple partitions2–4 that can be further subdivided into neuromuscular compartments representing small motor unit territories within each anatomical partition5,6. The individual masseter compartments have unique biomechanical properties that can generate a wide range of sagittal and off-sagittal torques about the temporomandibular joint7. In studies of regional masseter muscle activation during unrestricted mastication or during cortical stimulation to induce rhythmic jaw muscle activation, it has been shown that the individual compartments can be uniquely activated depending on the task, but more commonly are activated in discrete groups with other jaw muscles2,8. The independent activation of masseter compartments is more apparent during production of off-sagittal torques about the TMJ compared to mid-sagittal, high level torques. The motor control of these output elements has been examined at the level of the organization of the motoneurons, yet no distinct clustering of motoneurons representing each compartment has been observed9. Generation of complex behaviors requires coordination of discrete functional elements of multiple muscles on both sides of the jaw. This ability may be dependent on the functional organization of pre-motoneurons into movement control modules located in the brainstem that can be differentially sequenced and summed (see review by Mussa-Ivaldi and Bizzi10). However, this hypothesis remains to be tested for the masticatory muscle system.
Masseter Muscle Sexual Dimorphism
The myosin heavy chain (MyHC) fiber type distribution in the adult rabbit masseter generally does not vary among the different compartments (except for the faster contracting fibers in the deep compartment) but does differ between sexes11. A consistent finding of sex differences across species for the masseter muscle has been reported with a higher proportion of fibers with faster MyHC isoforms found in the adult male compared to the adult female11–13. In the rabbit, the male masseter has approximately 80% of the fibers containing MyHC type IIa phenotype. In contrast, the female masseter is composed of approximately 50% MyHC IIa and is similar to young adult rabbits (2 months old) where no sexual dimorphism of the MyHC protein can be observed14. In the adult mouse, an increased proportion of MyHC IIb isoform is found in the male mouse masseter while the female has a higher proportion of IIa4,13 and these isoforms are regionally distributed. These masseter muscle sex differences can be observed early postnatally (days 1, 8) in mice with females having higher levels of developmental and α-cardiac MyHC message expression compared to males15. In the early adult at postnatal day (pn) 28, the female mouse masseter is characterized by a slightly increased expression of α-cardiac and IIa isoform message, whereas the male masseter has an increased IIx and IIb expression. This differential expression of faster MyHC isoform message is consistent with hormonal changes observed during maturation. The male masseter MyHC fiber type is influenced by testosterone causing a proportional phenotype transition from slower to faster fiber-types13,16. The functional significance of faster contracting muscle fibers in males is unclear but may be based on the need for high force generation by jaw muscles for survival during food gathering and defense mechanisms.
Masseter Muscle Development
The development of the complex organization of the masseter muscle has been recently studied to further refine our understanding of this intricate and multifaceted process. Muscle-nerve interactions have a predominant role in postnatal muscle maturation and plasticity, but the role of these interactions in masticatory muscle development is unknown. All jaw muscles are derived from the segmentation of a single muscle mass at gestational day (gd) 11. The partitioning of this mass into specific jaw muscles and compartments is concomitant with the branching of the trigeminal nerve in intact embryos17. In our studies of the role of the muscle nerve during development, we evaluate an embryo mouse model lacking sensory and motor innervation. Aneural embryos are generated by the injection of a 1μl solution (0.5μg/μl) of β-bungarotoxin into the amnionic sac at gd12 and compared to a littermate that receives a control injection of PBS. At gd18, patterning of different jaw muscles and their anatomical layers is not found to be dependent on muscle-nerve interactions but appears to be pre-programmed18. These findings support the concept that the complexity of the masseter muscle architecture is established prior to birth. However, the muscle volume of different jaw muscles in aneural embryos is smaller and may be related to the limited ability to generate secondary myotubes in the absence of innervation.
