Abstract
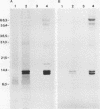
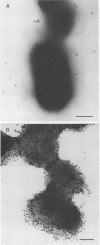
Affinity for porcine respiratory tract secretions was found in some isolates of Actinobacillus pleuropneumoniae and involved lipopolysaccharides (LPS) (M. Bélanger, S. Rioux, B. Foiry, and M. Jacques, FEMS Microbiol. Lett. 97:119-126, 1992). In the present study, the affinity for a crude preparation of porcine respiratory tract mucus of isolates of the Pasteurellaceae family, i.e., Actinobacillus, Haemophilus, and Pasteurella spp., and of some unrelated gram-negative bacteria was examined. Affinity for crude porcine respiratory tract mucus was not a property shared by all Pasteurellaceae isolates tested. Furthermore, affinity for the porcine crude mucus preparation was not unique to the Pasteurellaceae group and did not seem to be restricted to bacteria originating from pigs. Different surface properties of A. pleuropneumoniae isolates in relation to their adherence to crude mucus were examined. The capsular layer seemed to mask the adhesin and interfered with adherence to crude mucus. Two poorly capsulated isolates, which had a more hydrophobic surface and bound Congo red, were also heavily labeled by gold particles coated with polymyxin, which is known to interact with the lipid A-core region of LPS, and adhered strongly to respiratory tract secretions. Tetramethylurea, charged polymers, divalent cations, chelators, monosaccharides and amino sugars, or lectins were unable to inhibit adherence of A. pleuropneumoniae to the crude mucus preparation. To identify the receptor(s) recognized by the lipopolysaccharidic adhesin of A. pleuropneumoniae, affinity chromatography was used. Two bands, which were proteinaceous in nature, of 10 and 11 kDa were recovered. Our results suggest that two low-molecular-mass proteins present in porcine respiratory tract secretions bind A. pleuropneumoniae LPS.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beachey E. H. Bacterial adherence: adhesin-receptor interactions mediating the attachment of bacteria to mucosal surface. J Infect Dis. 1981 Mar;143(3):325–345. doi: 10.1093/infdis/143.3.325. [DOI] [PubMed] [Google Scholar]
- Bélanger M., Dubreuil D., Harel J., Girard C., Jacques M. Role of lipopolysaccharides in adherence of Actinobacillus pleuropneumoniae to porcine tracheal rings. Infect Immun. 1990 Nov;58(11):3523–3530. doi: 10.1128/iai.58.11.3523-3530.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bélanger M., Rioux S., Foiry B., Jacques M. Affinity for porcine respiratory tract mucus is found in some isolates of Actinobacillus pleuropneumoniae. FEMS Microbiol Lett. 1992 Oct 1;76(1-2):119–125. doi: 10.1111/j.1574-6968.1992.tb05450.x. [DOI] [PubMed] [Google Scholar]
- Darveau R. P., Hancock R. E. Procedure for isolation of bacterial lipopolysaccharides from both smooth and rough Pseudomonas aeruginosa and Salmonella typhimurium strains. J Bacteriol. 1983 Aug;155(2):831–838. doi: 10.1128/jb.155.2.831-838.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deneer H. G., Potter A. A. Effect of iron restriction on the outer membrane proteins of Actinobacillus (Haemophilus) pleuropneumoniae. Infect Immun. 1989 Mar;57(3):798–804. doi: 10.1128/iai.57.3.798-804.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golenbock D. T., Hampton R. Y., Raetz C. R., Wright S. D. Human phagocytes have multiple lipid A-binding sites. Infect Immun. 1990 Dec;58(12):4069–4075. doi: 10.1128/iai.58.12.4069-4075.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant M. M., Niederman M. S., Poehlman M. A., Fein A. M. Characterization of Pseudomonas aeruginosa adherence to cultured hamster tracheal epithelial cells. Am J Respir Cell Mol Biol. 1991 Dec;5(6):563–570. doi: 10.1165/ajrcmb/5.6.563. [DOI] [PubMed] [Google Scholar]
