Abstract
Angiogenesis has been demonstrated to be essential for tumor growth and metastasis, and inhibition of angiogenesis is emerging as a promising strategy for treating cancer. Among the most potent inhibitors of angiogenesis is the fumagillin family of natural products. An analog of fumagillin, known as TNP-470 or AGM-1470, has been undergoing clinical trials for treating a variety of cancers. TNP-470 has been shown to block endothelial cell cycle progression in the late G1 phase. Although the direct molecular target for TNP-470 has been identified as the type 2 methionine aminopeptidase (MetAP2), how inhibition of this enzyme leads to cell cycle arrest has remained unclear. We report that treatment of endothelial and other drug-sensitive cell types leads to the activation of the p53 pathway, causing an accumulation of the G1 cyclin-dependent kinase inhibitor p21WAF1/CIP1. The requirement of p53 and p21WAF1/CIP1 for the cell cycle inhibition by TNP-470 is underscored by the observation that cells deficient in p53 and p21WAF1/CIP1 are resistant to TNP-470. These results shed significant light on the mechanism of cell cycle inhibition by TNP-470 and suggest an alternative method of activating p53 in endothelial cells to halt angiogenesis and tumor progression.
Angiogenesis, the formation of new blood vessels from preexisting vessels, has been shown to be necessary for tumor growth and metastasis (1, 2). Endothelial cells, which form the inner lining of all blood vessels, play an essential role in angiogenesis. Endothelial cells are largely quiescent in adult mammals. In response to angiogenic factors secreted by a tumor, however, they can rapidly switch to a highly proliferative state, invade the surrounding tissues, and eventually undergo differentiation to form new blood vessels that supply the tumor with oxygen and nutrients. As a result, inhibition of endothelial cell growth can effectively block angiogenesis. In comparison with conventional chemotherapy, anti-angiogenic therapy has a number of advantages, including low toxicity, lack of drug resistance, and easy access of drugs to the targeted endothelial cells (3).
Among the most potent inhibitors of angiogenesis is the fumagillin family of natural products. The anti-angiogenic activity of fumagillin was discovered serendipitously from a fungal contamination of an endothelial cell culture (4). An analog of fumagillin, known as TNP-470 or AGM-1470, has been undergoing clinical trials for treating a variety of cancers. Ovalicin, a structurally related natural product initially identified as an immunosuppressive agent, was found to inhibit endothelial cell proliferation with potency comparable to that of TNP-470 (5). Although short serum half-life and dose-limiting side effects limit the potential of TNP-470 when given as the sole therapy, it has shown great promise when used in combination with other chemotherapeutic drugs (6). TNP-470 has been shown to inhibit endothelial cell growth with high potency both in culture and in vivo (4, 7). In contrast, the growth of cultured tumor cells is largely resistant to drug treatment, suggesting that the antitumor effects of this compound are likely to be largely mediated by inhibition of angiogenesis (8, 9). Despite the significant clinical advances made with TNP-470 as a potential therapeutic agent for cancer treatment, little was known until recently about the molecular mechanism of action of the fumagillin family of angiogenesis inhibitors.
In an effort to decipher the molecular mechanism of angiogenesis inhibition by TNP-470, we and others isolated a common target shared by fumagillin, TNP-470, and ovalicin (10, 11). This target was identified as the type 2 methionine aminopeptidase (MetAP2), one of two enzymes that catalyze the removal of the initiator methionine during protein translation (12). These inhibitors were found to bind MetAP2 covalently, leading to specific inhibition of its methionine aminopeptidase (MetAP) activity. It was shown that these inhibitors are highly specific for MetAP2, compared with its closely related isozyme MetAP1, both in vitro and in a yeast assay in vivo. Using a combination of chemical modification of fumagillin and site-directed mutagenesis, we identified and confirmed the covalent modification of histidine-231 of human MetAP2 by the ring epoxide group on fumagillin (13). This result is in agreement with the identification of the equivalent histidine in bacterial MetAP (14) and a high-resolution crystal structure of the human MetAP2-fumagillin complex (15). Several lines of evidence strongly support the notion that MetAP2 is a physiologically relevant target for TNP-470. First, by using a series of analogs of fumagillin and ovalicin, a strong correlation has been found between inhibition of MetAP2 enzymatic activity and inhibition of endothelial cell proliferation (10, 16). Second, by using a biotin-fumagillin conjugate as a probe, a correlation was observed between the drug concentrations required to inhibit endothelial cell proliferation and the concentrations needed to inactivate the endogenous MetAP2 (17). Third, down-regulation of MetAP2 expression by an antisense MetAP2 oligonucleotide was shown to specifically inhibit the proliferation of endothelial cells, as well as other drug-sensitive cell types [Wang, J., Quan, N. & Henkin, J. (1998) Proc. Am. Assoc. Cancer Res. 39, 98 (abstr.)]. Finally, the structural information from the MetAP2 crystal structure has now been used to rationally design fumagillin analogs with dramatically increased potency in cell culture (19). Thus, inhibition of MetAP2 is likely to underlie endothelial cell inhibition by TNP-470 at the molecular level.
