Abstract
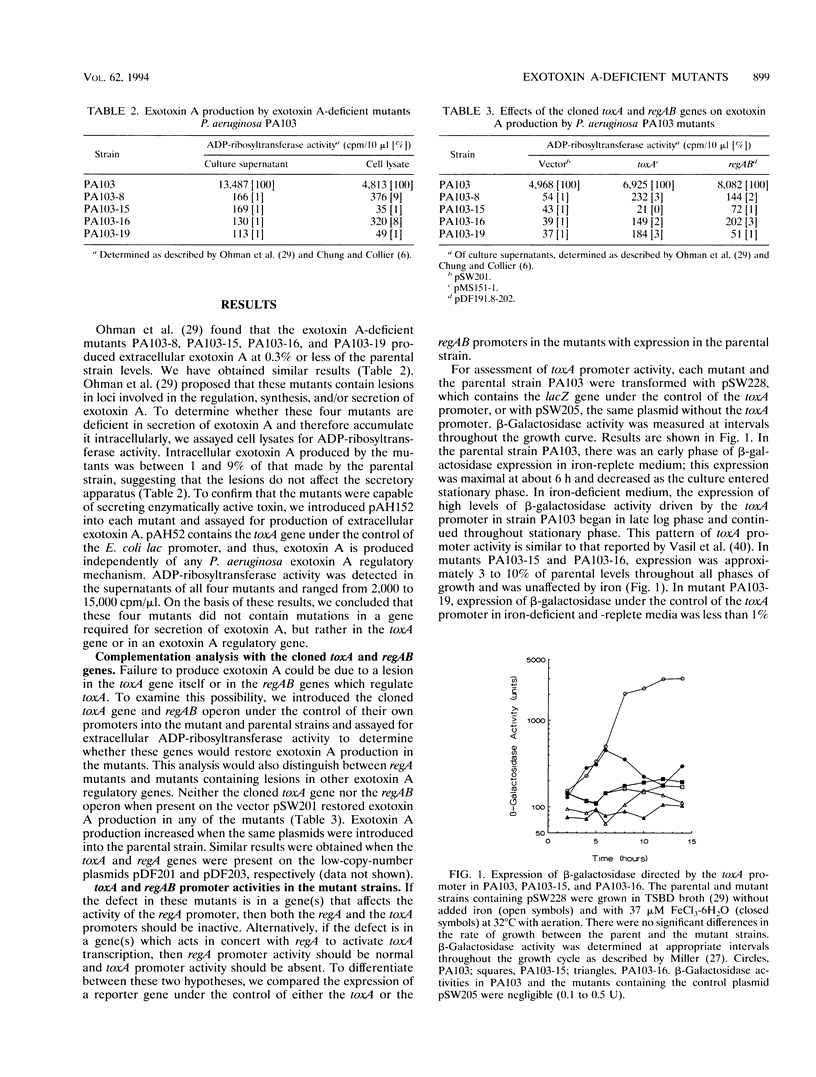
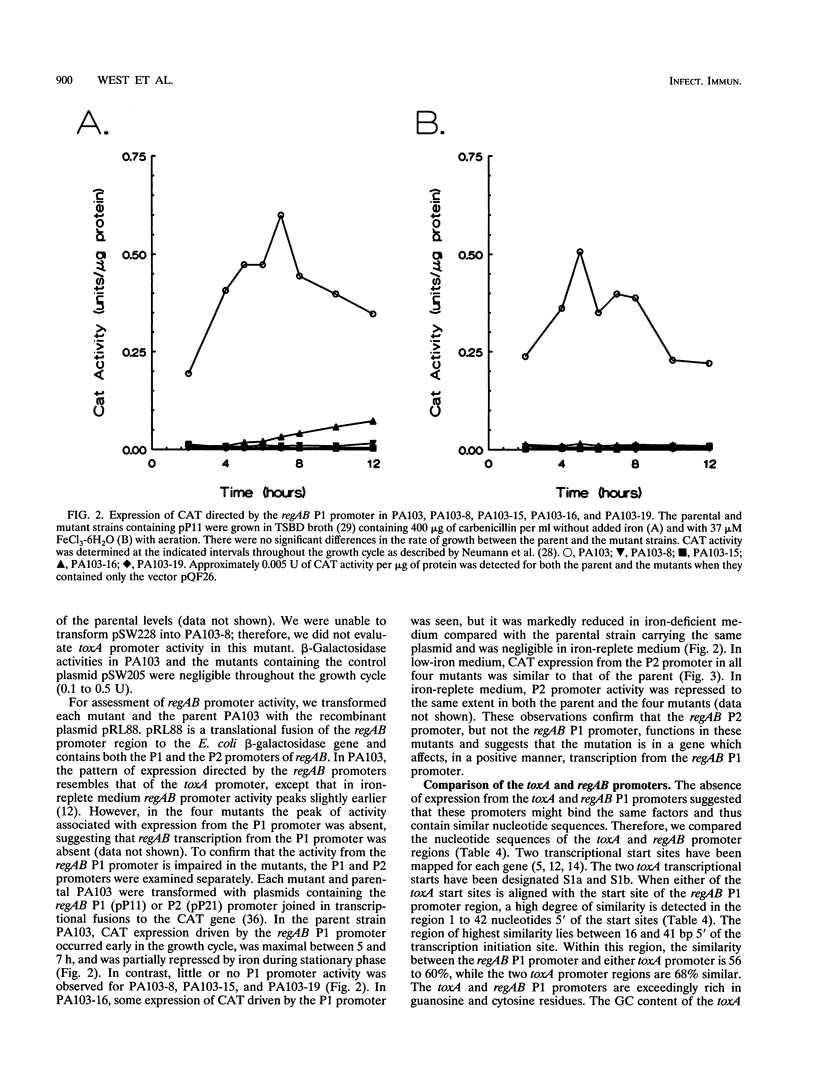
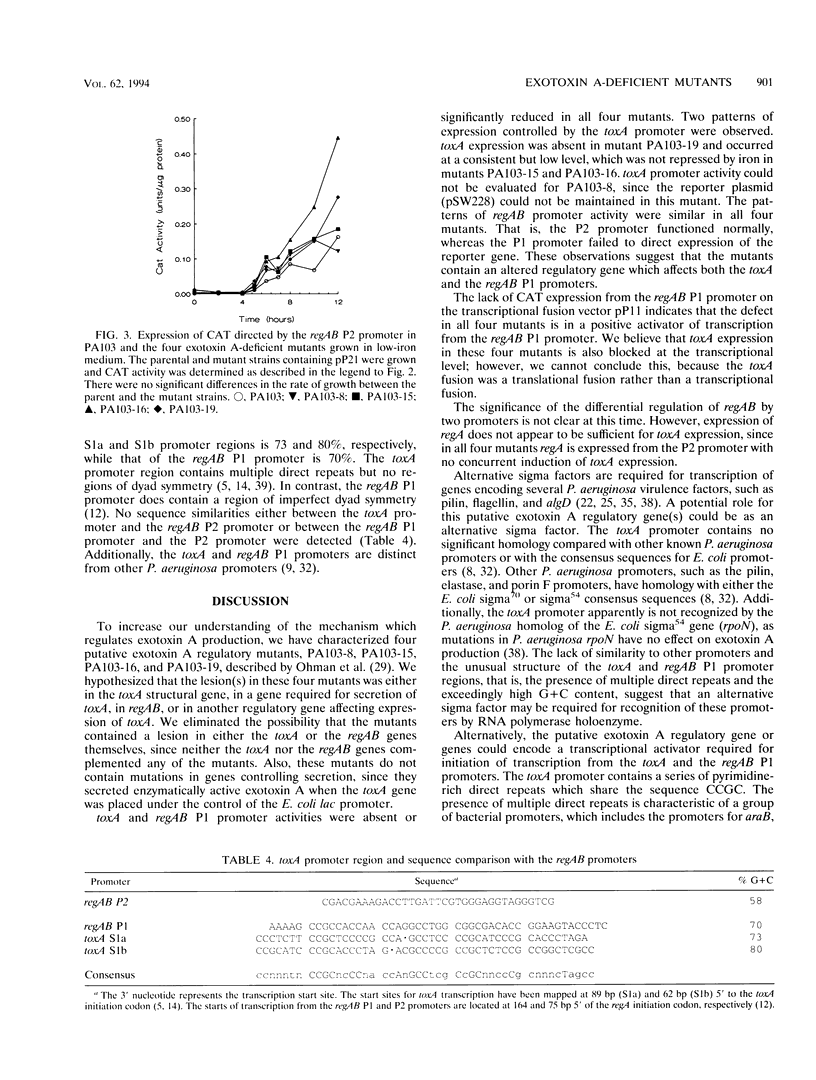
In Pseudomonas aeruginosa, production of exotoxin A, an ADP-ribosyltransferase, is a complex and highly regulated process. Two positively acting regulatory genes, regA and regB, have been cloned and characterized. To identify additional exotoxin A regulatory genes, we have characterized four N-methyl-N'-nitro-N-nitrosoguanidine-generated mutants of P. aeruginosa PA103 which are deficient in exotoxin A production. These mutants (PA103-8, PA103-15, PA103-16, and PA103-19) do not accumulate intracellular exotoxin A and are not complemented by the cloned toxA or regAB genes. This observation indicates that the lesion(s) in the mutants is probably in an exotoxin A regulatory gene(s) and is not in the genes for secretion of exotoxin A or in the toxA or regAB genes. To assess the effect of the putative regulatory mutations on the toxA and regAB genes, we compared the activity of the toxA and regAB promoters in the mutant and parental strains using plasmids containing the genes for beta-galactosidase or chloramphenicol acetyltransferase under the control of either the toxA or the regAB promoter. The toxA promoter-beta-galactosidase fusion plasmid could not be maintained in PA103-8. beta-Galactosidase expression driven by the toxA promoter was absent in the mutant PA103-19 and occurred at a low level, which was not repressed by iron in mutants PA103-15 and PA103-16. The regAB genes are temporally controlled by two promoters, P1 and P2. In all four