Abstract
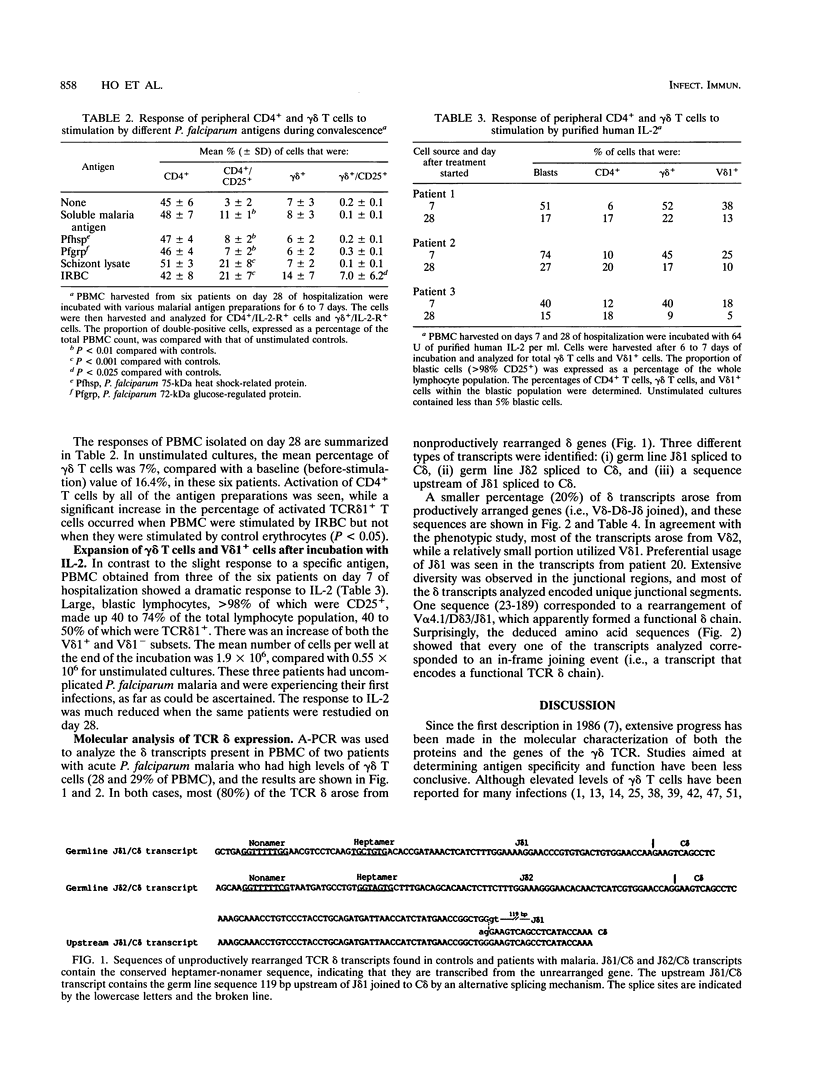
Plasmodium falciparum malaria in humans is associated with an increase in the percentage and absolute number of gamma delta T cells in the peripheral blood. This increase begins during the acute infection phase and persists for at least 4 weeks during convalescence. In the present study, 25 to 30% of the gamma delta T cells expressed HLA-DR antigens in vivo and in some patients they proliferated in response to further stimulation by purified human interleukin 2 in vitro. However, there was no in vitro proliferative response to various malarial antigens, including a 75-kDa heat shock protein and a 72-kDa glucose-regulated protein of P. falciparum during the acute infection phase. Cytofluorographic studies showed that although an increase of V delta 1- gamma delta T cells was largely responsible for the expansion of the total number of gamma delta T cells, there was also a proportional increase in V delta 1+ cells. These results were confirmed with anchored PCR and by DNA sequencing to characterize at the molecular level the set of T-cell receptor (TCR) delta mRNAs expressed in the peripheral blood of two patients with high levels of gamma delta T cells. In each case, most of the TCR delta mRNA transcripts corresponded to nonproductively rearranged delta genes (unrearranged J delta or near J delta spliced to C delta). In those sequences which did represent productively rearranged genes, most of the transcripts originated from a V delta 2/J delta 1 joining, as in normal individuals. A minority of transcripts originated from a V delta 1/J delta 1 rearrangement, and one originated from a V alpha 4/J delta 1 rearrangement. Polyclonal activation of gamma delta T cells was inferred from the extensive junctional diversity seen in the delta mRNAs analyzed. Expansion of a heterogeneous set of both V delta 1(-)- and V delta 1(+)-bearing T cells suggests that the elevated levels of gamma delta T cells seen during acute P. falciparum malaria arose from immune responses to multiple distinct parasite antigens or unidentified host factors.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Autran B., Triebel F., Katlama C., Rozenbaum W., Hercend T., Debre P. T cell receptor gamma/delta+ lymphocyte subsets during HIV infection. Clin Exp Immunol. 1989 Feb;75(2):206–210. [PMC free article] [PubMed] [Google Scholar]
- Behr C., Dubois P. Preferential expansion of V gamma 9 V delta 2 T cells following stimulation of peripheral blood lymphocytes with extracts of Plasmodium falciparum. Int Immunol. 1992 Mar;4(3):361–366. doi: 10.1093/intimm/4.3.361. [DOI] [PubMed] [Google Scholar]
- Bordessoule D., Gaulard P., Mason D. Y. Preferential localisation of human lymphocytes bearing gamma delta T cell receptors to the red pulp of the spleen. J Clin Pathol. 1990 Jun;43(6):461–464. doi: 10.1136/jcp.43.6.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borst J., van de Griend R. J., van Oostveen J. W., Ang S. L., Melief C. J., Seidman J. G., Bolhuis R. L. A T-cell receptor gamma/CD3 complex found on cloned functional lymphocytes. Nature. 1987 Feb 19;325(6106):683–688. doi: 10.1038/325683a0. [DOI] [PubMed] [Google Scholar]