Although MetAP2 serves as the direct molecular target for TNP-470, it is unknown how inhibition of this enzyme affects endothelial cell growth. It has been reported that TNP-470 blocks the endothelial cell cycle at the G1 phase (9, 20–22), and this arrest was suggested to involve the inhibition of accumulation of hyperphosphorylated form of the retinoblastoma gene product (pRB) (21). The effects of the compounds on cell cycle events preceding pRB phosphorylation have remained somewhat controversial. Using human umbilical vein endothelial cells (HUVECs), we have found that TNP-470 blocks S-phase entry and that this cell cycle blockage is characterized by the hypophosphorylation of pRB, likely because of a dramatic inhibition of cyclin E-dependent kinase activity. We further demonstrate that the inhibition of cyclin-dependent kinase (CDK) activity is caused by up-regulation of the CDK inhibitor p21WAF1/CIP1 (hereafter p21), which in turn is activated by p53. The essential role of p53 in mediating the action of TNP-470 was underscored by the use of p53-null mouse cells, which were nearly completely resistant to TNP-470. Thus, the p53 pathway appears to be specifically activated by TNP-470 in drug-sensitive cell types, including endothelial cells.
Materials and Methods
Cell Cultures.
HUVECs were obtained from Clonetics (San Diego) and cultured in EGM-2 medium. HUVECs were synchronized in the G0–G1 phase by contact inhibition of the confluent monolayers. Wild-type and knockout mouse embryo fibroblasts (MEFs) were prepared from day 13.5 mouse embryos as previously described (23) and maintained in DMEM containing 10% FBS and 50 units/ml penicillin plus 50 μg/ml streptomycin (P/S). NIH 3T3 cells were obtained from the American Type Culture Collection (ATCC) and cultured in DMEM (10% FBS plus P/S). HUVECs were used in the second and third passage, and MEFs were used before passage 7. All cells were grown in a humidified incubator at 37°C in an atmosphere of 5% CO2.
Western Blot.
Western blotting was carried out as previously described (17).
In Vitro Kinase Assays.
HUVECs were treated and harvested as described above. Lysates (200 μg) were precleared with protein A/G beads and incubated with 2 μg of anti-cyclin E Ab (Santa Cruz Biotechnology) at 4°C for 2 h followed by a 1-h incubation with 40 μl of protein A/G agarose beads. The precipitates were pelleted by centrifugation, washed three times with lysis buffer, and washed once with kinase buffer [50 mM Hepes (pH 7.4)/10 mM MgCl2/1 mM DTT/10 μM ATP). Samples were resuspended in 25 μl of kinase buffer containing [γ-32P]ATP (3,000 Ci/mmol, 10 μCi) and 2 μg of histone H1 (Boehringer Mannheim). Mixtures were incubated at 30°C for 30 min and boiled in SDS/PAGE loading buffer. Samples were resolved by 12% SDS/PAGE, dried, and analyzed by autoradiography.
Reverse Transcription-PCR (RT-PCR).
Total RNA was isolated from HUVECs by using Tri Reagent (Molecular Research Center, Cincinnati) as per the manufacturer's instructions. RT-PCR reactions were performed by using the Titan one tube RT-PCR system (Boehringer Mannheim). The following primer pairs were used: p21, 5′ primer, 5′-GATGTCCGTCAGAACCCATG-3′, p21, 3′ primer, 5′-TTAGGGCTTCCTCTTGGAGA-3′; p27, 5′ primer, 5′-ATGTCAAACGTGCGAGTGTC-3′, p27, 3′ primer, 5′-TTACGTTTGACGTCTTCTGAG-3′; p53, 5′ primer, 5′-GGGACAGCCAAGTCTGTG-3′, p53, 3′ primer, 5′-TTCCAGTGTGATGATGGT-3′. Samples were resolved on a 1.2% agarose gel and detected by ethidium bromide staining.
Cell Proliferation Assay.
Proliferating cells were seeded into a 96-well plate and grown in the presence of varying concentrations of TNP-470 or carrier solvent (0.5% ethanol) for 24 h. Cells were pulsed with [3H]thymidine (6.7 Ci/mmol, 1 μCi per well) for an additional 6 h and harvested with a semiautomated cell harvester onto glass fiber papers for scintillation counting. All assays were performed in triplicate with a given litter and are presented as the mean ± SD. Results were later confirmed by using MEFs isolated from two or more additional mouse litters.
Reporter Gene Assay.
The reporter gene assay was carried out by using a procedure slightly modified from that previously described by Geng et al. (24). NIH 3T3 cells were seeded into six-well plates and cotransfected with 3 μg of PG13py Luc (25) and 0.6 μg of cytomegalovirus-driven β-galactosidase expression plasmid by using Lipofectin reagent (GIBCO) as per manufacturer's instructions. After transfection, cells were allowed to recover in fresh DMEM (10% FBS plus P/S) for 4 h and then starved for 36 h in DMEM containing 0.1% FBS. The cells were restimulated with 10% FBS and harvested in a reporter lysis buffer (Promega) at the indicated time points. Luciferase activity assays were performed on a luminometer by using the luciferase assay system (Promega). The luciferase activities shown in Fig. 3B were normalized to β-galactosidase activity to control for variation in transfection. All assays were performed in triplicate.
