Abstract
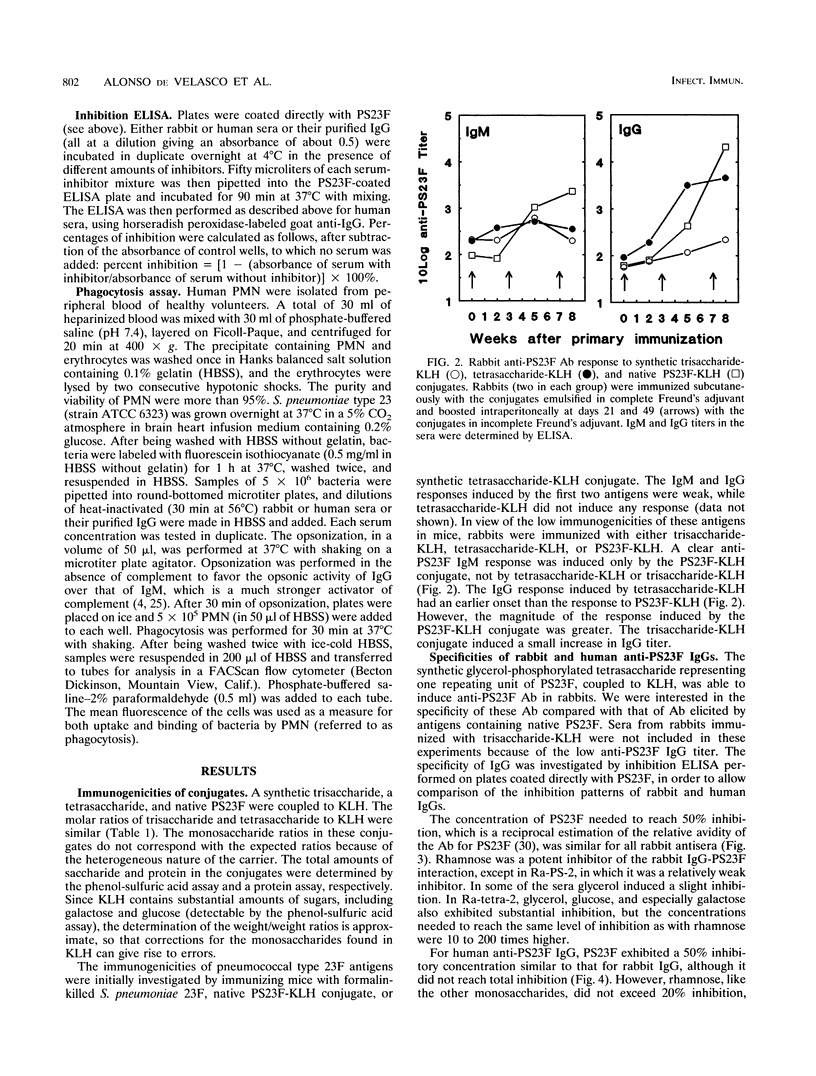
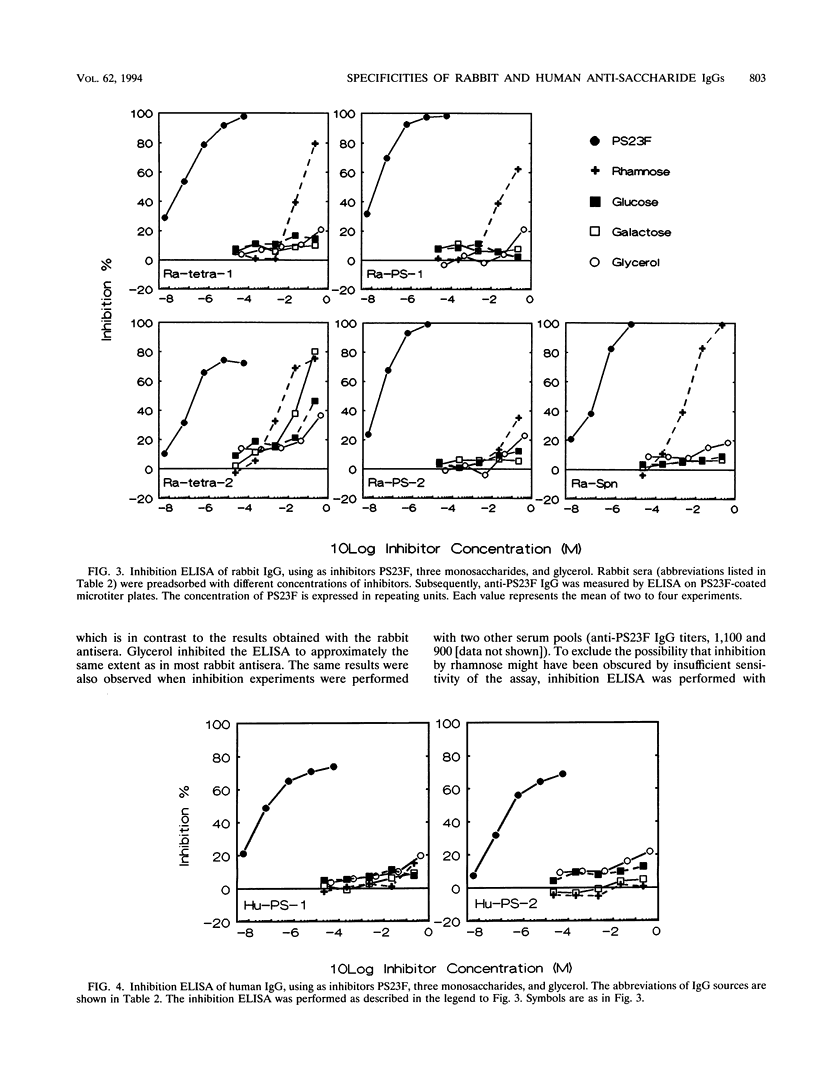
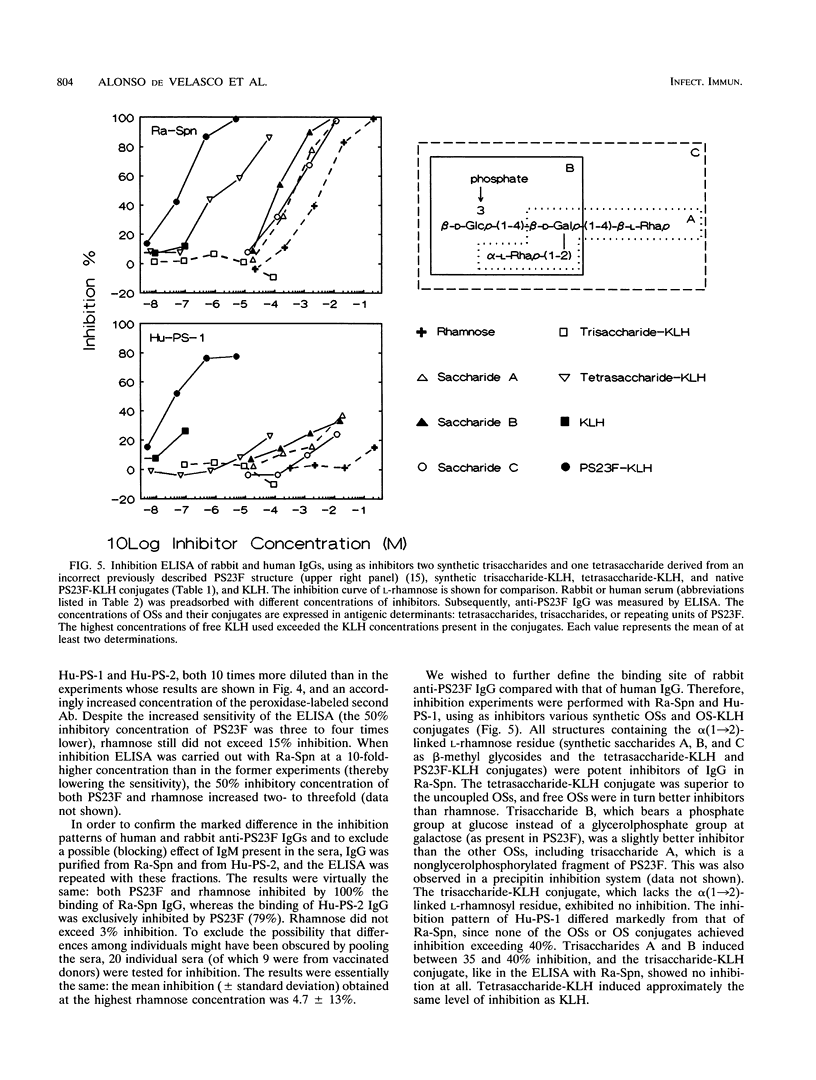
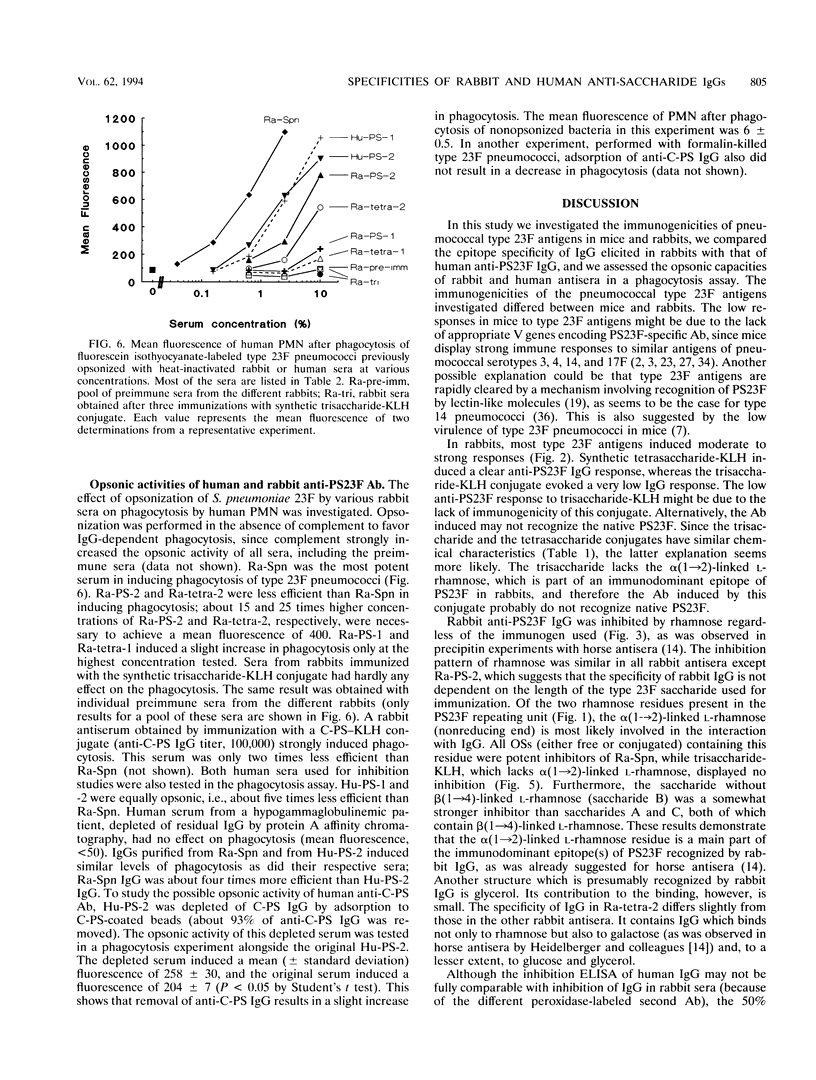
Streptococcus pneumoniae type 23F capsular polysaccharide (PS23F) consitss of a repeating glycerol-phosphorylated branched tetrasaccharide. The immunogenicities of the following related antigens were investigated: (i) a synthetic trisaccharide comprising the backbone of one repeating unit, (ii) a synthetic tetrasaccharide comprising the complete repeating unit, and (iii) native PS23F (all three conjugated to keyhole limpet hemocyanin [KLH]) and (iv) formalin-killed S. pneumoniae 23F. All antigens except the trisaccharide-KLH conjugate induced relatively high anti-PS23F antibody levels in rabbits. The epitope specificity of such antibodies was then studied by means of an inhibition immunoassay. The alpha(1-->2)-linked L-rhamnose branch was shown to be immunodominant for immunoglobulin G (IgG) induced by tetrasaccharide-KLH, PS23F-KLH, and killed S. pneumoniae 23F: in most sera L-rhamnose totally inhibited the binding of IgG to PS23F. Thus, there appears to be no major difference in epitope specificity between IgG induced by tetrasaccharide-KLH and that induced by antigens containing the polymeric form of PS23F. Human anti-PS23F IgG (either vaccine induced or naturally acquired) had a different epitope specificity: none of the inhibitors used, including L-rhamnose and tetrasaccharide-KLH, exhibited substantial inhibition. These observations suggest that the epitope recognized by human IgG on PS23F is larger than the epitope recognized by rabbit IgG. Both human and rabbit antisera efficiently opsonized type 23F pneumococci, as measured in a phagocytosis assay using human polymorphonuclear leukocytes.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alonso de Velasco E., Verheul A. F., Veeneman G. H., Gomes L. J., van Boom J. H., Verhoef J., Snippe H. Protein-conjugated synthetic di- and trisaccharides of pneumococcal type 17F exhibit a different immunogenicity and antigenicity than tetrasaccharide. Vaccine. 1993 Nov;11(14):1429–1436. doi: 10.1016/0264-410x(93)90172-t. [DOI] [PubMed] [Google Scholar]