Message for all MyHC isoforms (embryonic, neonatal, slow, α-cardiac, IIa, IIx, IIb) is detected within the mouse masseter at gd13/14 with adult isoforms co-expressed with developmental isoforms19–21. Embryonic and neonatal isoforms increase in expression with developmental age and then decrease postnatally22,23. These developmental MyHC isoforms have a prolonged postnatal expression in the masseter compared to limb and tongue muscles and are gradually replaced by the adult isoforms in the mouse21. Interestingly, the neonatal MyHC isoform has been found to be co-expressed with slow or fast isoforms in a significant proportion of adult masseter fibers in rabbit and humans24–26. The expression of slow MyHC isoforms, observed throughout the masseter at gd14, is also gradually replaced postnatally by fast isoforms27. MyHC IIb has an early and transient appearance at gd14, but is a prominent isoform in the masseter just prior to birth. In contrast, IIa expression showed a steady decline during prenatal development to where it was barely detected at birth. Expression of IIa is again detected only after several days postnatally22,23. MyHC fiber type message and protein expression in the embryo appear not to be influenced by the absence of the muscle nerve. No differences are observed in the distribution of MyHC isoforms within the masseter between aneural and intact embryos suggesting that the patterning of fiber types is not nerve dependent20. The preprogrammed expression of masseter MyHC isoforms during development and early postnatally becomes influenced by extrinsic influences such as the muscle nerve and hormonal regulation as the functional needs for mastication are required.
Neuromuscular junction development within the masseter muscle has been shown to be influenced by the muscle nerve and differs temporally compared to digastric muscle and tongue. In the masseter, end-plates form in the appropriate location in the absence of the muscle nerve but maturation of the synapse organization is nerve dependent28. In aneural fetuses, the observed endplates are characterized by diffuse α-BTX staining and a lack of associated synaptic carbohydrates. Synaptic carbohydrates such as N-acetylgalactosamine are located only at synapses in the adult endplate and are indicative of a mature synapse29. Interestingly, in normal development, maturation of endplates within different regions of the masseter is shown not to be uniform and may be an indicator of functional partitioning. Endplate formation and maturation is advanced in tongue and hyoid muscles compared to jaw closers by approximately one gestational day (fig. 1). The delayed development of the masseter muscle compared to the tongue and jaw opening muscles may be related to the delayed functional requirements for chewing.
Figure 1.
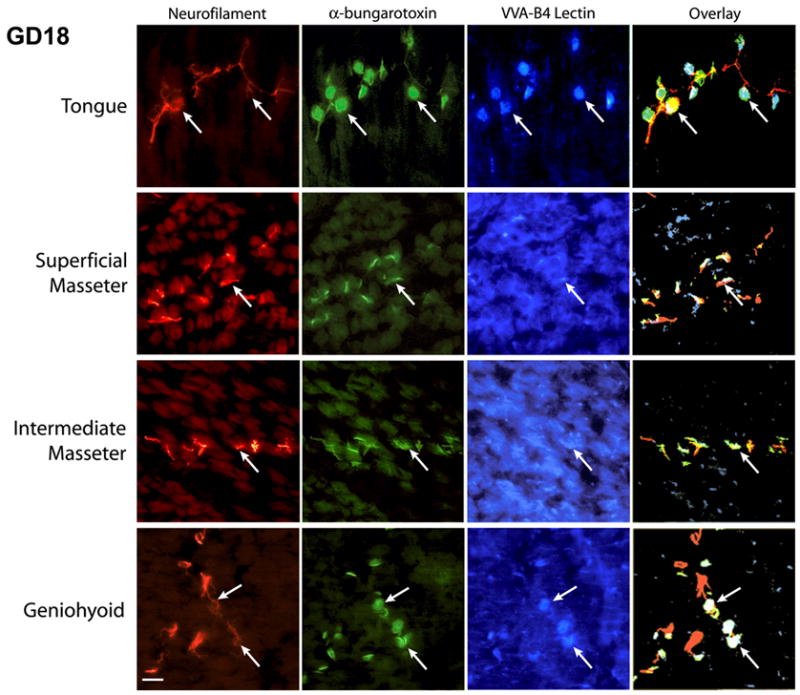
The neuromuscular endplate development in the masseter is delayed compared to development in the tongue and geniohyoid muscles. Frontal cryosections (14 μm thickness) of gestational day 18 mice were immuno/lectin-labeled for nerve (NF, neurofilament), endplate (α-BTX, α-bungarotoxin) and synaptic carbohydrates (VVA, Vicia villosa agglutinin isolectin B4). It can be observed that muscle nerve efferents have reached their target muscle fibers in all the muscles (neurofilament label). Arrows indicate selected endplates for comparison. Well-defined, mature endplates are seen in tongue and geniohyoid muscles while modest endplate development is observed in the superficial and intermediate masseter. VVA-binding specifically at synapses, an indicator of endplate maturation, is distinct in tongue and geniohyoid muscles. However, in masseter, synaptic carbohydrates are found to be diffusely distributed on the muscle fibers. The overlays of immuno/lectin-labeled images further demonstrate the co-localization of VVA-binding at the endplate in tongue and geniohyoid muscle. The scale bar represents 50 μm.