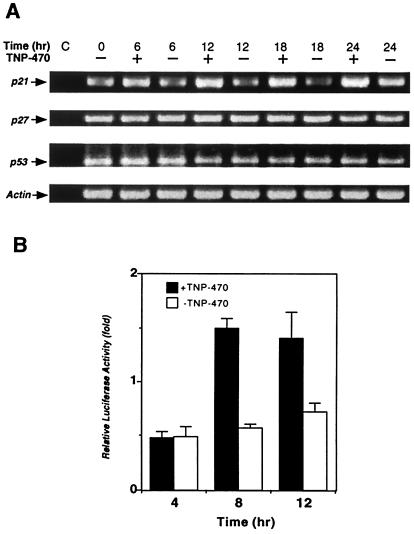
Figure 3.
TNP-470 treatment up-regulates p21 mRNA and stimulates p53 transcriptional activity. (A) RT-PCR analysis was carried out with total RNA and appropriate primers specific for p21, p27, p53, and actin. RNA samples treated with RNase were used as negative control (C). (B) NIH 3T3 cells were transiently transfected with the luciferase reporter construct pG13. Transfected cells were grown in the presence or absence of TNP-470. The relative luciferase activity is shown as the fold change from that of quiescent cells.
Isolation of Mouse Lung Endothelial Cells (MLECs).
MLECs were isolated by using a previously described procedure with slight modifications (26).
Results
To systematically characterize the effect of TNP-470 on endothelial cells, we used cultured HUVECs synchronized at G0–G1 by density arrest as a model system. Although HUVECs are not targets of anti-angiogenic action of TNP-470 in vivo, their proliferation has been shown to be potently suppressed by TNP-470 (9). The cells were then allowed to reenter the cell cycle by replating at a low density in the presence and absence of 10 nM TNP-470, a concentration well above the IC50 (50 pM) but well below cytotoxic concentrations (4). Cell cycle progression was followed by FACS analysis upon staining the chromosomal DNA with propidium iodide. Under these conditions, we observed a significant decrease in the accumulation of HUVECs in S phase upon TNP-470 treatment. TNP-470 significantly inhibited hyperphosphorylation of pRB without affecting the pRB levels (Fig. 1A), as reported previously for other systems (21). Given the effect of TNP-470 treatment on pRB phosphorylation, we then examined the effects of TNP-470 treatment on G1 CDK activity. Although we observed some minor inhibition of the cyclin D-Cdk4 complex, no inhibition of cyclin D-Cdk6 activity was detectable (data not shown). In contrast, cyclin E-dependent kinase activity was found to be dramatically inhibited after treatment with TNP-470 (Fig. 1B). Despite some minor reduction in cyclin D-Cdk4 activity, we chose to examine the cyclin E-Cdk2 complex in greater detail given its dramatic inhibition by TNP-470.
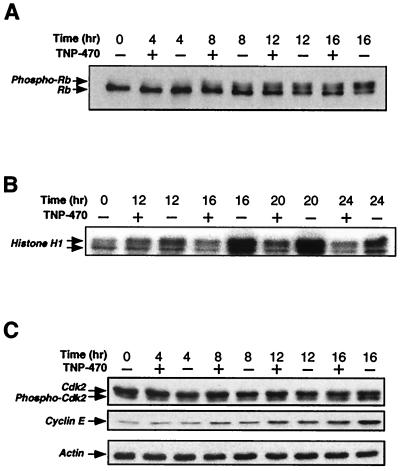
Figure 1.
Effect of TNP-470 on pRB phosphorylation, cyclin E-Cdk2 activity, and expression of cyclin E and Cdk2. HUVECs were synchronized by density arrest and replated at low density in the presence (+) or absence (−) of TNP-470 (10 nM), and harvested as described in Materials and Methods. (A) Western blot analysis of immunoprecipitated pRB. (B) In vitro kinase assay for cyclin E-dependent kinase activity. Samples were immunoprecipitated from lysates by using an anti-cyclin E Ab. Histone H1 was used as a substrate in the kinase reaction. (C) Western blot analysis of Cdk2, cyclin E, and actin expression levels.
The activity of cyclin E-Cdk2 is known to be regulated at multiple levels during the G1–S transition, including the synthesis of cyclin E, the phosphorylation state of Cdk2, and the synthesis of G1 CDK inhibitors. We determined the level of cyclin E by Western blot and found that TNP-470 treatment led to only a partial inhibition of cyclin E accumulation at later times (Fig. 1C). Because cyclin E synthesis is regulated by the RB-E2F pathway, this inhibition could be attributed to the inhibition of cyclin E-Cdk2 itself (27). Alternatively, TNP-470 may directly affect the expression of cyclin E. It remains unresolved whether this partial decrease represents a direct effect of the drug or a secondary effect of CDK inhibition because of other mechanisms. TNP-470 did not significantly affect the expression level of Cdk2, or the appearance of a faster-migrating phosphorylated form (Fig. 1C). In addition, we also determined the activity of cyclin H-Cdk7 from drug-treated extracts as well as the phosphorylation state of tyrosine-15 on Cdk2, neither of which was affected by TNP-470 (data not shown).