- Amir J., Scott M. G., Nahm M. H., Granoff D. M. Bactericidal and opsonic activity of IgG1 and IgG2 anticapsular antibodies to Haemophilus influenzae type b. J Infect Dis. 1990 Jul;162(1):163–171. doi: 10.1093/infdis/162.1.163. [DOI] [PubMed] [Google Scholar]
- Bernatowicz M. S., Matsueda G. R. Preparation of peptide-protein immunogens using N-succinimidyl bromoacetate as a heterobifunctional crosslinking reagent. Anal Biochem. 1986 May 15;155(1):95–102. doi: 10.1016/0003-2697(86)90231-9. [DOI] [PubMed] [Google Scholar]
- Briles D. E., Crain M. J., Gray B. M., Forman C., Yother J. Strong association between capsular type and virulence for mice among human isolates of Streptococcus pneumoniae. Infect Immun. 1992 Jan;60(1):111–116. doi: 10.1128/iai.60.1.111-116.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chudwin D. S., Artrip S. G., Korenblit A., Schiffman G., Rao S. Correlation of serum opsonins with in vitro phagocytosis of Streptococcus pneumoniae. Infect Immun. 1985 Oct;50(1):213–217. doi: 10.1128/iai.50.1.213-217.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray B. M., Converse G. M., 3rd, Dillon H. C., Jr Serotypes of Streptococcus pneumoniae causing disease. J Infect Dis. 1979 Dec;140(6):979–983. doi: 10.1093/infdis/140.6.979. [DOI] [PubMed] [Google Scholar]
- Heidelberger M., Davie J. M., Krause R. M. Cross-reactions of the group-specific polysaccharides of streptococcal groups B and G in anti-pneumococcal sera with especial reference to type 23 and its determinants. J Immunol. 1967 Oct;99(4):794–796. [PubMed] [Google Scholar]