In summary, masticatory muscle form is pre-programmed prior to birth while muscle fiber contractile characteristics are refined postnatally in response to functional requirements. However, the motor control mechanisms required to coordinate the activation of discrete functional elements of these muscles remain to be determined.
Acknowledgments
Supported by NIH/NIDCR DE00333, DE10130, DE12207 and DE11536.
Reference List
- 1.Schumacher GH. Funktionelle morphologie der kaumuskulatur. Jena: VEB Gustav Fischer; 1961. [Google Scholar]
- 2.Weijs WA, Dantuma R. Functional anatomy of the masticatory apparatus in the rabbit. Neth J Zool. 1981;31:99–147. [Google Scholar]
- 3.Widmer CG, Klugman D, English AW. Anatomical partitioning and nerve branching patterns in the adult rabbit masseter. Acta Anat (Basel) 1997;159:222–232. doi: 10.1159/000147988. [DOI] [PubMed] [Google Scholar]
- 4.Widmer CG, Morris-Wiman JA, Nekula C. Spatial distribution of myosin heavy-chain isoforms in mouse masseter. J Dent Res. 2002;81:33–38. doi: 10.1177/002203450208100108. [DOI] [PubMed] [Google Scholar]
- 5.Weijs WA, Juch PJ, Kwa SH, Korfage JA. Motor unit territories and fiber types in rabbit masseter muscle. J Dent Res. 1993;72:1491–1498. doi: 10.1177/00220345930720110601. [DOI] [PubMed] [Google Scholar]
- 6.McMillan AS, Hannam AG. Motor-unit territory in the human masseter muscle. Arch Oral Biol. 1991;36:435–441. doi: 10.1016/0003-9969(91)90134-g. [DOI] [PubMed] [Google Scholar]
- 7.English AW, Carrasco DI, Widmer CG. Torques produced by different compartments of the rabbit masseter muscle. J Appl Biomech. 1999;15:348–360. [Google Scholar]
- 8.Widmer CG, Carrasco DI, English AW. Differential activation of neuromuscular compartments in the rabbit masseter muscle during different oral behaviors. Exp Brain Res. 2003;150:297–307. doi: 10.1007/s00221-003-1464-y. [DOI] [PubMed] [Google Scholar]
- 9.Saad M, Dubuc R, Widmer CG, Westberg KG, Lund JP. Anatomical organization of efferent neurons innervating various regions of the rabbit masseter muscle. J Comp Neurol. 1997;383:428–438. [PubMed] [Google Scholar]
- 10.Mussa-Ivaldi FA, Bizzi E. Motor learning through the combination of primitives. Philos Trans R Soc Lond B Biol Sci. 2000;355:1755–1769. doi: 10.1098/rstb.2000.0733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.English AW, Eason J, Schwartz G, Shirley A, Carrasco DI. Sexual dimorphism in the rabbit masseter muscle: myosin heavy chain composition of neuromuscular compartments. Cells Tissues Organs. 1999;164:179–191. doi: 10.1159/000016658. [DOI] [PubMed] [Google Scholar]
- 12.Maxwell LC, Carlson DS, McNamara JA, Jr, Faulkner JA. Histochemical characteristics of the masseter and temporalis muscles of the rhesus monkey (Macaca mulatta) Anat Rec. 1979;193:389–402. doi: 10.1002/ar.1091930306. [DOI] [PubMed] [Google Scholar]
- 13.Eason JM, Schwartz GA, Pavlath GK, English AW. Sexually dimorphic expression of myosin heavy chains in the adult mouse masseter. J Appl Physiol. 2000;89:251–258. doi: 10.1152/jappl.2000.89.1.251. [DOI] [PubMed] [Google Scholar]
- 14.Eason JM, Schwartz G, Shirley KA, English AW. Investigation of sexual dimorphism in the rabbit masseter muscle showing different effects of androgen deprivation in adult and young adult animals. Arch Oral Biol. 2000;45:683–690. doi: 10.1016/s0003-9969(00)00030-3. [DOI] [PubMed] [Google Scholar]