- Bottino C., Tambussi G., Ferrini S., Ciccone E., Varese P., Mingari M. C., Moretta L., Moretta A. Two subsets of human T lymphocytes expressing gamma/delta antigen receptor are identifiable by monoclonal antibodies directed to two distinct molecular forms of the receptor. J Exp Med. 1988 Aug 1;168(2):491–505. doi: 10.1084/jem.168.2.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner M. B., McLean J., Dialynas D. P., Strominger J. L., Smith J. A., Owen F. L., Seidman J. G., Ip S., Rosen F., Krangel M. S. Identification of a putative second T-cell receptor. Nature. 1986 Jul 10;322(6075):145–149. doi: 10.1038/322145a0. [DOI] [PubMed] [Google Scholar]
- Brenner M. B., McLean J., Scheft H., Riberdy J., Ang S. L., Seidman J. G., Devlin P., Krangel M. S. Two forms of the T-cell receptor gamma protein found on peripheral blood cytotoxic T lymphocytes. Nature. 1987 Feb 19;325(6106):689–694. doi: 10.1038/325689a0. [DOI] [PubMed] [Google Scholar]
- Carbonari M., Cherchi M., Paganelli R., Giannini G., Galli E., Gaetano C., Papetti C., Fiorilli M. Relative increase of T cells expressing the gamma/delta rather than the alpha/beta receptor in ataxia-telangiectasia. N Engl J Med. 1990 Jan 11;322(2):73–76. doi: 10.1056/NEJM199001113220201. [DOI] [PubMed] [Google Scholar]
- Casorati G., De Libero G., Lanzavecchia A., Migone N. Molecular analysis of human gamma/delta+ clones from thymus and peripheral blood. J Exp Med. 1989 Nov 1;170(5):1521–1535. doi: 10.1084/jem.170.5.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccone E., Viale O., Bottino C., Pende D., Migone N., Casorati G., Tambussi G., Moretta A., Moretta L. Antigen recognition by human T cell receptor gamma-positive lymphocytes. Specific lysis of allogeneic cells after activation in mixed lymphocyte culture. J Exp Med. 1988 Apr 1;167(4):1517–1522. doi: 10.1084/jem.167.4.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. M., Bjorkman P. J. T-cell antigen receptor genes and T-cell recognition. Nature. 1988 Aug 4;334(6181):395–402. doi: 10.1038/334395a0. [DOI] [PubMed] [Google Scholar]
- De Paoli P., Basaglia G., Gennari D., Crovatto M., Modolo M. L., Santini G. Phenotypic profile and functional characteristics of human gamma and delta T cells during acute toxoplasmosis. J Clin Microbiol. 1992 Mar;30(3):729–731. doi: 10.1128/jcm.30.3.729-731.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Paoli P., Gennari D., Martelli P., Cavarzerani V., Comoretto R., Santini G. Gamma delta T cell receptor-bearing lymphocytes during Epstein-Barr virus infection. J Infect Dis. 1990 May;161(5):1013–1016. doi: 10.1093/infdis/161.5.1013. [DOI] [PubMed] [Google Scholar]
- Emoto M., Danbara H., Yoshikai Y. Induction of gamma/delta T cells in murine salmonellosis by an avirulent but not by a virulent strain of Salmonella choleraesuis. J Exp Med. 1992 Aug 1;176(2):363–372. doi: 10.1084/jem.176.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falini B., Flenghi L., Pileri S., Pelicci P., Fagioli M., Martelli M. F., Moretta L., Ciccone E. Distribution of T cells bearing different forms of the T cell receptor gamma/delta in normal and pathological human tissues. J Immunol. 1989 Oct 15;143(8):2480–2488. [PubMed] [Google Scholar]
- Faure F., Jitsukawa S., Triebel F., Hercend T. Characterization of human peripheral lymphocytes expressing the CD3-gamma/delta complex with anti-receptor monoclonal antibodies. J Immunol. 1988 Nov 15;141(10):3357–3360. [PubMed] [Google Scholar]