- Heidelberger M. Precipitating cross-reactions among pneumococcal types. Infect Immun. 1983 Sep;41(3):1234–1244. doi: 10.1128/iai.41.3.1234-1244.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones C. Identification of the tetrasaccharide repeating-unit of the Streptococcus pneumoniae type 23 polysaccharide by high-field proton n.m.r. spectroscopy. Carbohydr Res. 1985 Jun 15;139:75–83. doi: 10.1016/0008-6215(85)90008-4. [DOI] [PubMed] [Google Scholar]
- Jorgenson J. H., Howell A. W., Maher L. A., Facklam R. R. Serotypes of respiratory isolates of Streptococcus pneumoniae compared with the capsular types included in the current pneumococcal vaccine. J Infect Dis. 1991 Mar;163(3):644–646. doi: 10.1093/infdis/163.3.644. [DOI] [PubMed] [Google Scholar]
- Kabat E. A., Liao J., Osserman E. F., Gamian A., Michon F., Jennings H. J. The epitope associated with the binding of the capsular polysaccharide of the group B meningococcus and of Escherichia coli K1 to a human monoclonal macroglobulin, IgMNOV. J Exp Med. 1988 Aug 1;168(2):699–711. doi: 10.1084/jem.168.2.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamerling J. P., Gerwig G. J., Vliegenthart J. F., Clamp J. R. Characterization by gas-liquid chromatography-mass spectrometry and proton-magnetic-resonance spectroscopy of pertrimethylsilyl methyl glycosides obtained in the methanolysis of glycoproteins and glycopeptides. Biochem J. 1975 Dec;151(3):491–495. doi: 10.1042/bj1510491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klerx J. P., Molendijk A. J., Van Dijk H., Vloet K. P., Willers J. M. Simple sugars with affinity for the macrophage asialoglycoprotein receptor are adjuvants for the humoral immune response to neuraminidase-treated sheep erythrocytes. J Immunol. 1986 Jan;136(1):73–75. [PubMed] [Google Scholar]
- Lai E., Kabat E. A. Immunochemical studies of conjugates of isomaltosyl oligosaccharides to lipid: production and characterization of mouse hybridoma antibodies specific for stearyl-isomaltosyl oligosaccharides. Mol Immunol. 1985 Sep;22(9):1021–1037. doi: 10.1016/0161-5890(85)90105-1. [DOI] [PubMed] [Google Scholar]