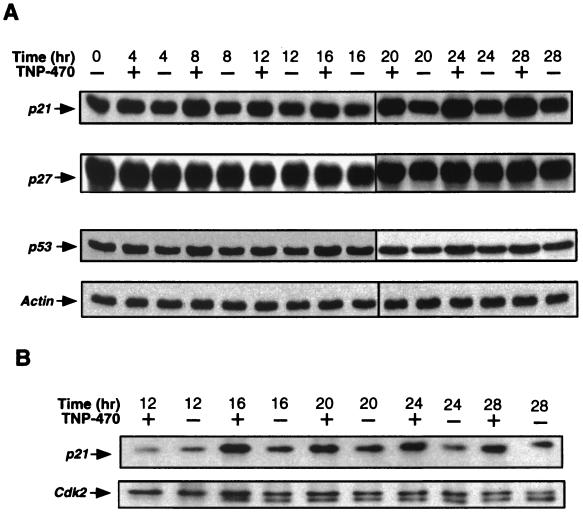
We next investigated the effect of TNP-470 treatment on the levels of several CDK inhibitors. We observed induction of p21 beginning 6–8 h after cell cycle reentry in the presence of TNP-470 (Fig. 2A). The higher levels of p21 persisted throughout the entire 28-h period examined. In contrast, there was little, if any, change in the level of p27KIP (Fig. 2A) or p57KIP2 (data not shown). To assess whether the increased expression of p21 was associated with a higher level of p21 bound to the cyclin E-Cdk2 complex, we immunoprecipitated the complex from treated HUVECs by using an anti-cyclin E Ab and determined the amount of associated p21. As shown in Fig. 2B, an increased amount of p21 was indeed found associated with cyclin E-Cdk2 upon treatment with TNP-470. These results suggest that the induction of p21 by TNP-470 affected cyclin E-Cdk2 and possibly to some extent cyclin D-Cdk4 activity.
Figure 2.
Induction of p21 and p53 by TNP-470. HUVECs were synchronized and treated as in Fig. 1. (A) HUVEC lysates were subjected to Western blot analysis to determine the expression levels of p21, p27, p53, and actin. (B) TNP-470 treatment increases the levels of p21 associated with the cyclin E-Cdk2 complex. The cyclin E-Cdk2 complex was immunoprecipitated from lysates by using an anti-cyclin E Ab, and the bound p21 and Cdk2 was detected by Western blot analysis.
p21 is known to be regulated at both transcriptional and translational levels (28). We therefore determined the level of p21 transcripts in the presence and absence of TNP-470 by using an RT-PCR assay. TNP-470 treatment was found to significantly up-regulate the level of the p21 transcript (Fig. 3A). This effect appears specific because TNP-470 treatment had no effect on the levels of the p27KIP transcript (Fig. 3A). Although it remains to be determined whether TNP-470 treatment also affects p21 at the translational level, drug treatment appears to significantly up-regulate p21 at the transcriptional level.
It has been demonstrated that p21 expression is regulated by at least two alternative mechanisms, p53-dependent and p53-independent (29). We wondered whether p53 was involved in the induction of p21 by TNP-470 treatment. When the expression level of p53 was determined, a slight, but significant, increase in p53 protein levels was observed in the presence of TNP-470 (Fig. 2A). Because p53 can serve to activate p21 transcription, this slight increase in p53 level could account for the level of p21 induction. In contrast to its protein level, the level of p53 transcript did not appear to be affected by TNP-470 (Fig. 3A), suggesting TNP-470 treatment is likely to activate p53 posttranslationally in a still undefined mechanism. To determine the effect of TNP-470 treatment on p53 transcriptional activity, we used a p53 reporter gene composed of multimerized p53 binding sites driving luciferase (25). Because HUVECs are difficult to transfect, we used NIH 3T3 cells, which have been shown to be sensitive to TNP-470, albeit to a lesser extent than HUVECs (E.C.G. and J.O.L., unpublished data). Upon transfection of the p53 reporter gene, NIH 3T3 cells were incubated in the presence or absence of TNP-470. TNP-470 treatment was found to activate the p53 reporter gene at both 8 and 12 h post restimulation (Fig. 3B). These results further suggested that induction of p21 was likely a consequence of p53 activation upon TNP-470 treatment.