- Forrester J. M., Newman L. S., Wang Y., King T. E., Jr, Kotzin B. L. Clonal expansion of lung V delta 1+ T cells in pulmonary sarcoidosis. J Clin Invest. 1993 Jan;91(1):292–300. doi: 10.1172/JCI116184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goerlich R., Häcker G., Pfeffer K., Heeg K., Wagner H. Plasmodium falciparum merozoites primarily stimulate the V gamma 9 subset of human gamma/delta T cells. Eur J Immunol. 1991 Oct;21(10):2613–2616. doi: 10.1002/eji.1830211045. [DOI] [PubMed] [Google Scholar]
- Goodier M., Fey P., Eichmann K., Langhorne J. Human peripheral blood gamma delta T cells respond to antigens of Plasmodium falciparum. Int Immunol. 1992 Jan;4(1):33–41. doi: 10.1093/intimm/4.1.33. [DOI] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Haregewoin A., Soman G., Hom R. C., Finberg R. W. Human gamma delta+ T cells respond to mycobacterial heat-shock protein. Nature. 1989 Jul 27;340(6231):309–312. doi: 10.1038/340309a0. [DOI] [PubMed] [Google Scholar]
- Hata S., Satyanarayana K., Devlin P., Band H., McLean J., Strominger J. L., Brenner M. B., Krangel M. S. Extensive junctional diversity of rearranged human T cell receptor delta genes. Science. 1988 Jun 10;240(4858):1541–1544. doi: 10.1126/science.3259726. [DOI] [PubMed] [Google Scholar]
- Ho M., Webster H. K., Looareesuwan S., Supanaranond W., Phillips R. E., Chanthavanich P., Warrell D. A. Antigen-specific immunosuppression in human malaria due to Plasmodium falciparum. J Infect Dis. 1986 Apr;153(4):763–771. doi: 10.1093/infdis/153.4.763. [DOI] [PubMed] [Google Scholar]
- Ho M., Webster H. K., Tongtawe P., Pattanapanyasat K., Weidanz W. P. Increased gamma delta T cells in acute Plasmodium falciparum malaria. Immunol Lett. 1990 Aug;25(1-3):139–141. doi: 10.1016/0165-2478(90)90105-y. [DOI] [PubMed] [Google Scholar]
- Holoshitz J., Koning F., Coligan J. E., De Bruyn J., Strober S. Isolation of CD4- CD8- mycobacteria-reactive T lymphocyte clones from rheumatoid arthritis synovial fluid. Nature. 1989 May 18;339(6221):226–229. doi: 10.1038/339226a0. [DOI] [PubMed] [Google Scholar]
- Holoshitz J., Vila L. M., Keroack B. J., McKinley D. R., Bayne N. K. Dual antigenic recognition by cloned human gamma delta T cells. J Clin Invest. 1992 Jan;89(1):308–314. doi: 10.1172/JCI115577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen O., Wesselborg S., Heckl-Ostreicher B., Pechhold K., Bender A., Schondelmaier S., Moldenhauer G., Kabelitz D. T cell receptor/CD3-signaling induces death by apoptosis in human T cell receptor gamma delta + T cells. J Immunol. 1991 Jan 1;146(1):35–39. [PubMed] [Google Scholar]
- Kabelitz D., Bender A., Schondelmaier S., Schoel B., Kaufmann S. H. A large fraction of human peripheral blood gamma/delta + T cells is activated by Mycobacterium tuberculosis but not by its 65-kD heat shock protein. J Exp Med. 1990 Mar 1;171(3):667–679. doi: 10.1084/jem.171.3.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjeldsen-Kragh J., Quayle A. J., Skålhegg B. S., Sioud M., Førre O. Selective activation of resting human gamma delta T lymphocytes by interleukin-2. Eur J Immunol. 1993 Sep;23(9):2092–2099. doi: 10.1002/eji.1830230908. [DOI] [PubMed] [Google Scholar]
- Krangel M. S., Band H., Hata S., McLean J., Brenner M. B. Structurally divergent human T cell receptor gamma proteins encoded by distinct C gamma genes. Science. 1987 Jul 3;237(4810):64–67. doi: 10.1126/science.2955517. [DOI] [PubMed] [Google Scholar]