mutants, regAB P1 promoter activity was reduced; however, expression under the control of the regAB P2 promoter was normal. These observations suggest the existence of one or more regulatory genes which directly affect expression of both the toxA and the regAB P1 promoters.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagdasarian M., Lurz R., Rückert B., Franklin F. C., Bagdasarian M. M., Frey J., Timmis K. N. Specific-purpose plasmid cloning vectors. II. Broad host range, high copy number, RSF1010-derived vectors, and a host-vector system for gene cloning in Pseudomonas. Gene. 1981 Dec;16(1-3):237–247. doi: 10.1016/0378-1119(81)90080-9. [DOI] [PubMed] [Google Scholar]
- Bagdasarian M., Timmis K. N. Host: vector systems for gene cloning in Pseudomonas. Curr Top Microbiol Immunol. 1982;96:47–67. doi: 10.1007/978-3-642-68315-2_4. [DOI] [PubMed] [Google Scholar]
- Bjorn M. J., Iglewski B. H., Ives S. K., Sadoff J. C., Vasil M. L. Effect of iron on yields of exotoxin A in cultures of Pseudomonas aeruginosa PA-103. Infect Immun. 1978 Mar;19(3):785–791. doi: 10.1128/iai.19.3.785-791.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumentals I. I., Kelly R. M., Gorziglia M., Kaufman J. B., Shiloach J. Development of a defined medium and two-step culturing method for improved exotoxin A yields from Pseudomonas aeruginosa. Appl Environ Microbiol. 1987 Sep;53(9):2013–2020. doi: 10.1128/aem.53.9.2013-2020.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S. T., Jordan E. M., Wilson R. B., Draper R. K., Clowes R. C. Transcription and expression of the exotoxin A gene of Pseudomonas aeruginosa. J Gen Microbiol. 1987 Nov;133(11):3081–3091. doi: 10.1099/00221287-133-11-3081. [DOI] [PubMed] [Google Scholar]