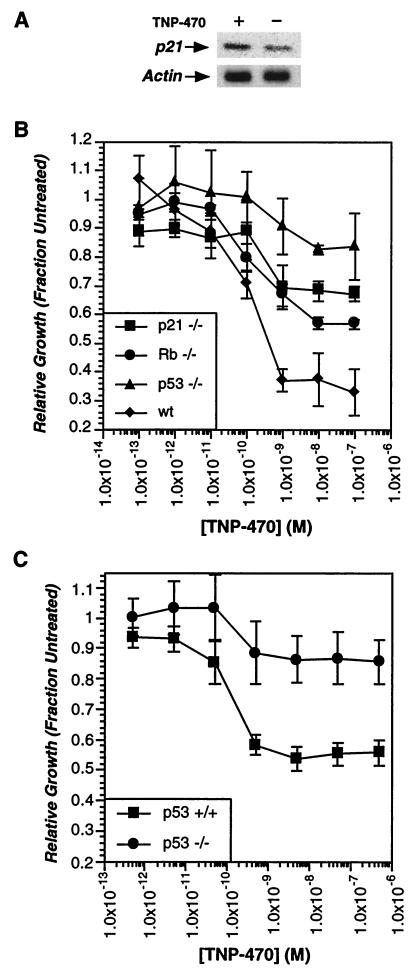
Given that TNP-470 treatment was known to block S-phase entry of cultured endothelial cells, it was possible that induction of p53 transcriptional activity and up-regulation of p21 levels was simply a consequence of prolonged G1 arrest. To determine whether these effects were causative or simply a consequence of prolonged cell cycle arrest by TNP-470, we examined the effects of TNP-470 treatment on cells deficient in pRB, p53, or p21. In addition to its effects on endothelial cells, TNP-470 has been shown to affect some other cell types, including fibroblasts (4, 9, 30). We therefore initially examined the effect of TNP-470 treatment on MEFs. As with HUVECs, TNP-470 treatment led to up-regulation of p21 (Fig. 4A), and the proliferation of MEFs was also found to be potently inhibited upon drug treatment in a dose-dependent manner (Fig. 4B). Previously, the phosphorylation state of pRB was shown to correlate with TNP-470-induced cell arrest. MEFs derived from Rb-deficient embryos showed a small but significant resistance to TNP-470 treatment, also implicating pRB in the cell cycle arrest (Fig. 4B). To further assess the importance of p53 and p21 in the cell cycle inhibition by TNP-470, we determined the sensitivity of MEFs isolated from wild-type, p53-null, and p21-null mice. Both p53- and p21-deficient MEFs showed significant resistance to TNP-470 treatment (Fig. 4B) similar to that observed for several drug-resistant transformed cell lines (17). Thus, the disruption of the p53 pathway, which frequently accompanies cellular transformation, may explain the drug resistance of many transformed cell lines.
Figure 4.
p53 and p21 are necessary for cell cycle arrest induced by TNP-470. (A) TNP-470 treatment induces p21 in MEFs. Subconfluent MEFs were incubated in the presence or absence of TNP-470 (10 nM) for 24 h. p21 levels were determined from crude cell lysates by Western blot. (B) p53 and p21-deficient MEFs show dramatic resistance to TNP-470. MEFs were incubated in the presence or absence of varying concentrations of TNP-470 for 24 h and pulsed with [ 3H]thymidine (1 μCi) for an additional 6 h. Cells were harvested and the amounts of incorporated tritium were determined by scintillation counting. The amount of tritium incorporation in the absence of drug is used as standard for normalization. Wild-type MEFs derived from two different strains of mice were used as control. MEFs from 129/Sv were used as control for p53- and p21-null cells, and those from 129/Sv × C57BL/6 were used as controls for Rb-null cells. The titration curves for the MEFs from both strains are similar. Only the curve with 129/Sv MEFs was shown. (C) p53-deficient MLECs are resistant to growth inhibition by TNP-470. MLECs were isolated from wild-type and p53-deficient mice, and drug sensitivity was determined as described above for MEFs.
Despite these results with embryo fibroblasts, drug-induced arrest in other cell types could occur by a mechanism different from that of endothelial cells. To address this possibility, MLECs were isolated from p53-deficient mice. Compared with MLECs isolated from wild-type littermates, p53-deficient MLECs showed almost complete resistance to TNP-470 treatment (Fig. 4C). Surprisingly, wild-type MLECs showed only partial inhibition when treated with the drug (56 ± 7%). This reduced sensitivity may be due to species-specific differences in endothelial cell sensitivity, because mouse neonatal atrial endothelial cells also showed only partial sensitivity to the drug (53 ± 3%). Similar species-specific differences have previously been observed in the immunosuppressive activity of ovalicin (16). Despite this complication, the loss of p53 clearly resulted in a dramatic resistance to TNP-470 treatment in endothelial cells. Thus, activation of the p53 pathway and induction of the CDK inhibitor p21 appear to be requisite steps for TNP-470-induced cell cycle arrest, leading to an inhibition of angiogenesis.