- Kranz D. M., Saito H., Heller M., Takagaki Y., Haas W., Eisen H. N., Tonegawa S. Limited diversity of the rearranged T-cell gamma gene. 1985 Feb 28-Mar 6Nature. 313(6005):752–755. doi: 10.1038/313752a0. [DOI] [PubMed] [Google Scholar]
- Kumar N., Zhao Y., Graves P., Perez Folgar J., Maloy L., Zheng H. Human immune response directed against Plasmodium falciparum heat shock-related proteins. Infect Immun. 1990 May;58(5):1408–1414. doi: 10.1128/iai.58.5.1408-1414.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langhorne J., Goodier M., Behr C., Dubois P. Is there a role for gamma delta T cells in malaria? Immunol Today. 1992 Aug;13(8):298–300. doi: 10.1016/0167-5699(92)90041-5. [DOI] [PubMed] [Google Scholar]
- Loh E. Y., Elliott J. F., Cwirla S., Lanier L. L., Davis M. M. Polymerase chain reaction with single-sided specificity: analysis of T cell receptor delta chain. Science. 1989 Jan 13;243(4888):217–220. doi: 10.1126/science.2463672. [DOI] [PubMed] [Google Scholar]
- Meuer S. C., Fitzgerald K. A., Hussey R. E., Hodgdon J. C., Schlossman S. F., Reinherz E. L. Clonotypic structures involved in antigen-specific human T cell function. Relationship to the T3 molecular complex. J Exp Med. 1983 Feb 1;157(2):705–719. doi: 10.1084/jem.157.2.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minoprio P., Itohara S., Heusser C., Tonegawa S., Coutinho A. Immunobiology of murine T. cruzi infection: the predominance of parasite-nonspecific responses and the activation of TCRI T cells. Immunol Rev. 1989 Dec;112:183–207. doi: 10.1111/j.1600-065x.1989.tb00558.x. [DOI] [PubMed] [Google Scholar]
- Modlin R. L., Pirmez C., Hofman F. M., Torigian V., Uyemura K., Rea T. H., Bloom B. R., Brenner M. B. Lymphocytes bearing antigen-specific gamma delta T-cell receptors accumulate in human infectious disease lesions. Nature. 1989 Jun 15;339(6225):544–548. doi: 10.1038/339544a0. [DOI] [PubMed] [Google Scholar]
- Morio T., Nagasawa M., Nonoyama S., Okawa H., Yata J. Phenotypic profile and functions of T cell receptor-gamma delta-bearing cells from patients with primary immunodeficiency syndrome. J Immunol. 1990 Feb 15;144(4):1270–1275. [PubMed] [Google Scholar]
- Morita C. T., Verma S., Aparicio P., Martinez C., Spits H., Brenner M. B. Functionally distinct subsets of human gamma/delta T cells. Eur J Immunol. 1991 Dec;21(12):2999–3007. doi: 10.1002/eji.1830211215. [DOI] [PubMed] [Google Scholar]
- O'Brien R. L., Fu Y. X., Cranfill R., Dallas A., Ellis C., Reardon C., Lang J., Carding S. R., Kubo R., Born W. Heat shock protein Hsp60-reactive gamma delta cells: a large, diversified T-lymphocyte subset with highly focused specificity. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4348–4352. doi: 10.1073/pnas.89.10.4348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmen J. D., Barnes P. F., Uyemura K., Lu S. Z., Grisso C. L., Modlin R. L. The T cell receptors of human gamma delta T cells reactive to Mycobacterium tuberculosis are encoded by specific V genes but diverse V-J junctions. J Immunol. 1991 Nov 15;147(10):3353–3359. [PubMed] [Google Scholar]
- Patel S. S., Wacholtz M. C., Duby A. D., Thiele D. L., Lipsky P. E. Analysis of the functional capabilities of CD3+CD4-CD8- and CD3+CD4+CD8+ human T cell clones. J Immunol. 1989 Aug 15;143(4):1108–1117. [PubMed] [Google Scholar]