- Lee C. J., Koizumi K. Immunochemical relations between pneumococcal group 19 and Klebsiella capsular polysaccharides. J Immunol. 1981 Oct;127(4):1619–1623. [PubMed] [Google Scholar]
- Paoletti L. C., Kasper D. L., Michon F., DiFabio J., Jennings H. J., Tosteson T. D., Wessels M. R. Effects of chain length on the immunogenicity in rabbits of group B Streptococcus type III oligosaccharide-tetanus toxoid conjugates. J Clin Invest. 1992 Jan;89(1):203–209. doi: 10.1172/JCI115564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeters C. C., Tenbergen-Meekes A. M., Evenberg D. E., Poolman J. T., Zegers B. J., Rijkers G. T. A comparative study of the immunogenicity of pneumococcal type 4 polysaccharide and oligosaccharide tetanus toxoid conjugates in adult mice. J Immunol. 1991 Jun 15;146(12):4308–4314. [PubMed] [Google Scholar]
- Richards J. C., Perry M. B. Structure of the specific capsular polysaccharide of Streptococcus pneumoniae type 23F (American type 23). Biochem Cell Biol. 1988 Jul;66(7):758–771. doi: 10.1139/o88-087. [DOI] [PubMed] [Google Scholar]
- Shyur S. D., Raff H. V., Bohnsack J. F., Kelsey D. K., Hill H. R. Comparison of the opsonic and complement triggering activity of human monoclonal IgG1 and IgM antibody against group B streptococci. J Immunol. 1992 Mar 15;148(6):1879–1884. [PubMed] [Google Scholar]
- Smit P., Oberholzer D., Hayden-Smith S., Koornhof H. J., Hilleman M. R. Protective efficacy of pneumococcal polysaccharide vaccines. JAMA. 1977 Dec 12;238(24):2613–2616. [PubMed] [Google Scholar]
- Snippe H., van Houte A. J., van Dam J. E., De Reuver M. J., Jansze M., Willers J. M. Immunogenic properties in mice of hexasaccharide from the capsular polysaccharide of Streptococcus pneumoniae type 3. Infect Immun. 1983 Jun;40(3):856–861. doi: 10.1128/iai.40.3.856-861.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein K. E., Zopf D. A., Johnson B. M., Miller C. B., Paul W. E. The immune response to an isomaltohexosyl-protein conjugate, a thymus-dependent analogue of alpha(1 replaced by 6) dextran. J Immunol. 1982 Mar;128(3):1350–1354. [PubMed] [Google Scholar]