- Jacques M., Foiry B., Higgins R., Mittal K. R. Electron microscopic examination of capsular material from various serotypes of Actinobacillus pleuropneumoniae. J Bacteriol. 1988 Jul;170(7):3314–3318. doi: 10.1128/jb.170.7.3314-3318.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacques M., Gottschalk M., Foiry B., Higgins R. Ultrastructural study of surface components of Streptococcus suis. J Bacteriol. 1990 Jun;172(6):2833–2838. doi: 10.1128/jb.172.6.2833-2838.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay W. W., Phipps B. M., Ishiguro E. E., Trust T. J. Porphyrin binding by the surface array virulence protein of Aeromonas salmonicida. J Bacteriol. 1985 Dec;164(3):1332–1336. doi: 10.1128/jb.164.3.1332-1336.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Letellier A., Dubreuil D., Roy G., Fairbrother J. M., Jacques M. Determination of affinity of Pasteurella multocida isolates for porcine respiratory tract mucus, and partial characterization of the receptors. Am J Vet Res. 1991 Jan;52(1):34–39. [PubMed] [Google Scholar]
- Lindahl M., Faris A., Wadström T., Hjertén S. A new test based on 'salting out' to measure relative surface hydrophobicity of bacterial cells. Biochim Biophys Acta. 1981 Nov 5;677(3-4):471–476. doi: 10.1016/0304-4165(81)90261-0. [DOI] [PubMed] [Google Scholar]
- Marcus H., Austria A., Baker N. R. Adherence of Pseudomonas aeruginosa to tracheal epithelium. Infect Immun. 1989 Apr;57(4):1050–1053. doi: 10.1128/iai.57.4.1050-1053.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morioka H., Tachibana M., Machino M., Suganuma A. Polymyxin B binding sites in Escherichia coli as revealed by polymyxin B-gold labeling. J Histochem Cytochem. 1987 Feb;35(2):229–231. doi: 10.1177/35.2.3025293. [DOI] [PubMed] [Google Scholar]
- Morrison D. C. The case for specific lipopolysaccharide receptors expressed on mammalian cells. Microb Pathog. 1989 Dec;7(6):389–398. doi: 10.1016/0882-4010(89)90019-3. [DOI] [PubMed] [Google Scholar]
- Ramphal R., Pyle M. Evidence for mucins and sialic acid as receptors for Pseudomonas aeruginosa in the lower respiratory tract. Infect Immun. 1983 Jul;41(1):339–344. doi: 10.1128/iai.41.1.339-344.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozee K. R., Cooper D., Lam K., Costerton J. W. Microbial flora of the mouse ileum mucous layer and epithelial surface. Appl Environ Microbiol. 1982 Jun;43(6):1451–1463. doi: 10.1128/aem.43.6.1451-1463.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter J. M. Atrophic rhinitis in swine. Adv Vet Sci Comp Med. 1985;29:239–279. [PubMed] [Google Scholar]
- Sajjan U. S., Corey M., Karmali M. A., Forstner J. F. Binding of Pseudomonas cepacia to normal human intestinal mucin and respiratory mucin from patients with cystic fibrosis. J Clin Invest. 1992 Feb;89(2):648–656. doi: 10.1172/JCI115631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajjan U., Reisman J., Doig P., Irvin R. T., Forstner G., Forstner J. Binding of nonmucoid Pseudomonas aeruginosa to normal human intestinal mucin and respiratory mucin from patients with cystic fibrosis. J Clin Invest. 1992 Feb;89(2):657–665. doi: 10.1172/JCI115632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Weerkamp A. H., van der Mei H. C., Slot J. W. Relationship of cell surface morphology and composition of Streptococcus salivarius K+ to adherence and hydrophobicity. Infect Immun. 1987 Feb;55(2):438–445. doi: 10.1128/iai.55.2.438-445.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S. D. Multiple receptors for endotoxin. Curr Opin Immunol. 1991 Feb;3(1):83–90. doi: 10.1016/0952-7915(91)90082-c. [DOI] [PubMed] [Google Scholar]