- Pfeffer K., Schoel B., Gulle H., Kaufmann S. H., Wagner H. Primary responses of human T cells to mycobacteria: a frequent set of gamma/delta T cells are stimulated by protease-resistant ligands. Eur J Immunol. 1990 May;20(5):1175–1179. doi: 10.1002/eji.1830200534. [DOI] [PubMed] [Google Scholar]
- Porcelli S., Brenner M. B., Band H. Biology of the human gamma delta T-cell receptor. Immunol Rev. 1991 Apr;120:137–183. doi: 10.1111/j.1600-065x.1991.tb00591.x. [DOI] [PubMed] [Google Scholar]
- Raulet D. H. The structure, function, and molecular genetics of the gamma/delta T cell receptor. Annu Rev Immunol. 1989;7:175–207. doi: 10.1146/annurev.iy.07.040189.001135. [DOI] [PubMed] [Google Scholar]
- Roussilhon C., Agrapart M., Ballet J. J., Bensussan A. T lymphocytes bearing the gamma delta T cell receptor in patients with acute Plasmodium falciparum malaria. J Infect Dis. 1990 Jul;162(1):283–285. doi: 10.1093/infdis/162.1.283-a. [DOI] [PubMed] [Google Scholar]
- Rust C. J., Verreck F., Vietor H., Koning F. Specific recognition of staphylococcal enterotoxin A by human T cells bearing receptors with the V gamma 9 region. Nature. 1990 Aug 9;346(6284):572–574. doi: 10.1038/346572a0. [DOI] [PubMed] [Google Scholar]
- Savilahti E., Reunala T., Mäki M. Increase of lymphocytes bearing the gamma/delta T cell receptor in the jejunum of patients with dermatitis herpetiformis. Gut. 1992 Feb;33(2):206–211. doi: 10.1136/gut.33.2.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snodgrass H. R., Dembić Z., Steinmetz M., von Boehmer H. Expression of T-cell antigen receptor genes during fetal development in the thymus. Nature. 1985 May 16;315(6016):232–233. doi: 10.1038/315232a0. [DOI] [PubMed] [Google Scholar]
- Sumida T., Maeda T., Takahashi H., Yoshida S., Yonaha F., Sakamoto A., Tomioka H., Koike T., Yoshida S. Predominant expansion of V gamma 9/V delta 2 T cells in a tularemia patient. Infect Immun. 1992 Jun;60(6):2554–2558. doi: 10.1128/iai.60.6.2554-2558.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumida T., Yonaha F., Maeda T., Tanabe E., Koike T., Tomioka H., Yoshida S. T cell receptor repertoire of infiltrating T cells in lips of Sjögren's syndrome patients. J Clin Invest. 1992 Feb;89(2):681–685. doi: 10.1172/JCI115635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura N., Holroyd K. J., Banks T., Kirby M., Okayama H., Crystal R. G. Diversity in junctional sequences associated with the common human V gamma 9 and V delta 2 gene segments in normal blood and lung compared with the limited diversity in a granulomatous disease. J Exp Med. 1990 Jul 1;172(1):169–181. doi: 10.1084/jem.172.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uyemura K., Deans R. J., Band H., Ohmen J., Panchamoorthy G., Morita C. T., Rea T. H., Modlin R. L. Evidence for clonal selection of gamma/delta T cells in response to a human pathogen. J Exp Med. 1991 Sep 1;174(3):683–692. doi: 10.1084/jem.174.3.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wucherpfennig K. W., Newcombe J., Li H., Keddy C., Cuzner M. L., Hafler D. A. Gamma delta T-cell receptor repertoire in acute multiple sclerosis lesions. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4588–4592. doi: 10.1073/pnas.89.10.4588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zevering Y., Amante F., Smillie A., Currier J., Smith G., Houghten R. A., Good M. F. High frequency of malaria-specific T cells in non-exposed humans. Eur J Immunol. 1992 Mar;22(3):689–696. doi: 10.1002/eji.1830220311. [DOI] [PubMed] [Google Scholar]