Discussion
TNP-470 has been previously shown to inhibit endothelial cell proliferation (4) and cell cycle progression in G1 (9, 20–22). There have been disagreements, however, on the underlying mechanism of this inhibition. TNP-470 was reported to inhibit growth factor-induced activation of cdc2 and Cdk2 and the phosphorylation of pRB in vascular endothelial cells (21). In agreement with this finding, TNP-470 has also been reported to block activation of cyclin E-Cdk2 stimulated by platelet-derived growth factor (PDGF) and insulin-like growth factor (IGF) in vascular smooth muscle cells (30). However, another group has reported that TNP-470 inhibited the expression of cyclin D1, implicating cyclin D-dependent kinase activity as a possible target for the drug (22). In the present study, we examined the effect of TNP-470 on the cell cycle progression of endothelial cells after cell cycle reentry. We found that pRB hyperphosphorylation and cyclin E-dependent kinase activity were inhibited by TNP-470, in agreement with some previous reports (21, 22). Of the several known regulatory processes that affect cyclin E-Cdk2 activity, only the dramatic increase in p21 associated with the complex appeared likely to account for the potent inhibition of the CDK activity. Subsequently, we found that up-regulation of p21 was accompanied by p53 activation in the presence of TNP-470. We further demonstrated that p53 and p21 are necessary for the cell cycle inhibition by TNP-470 by using cells derived from the respective knockout mice.
We and others (10, 11, 13–15) have previously shown that TNP-470 binds covalently to MetAP2, inhibiting its enzymatic activity with high specificity. The direct inhibition of MetAP2 by TNP-470 suggests a possible causal relationship between inhibition of MetAP2 and activation of the p53 pathway. Over the past few years, evidence has begun to emerge that p53 is activated in response not only to DNA damage but also to a wide variety of stress stimuli, including hypoxia, oncogenic stimuli, NTP deprivation, cytoskeletal disruption, and redox stress (31–33). The present study suggests that the p53 pathway responds to yet another stress signal resulting from specific defects in the N-terminal processing of nascent polypeptides upon TNP-470 treatment.
How might defects in N-terminal processing serve to activate p53? N-terminal processing is required for a number of posttranslational modifications, including N-terminal myristoylation and N-terminal acetylation. These modifications are known to regulate the localization and stability of some cellular proteins. In addition, the identity of the N-terminal residue can dramatically affect the turnover rate of a protein, modulating its degradation by the N-end rule pathway. Defects in methionine removal caused by inhibition of MetAP2 could therefore aberrantly stabilize short-lived proteins, resulting in cellular stress. The effects of N-terminal processing defects on p53 and p21 do not appear to be direct but seem to be mediated by the inactivation of as yet unidentified proteins, which generate a stress signal. p53 itself is not a substrate for methionine processing because its second translated residue is a conserved glutamate residue. p21 does have a conserved serine as its second translated residue, making it a potential MetAP substrate. However, the requirement of intact p53 for cell arrest argues against a direct stabilization of the p21 protein by methionine retention. It remains to be determined whether p53 activation is due to a small subset of specific MetAP2 substrates or a result of global effects on N-terminal processing.
Before our current studies, the prevailing model to explain TNP-470-induced cell cycle arrest was the inhibition of pRB phosphorylation. Experiments using Rb-deficient MEFs did reveal a role for Rb in this process. However, p53 activation appeared to play a more significant role. Loss of p53 renders cells almost completely resistant to TNP-470 treatment, similar to the resistance observed for a number of tumor cell lines (17). Cells deficient in p53 still retained some, although slight, sensitivity to TNP-470. These effects may be due to other antiproliferative effects of defective methionine processing. The dramatic resistance resulting from loss of p53, however, clearly defines p53 as the major mediator of the cell cycle arrest in response to TNP-470.
The importance of the p53 pathway in cell arrest resulting from TNP-470 treatment explains a number of observations made concerning the cellular effects of the drug. A long-standing discrepancy in the cell culture studies of TNP-470 treatment has been whether drug treatment results in cell apoptosis or arrest. We have consistently observed cell arrest in our studies, but it is possible that under slightly different culture conditions the p53 activation resulting from drug treatment could result in cell death. Endothelial cells have also been shown to be particularly sensitive to TNP-470 treatment. This sensitivity may be due to the presence of additional stress-inducing signals resulting from aberrant methionine processing in this cell type, or endothelial cells may be sensitized to the effects of the p53 pathway itself. The latter possibility is particularly interesting given the observation that the proteosome inhibitor lactacystin has also been found to activate the p53 pathway in endothelial cells and does so with dramatically increased potency when compared with other cell types (34). Other proteosome inhibitors have also been shown to inhibit endothelial cell proliferation (35). This sensitization could result from an up-regulation of the p53 pathway in this cell type. Alternatively, increased sensitivity in endothelial cells may be due to the presence of additional p53 responsive genes. Elevated p53 activity has been shown to inhibit angiogenesis in vivo by enhancing the synthesis of thrombospondin-1, a potent inhibitor of angiogenesis (36) and down-regulating vascular endothelial growth factor (VEGF) expression (37). The activation of other p53 targets may be relevant, given the difference in TNP-470 sensitivity between p53- and p21-deficient MEFs. It remains to be seen whether TNP-470 treatment has similar effects in vivo.