- 15.Morris-Wiman J, Basco E, Widmer CG. Temporal patterns of myosin heavy chain message in mouse masseter during development and maturation. Soc Neurosci. 1999;25:2010. (abstract) [Google Scholar]
- 16.Reader M, Schwartz G, English AW. Brief exposure to testosterone is sufficient to induce sex differences in the rabbit masseter muscle. Cells Tissues Organs. 2001;169:210–217. doi: 10.1159/000047884. [DOI] [PubMed] [Google Scholar]
- 17.Melzer J, Morris-Wiman J, Widmer CG. Temporospatial patterning of mouse jaw muscles during development. J Dent Res. 2000;79:499. (abstract) [Google Scholar]
- 18.Morris-Wiman JA, Widmer CG. Early nerve-muscle interactions influence jaw muscle architecture. J Dent Res. 2001;80:130. (abstract) [Google Scholar]
- 19.Soussi-Yanicostas N, Barbet JP, Laurent-Winter C, Barton P, Butler-Browne GS. Transition of myosin isozymes during development of human masseter muscle. Persistence of developmental isoforms during postnatal stage. Development. 1990;108:239–249. doi: 10.1242/dev.108.2.239. [DOI] [PubMed] [Google Scholar]
- 20.Morris-Wiman J, Widmer CG. Myosin heavy chain (MyHC) isoform expression in early mouse masseter development. J Dent Res. 2003;82:1770. doi: 10.1177/002203450208100108. (abstract) [DOI] [PubMed] [Google Scholar]
- 21.Agbulut O, Noirez P, Beaumont F, Butler-Browne G. Myosin heavy chain isoforms in postnatal muscle development of mice. Biol Cell. 2003;95:399–406. doi: 10.1016/s0248-4900(03)00087-x. [DOI] [PubMed] [Google Scholar]
- 22.Weydert A, Barton P, Harris AJ, Pinset C, Buckingham M. Developmental pattern of mouse skeletal myosin heavy chain gene transcripts in vivo and in vitro. Cell. 1987;49:121–129. doi: 10.1016/0092-8674(87)90762-8. [DOI] [PubMed] [Google Scholar]
- 23.Lu BD, Allen DL, Leinwand LA, Lyons GE. Spatial and temporal changes in myosin heavy chain gene expression in skeletal muscle development. Dev Biol. 1999;216:312–326. doi: 10.1006/dbio.1999.9488. [DOI] [PubMed] [Google Scholar]
- 24.Bredman JJ, Weijs WA, Korfage HAM, Brugman P, Moorman AFM. Myosin heavy chain expression in rabbit masseter muscle during postnatal development. J Anat. 1992;180:263–274. [PMC free article] [PubMed] [Google Scholar]
- 25.Monemi M, Eriksson P-O, Dubail I, Butler-Browne GS, Thornell LE. Fetal myosin heavy chain increases in the human masseter muscle during aging. FEBS Letters. 1996;386:87–90. doi: 10.1016/0014-5793(96)00402-4. [DOI] [PubMed] [Google Scholar]
- 26.Monemi M, Eriksson PO, Kadi F, Butler-Browne GS, Thornell LE. Opposite changes in myosin heavy chain composition of human masseter and biceps brachii muscles during aging. J Muscle Res Cell Motil. 1999;20:351–361. doi: 10.1023/a:1005421604314. [DOI] [PubMed] [Google Scholar]
- 27.Gojo K, Abe S, Ide Y. Characteristics of myofibres in the masseter muscle of mice during postnatal growth period. Anat Histol Embryol. 2002;31:105–112. doi: 10.1046/j.1439-0264.2002.00368.x. [DOI] [PubMed] [Google Scholar]
- 28.Widmer CG, Morris-Wiman J. Do early nerve-muscle interactions specify innervation patterns in jaw muscle? J Dent Res. 2004;83:2570. (abstract) [Google Scholar]
- 29.Martin PT. Glycobiology of the neuromuscular junction. J Neurocytol. 2003;32:915–929. doi: 10.1023/B:NEUR.0000020632.41508.83. [DOI] [PubMed] [Google Scholar]