- Darzins A., Chakrabarty A. M. Cloning of genes controlling alginate biosynthesis from a mucoid cystic fibrosis isolate of Pseudomonas aeruginosa. J Bacteriol. 1984 Jul;159(1):9–18. doi: 10.1128/jb.159.1.9-18.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deretic V., Konyecsni W. M. A procaryotic regulatory factor with a histone H1-like carboxy-terminal domain: clonal variation of repeats within algP, a gene involved in regulation of mucoidy in Pseudomonas aeruginosa. J Bacteriol. 1990 Oct;172(10):5544–5554. doi: 10.1128/jb.172.10.5544-5554.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farinha M. A., Kropinski A. M. Construction of broad-host-range vectors for general cloning and promoter selection in Pseudomonas and Escherichia coli. Gene. 1989 Apr 30;77(2):205–210. doi: 10.1016/0378-1119(89)90068-1. [DOI] [PubMed] [Google Scholar]
- Frank D. W., Iglewski B. H. Kinetics of toxA and regA mRNA accumulation in Pseudomonas aeruginosa. J Bacteriol. 1988 Oct;170(10):4477–4483. doi: 10.1128/jb.170.10.4477-4483.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank D. W., Storey D. G., Hindahl M. S., Iglewski B. H. Differential regulation by iron of regA and toxA transcript accumulation in Pseudomonas aeruginosa. J Bacteriol. 1989 Oct;171(10):5304–5313. doi: 10.1128/jb.171.10.5304-5313.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman A. M., Long S. R., Brown S. E., Buikema W. J., Ausubel F. M. Construction of a broad host range cosmid cloning vector and its use in the genetic analysis of Rhizobium mutants. Gene. 1982 Jun;18(3):289–296. doi: 10.1016/0378-1119(82)90167-6. [DOI] [PubMed] [Google Scholar]