Current therapeutic strategies for cancer treatment have begun to focus not only on the tumor cell population itself, but also on the complex interaction of the tumor with the nontransformed host tissues. Inhibition of tumor-induced angiogenesis has now been recognized as a valuable therapeutic strategy with several important advantages over conventional chemotherapeutics. Fumagillin was first identified as an anti-angiogenic agent because of its inhibition of endothelial cell growth. However, both fumagillin and TNP-470 were also reported to have similar effects on other cell types, including fibroblasts (4, 9). The related natural product ovalicin was in fact initially identified as an immunosuppressive agent affecting lymphoid cells (18), and TNP-470 has also been found to be immunosuppressive (16). It is worth noting that, of various cell types examined so far, endothelial cells are among the most sensitive cell type to TNP-470 treatment. Thus, TNP-470 is selective, rather than specific, for endothelial cells. The relative importance of direct effects on tumor cell growth and angiogenesis inhibition have yet to be directly determined. Our findings suggest that these effects should be explored systematically by using wild-type and p53-deficient hosts to assess the efficacy of TNP-470 treatment for tumor inhibition. The finding that TNP-470 acts to arrest endothelial cells by activation of the p53 pathway has important implications for anti-angiogenic therapy, because endothelial cells often maintain functional p53 in contrast to tumor cells. The sensitivity of endothelial cells to p53 activation suggests that other p53-activating stimuli could potentially be exploited in anti-angiogenic therapy.
Acknowledgments
We thank Dr. Judah Folkman for the helpful suggestion of using density arrest to synchronize endothelial cells, and Zhuang Su for the synthetic sample of TNP-470. We thank Ben Turk for initiating this work. We thank Julie Lively and Richard Hynes for instruction and assistance with the isolation of MLECs, Jay Edelberg and Bob Rosenberg for mouse neonatal atrial endothelial cells, and Scott Boyd for critical reading of the manuscript. We are grateful to Dr. David Morgan for cyclin H-Cdk7. This work was supported in part by the Rita Allen Foundation and the National Cancer Institute (J.O.L.). T.J. is an Associate Investigator of the Howard Hughes Medical Institute. Postdoctoral fellowships from the Anna Fuller Foundation (Y.Z.) and the Human Frontier Science Program (J.S.) and a Koch Graduate Fellowship (E.C.G.) are gratefully acknowledged.
Abbreviations
- MetAP
methionine aminopeptidase
- MetAP2
type 2 MetAP
- pRB
retinoblastoma gene product
- HUVEC
human umbilical vein endothelial cell
- CDK
cyclin-dependent kinase
- MEF
mouse embryo fibroblast
- RT-PCR
reverse transcription-PCR
- MLEC
mouse lung endothelial cell
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Folkman J. N Engl J Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 2.Hanahan D, Folkman J. Cell. 1996;86:353–364. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- 3.Boehm T, Folkman J, Browder T, O'Reilly M S. Nature (London) 1997;390:404–407. doi: 10.1038/37126. [DOI] [PubMed] [Google Scholar]
- 4.Ingber D, Fujita T, Kishmoto S, Sudo K, Kanamaru T, Brem H, Folkman J. Nature (London) 1990;348:555–557. doi: 10.1038/348555a0. [DOI] [PubMed] [Google Scholar]
- 5.Corey E J, Guzman-Perez A, Noe M C. J Am Chem Soc. 1994;116:12109–12110. [Google Scholar]
- 6.Bergers G, Javaherian K, Lo K M, Folkman J, Hanahan D. Science. 1999;284:808–812. doi: 10.1126/science.284.5415.808. [DOI] [PubMed] [Google Scholar]
- 7.Yamamoto T, Sudo K, Fujita T. Anticancer Res. 1994;14:1–4. [PubMed] [Google Scholar]
- 8.Yanase T, Tamura M, Fujita K, Kodama S, Tanaka K. Cancer Res. 1993;53:2566–2570. [PubMed] [Google Scholar]
- 9.Kusaka M, Sudo K, Matsutani E, Kozai Y, Marui S, Fujita T, Ingber D, Folman J. Brit J Cancer. 1993;69:212–216. doi: 10.1038/bjc.1994.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Griffith E C, Su Z, Turk B E, Chen S, Chang Y-W, Wu Z, Biemann K, Liu J O. Chem Biol. 1997;4:461–471. doi: 10.1016/s1074-5521(97)90198-8. [DOI] [PubMed] [Google Scholar]