- Verheul A. F., Versteeg A. A., De Reuver M. J., Jansze M., Snippe H. Modulation of the immune response to pneumococcal type 14 capsular polysaccharide-protein conjugates by the adjuvant Quil A depends on the properties of the conjugates. Infect Immun. 1989 Apr;57(4):1078–1083. doi: 10.1128/iai.57.4.1078-1083.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verheul A. F., Versteeg A. A., Westerdaal N. A., Van Dam G. J., Jansze M., Snippe H. Measurement of the humoral immune response against Streptococcus pneumoniae type 14-derived antigens by an ELISA and ELISPOT assay based on biotin-avidin technology. J Immunol Methods. 1990 Jan 24;126(1):79–87. doi: 10.1016/0022-1759(90)90014-m. [DOI] [PubMed] [Google Scholar]
- Wessels M. R., Kasper D. L. Antibody recognition of the type 14 pneumococcal capsule. Evidence for a conformational epitope in a neutral polysaccharide. J Exp Med. 1989 Jun 1;169(6):2121–2131. doi: 10.1084/jem.169.6.2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood C., Kabat E. A. Immunochemical studies of conjugates of isomaltosyl oligosaccharides to lipid: specificities and reactivities of the antibodies formed in rabbits to stearyl-isomaltosyl oligosaccharides. Arch Biochem Biophys. 1981 Nov;212(1):262–276. doi: 10.1016/0003-9861(81)90366-0. [DOI] [PubMed] [Google Scholar]
- van Dam G. J., Verheul A. F., Zigterman G. J., de Reuver M. J., Snippe H. Estimation of the avidity of antibodies in polyclonal antisera against Streptococcus pneumoniae type 3 by inhibition ELISA. Mol Immunol. 1989 Mar;26(3):269–274. doi: 10.1016/0161-5890(89)90080-1. [DOI] [PubMed] [Google Scholar]
- van Steijn A. M., Kamerling J. P., Vliegenthart J. F. Synthesis of a spacer-containing repeating unit of the capsular polysaccharide of Streptococcus pneumoniae type 23F. Carbohydr Res. 1991 Apr 24;211(2):261–277. doi: 10.1016/0008-6215(91)80096-6. [DOI] [PubMed] [Google Scholar]