- 11.Sin N, Meng L, Wang M Q W, Wen J J, Bornmann W G, Crews C M. Proc Natl Acad Sci USA. 1997;94:6099–6103. doi: 10.1073/pnas.94.12.6099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bradshaw R A, Brickey W W, Walker K W. Trends Biochem Sci. 1998;23:263–267. doi: 10.1016/s0968-0004(98)01227-4. [DOI] [PubMed] [Google Scholar]
- 13.Griffith E C, Su Z, Niwayama S, Ramsay C A, Chang Y H, Liu J O. Proc Natl Acad Sci USA. 1998;95:15183–15188. doi: 10.1073/pnas.95.26.15183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lowther W T, McMillen D A, Orville A M, Matthews B W. Proc Natl Acad Sci USA. 1998;95:12153–12157. doi: 10.1073/pnas.95.21.12153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu S, Widom J, Kemp C W, Crews C M, Clardy J. Science. 1998;282:1324–1327. doi: 10.1126/science.282.5392.1324. [DOI] [PubMed] [Google Scholar]
- 16.Turk B E, Su Z, Liu J O. Bioorg Med Chem. 1998;6:1163–1169. doi: 10.1016/s0968-0896(98)00078-9. [DOI] [PubMed] [Google Scholar]
- 17.Turk B E, Griffith E C, Wolf S, Biemann K, Chang Y H, Liu J O. Chem Biol. 1999;6:823–833. doi: 10.1016/s1074-5521(99)80129-x. [DOI] [PubMed] [Google Scholar]
- 18.Borel J F, Lazary S, Stahelin H. Agents Actions. 1974;4:357–363. doi: 10.1007/BF01964937. [DOI] [PubMed] [Google Scholar]
- 19.Han C K, Ahn S K, Choi N S, Hong R K, Moon S K, Chun H S, Lee S J, Kim J W, Hong C I, Kim D, et al. Bioorg Med Chem Lett. 2000;10:39–43. doi: 10.1016/s0960-894x(99)00577-6. [DOI] [PubMed] [Google Scholar]
- 20.Antoine N, Greimers R, M, D R, Kusaka M, Heinen E, Simar L J, Castronovo V. Cancer Res. 1994;54:2073–2076. [PubMed] [Google Scholar]
- 21.Abe J, Zhou W, Takuwa N, Taguchi J, Kurokawa K, Kumada M, Takuwa Y. Cancer Res. 1994;54:3407–3412. [PubMed] [Google Scholar]
- 22.Hori A, Ikeyama S, Sudo K. Biochem Biophys Res Commun. 1994;204:1067–1073. doi: 10.1006/bbrc.1994.2571. [DOI] [PubMed] [Google Scholar]
- 23.Jacks T, Remington L, Williams B O, Schmitt E M, Halachmi S, Bronson R T, Weinberg R A. Curr Biol. 1994;4:1–7. doi: 10.1016/s0960-9822(00)00002-6. [DOI] [PubMed] [Google Scholar]
- 24.Geng Y, Eaton E N, Picon M, Roberts J M, Lundberg A S, Gifford A, Sardet C, Weinberg R A. Oncogene. 1996;12:1173–1180. [PubMed] [Google Scholar]
- 25.Kern S E, Pietenpol J A, Thiagalingam S, Seymour A, Kinzler K W, Vogelstein B. Science. 1992;256:827–830. doi: 10.1126/science.1589764. [DOI] [PubMed] [Google Scholar]
- 26.Dong Q G, Bernasconi S, Lostaglio S, De Calmanovici R W, Martin-Padura I, Breviario F, Garlanda C, Ramponi S, Mantovani A, Vecchi A. Arterioscler Thromb Vasc Biol. 1997;17:1599–1604. doi: 10.1161/01.atv.17.8.1599. [DOI] [PubMed] [Google Scholar]
- 27.Sherr C J. Science. 1996;274:1672–1677. doi: 10.1126/science.274.5293.1672. [DOI] [PubMed] [Google Scholar]
- 28.Gartel A L, Tyner A L. Exp Cell Res. 1999;246:280–289. doi: 10.1006/excr.1998.4319. [DOI] [PubMed] [Google Scholar]
- 29.Macleod K F, Sherry N, Hannon G, Beach D, Tokino T, Kinzler K, Vogelstein B, Jacks T. Genes Dev. 1995;9:935–944. doi: 10.1101/gad.9.8.935. [DOI] [PubMed] [Google Scholar]
- 30.Koyama H, Nishizawa Y, Hosoi M, Fukumoto S, Kogawa K, Shioi A, Morii H. Circ Res. 1996;79:757–764. doi: 10.1161/01.res.79.4.757. [DOI] [PubMed] [Google Scholar]
- 31.Levine A J. Cell. 1997;88:323–331. doi: 10.1016/s0092-8674(00)81871-1. [DOI] [PubMed] [Google Scholar]
- 32.Giaccia A J, Kastan M B. Genes Dev. 1998;12:2973–2983. doi: 10.1101/gad.12.19.2973. [DOI] [PubMed] [Google Scholar]
- 33.Ashcroft M, Vousden K H. Oncogene. 1999;18:7637–7643. doi: 10.1038/sj.onc.1203012. [DOI] [PubMed] [Google Scholar]
- 34.Kumeda S I, Deguchi A, Toi M, Omura S, Umezawa K. Anticancer Res. 1999;19:3961–3968. [PubMed] [Google Scholar]
- 35.Sin N, Meng L, Auth H, Crews C M. Bioorg Med Chem. 1998;6:1209–1217. doi: 10.1016/s0968-0896(98)00089-3. [DOI] [PubMed] [Google Scholar]
- 36.Dameron K M, Volpert O V, Tainsky M A, Bouck N. Science. 1994;265:1582–1584. doi: 10.1126/science.7521539. [DOI] [PubMed] [Google Scholar]
- 37.Kieser A, Weich H A, Brandner G, Marme D, Kolch W. Oncogene. 1994;9:963–969. [PubMed] [Google Scholar]