- Grant C. C., Vasil M. L. Analysis of transcription of the exotoxin A gene of Pseudomonas aeruginosa. J Bacteriol. 1986 Dec;168(3):1112–1119. doi: 10.1128/jb.168.3.1112-1119.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray G. L., Smith D. H., Baldridge J. S., Harkins R. N., Vasil M. L., Chen E. Y., Heyneker H. L. Cloning, nucleotide sequence, and expression in Escherichia coli of the exotoxin A structural gene of Pseudomonas aeruginosa. Proc Natl Acad Sci U S A. 1984 May;81(9):2645–2649. doi: 10.1073/pnas.81.9.2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamood A. N., Olson J. C., Vincent T. S., Iglewski B. H. Regions of toxin A involved in toxin A excretion in Pseudomonas aeruginosa. J Bacteriol. 1989 Apr;171(4):1817–1824. doi: 10.1128/jb.171.4.1817-1824.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamood A. N., Wick M. J., Iglewski B. H. Secretion of toxin A from Pseudomonas aeruginosa PAO1, PAK, and PA103 by Escherichia coli. Infect Immun. 1990 May;58(5):1133–1140. doi: 10.1128/iai.58.5.1133-1140.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedstrom R. C., Funk C. R., Kaper J. B., Pavlovskis O. R., Galloway D. R. Cloning of a gene involved in regulation of exotoxin A expression in Pseudomonas aeruginosa. Infect Immun. 1986 Jan;51(1):37–42. doi: 10.1128/iai.51.1.37-42.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindahl M. S., Frank D. W., Hamood A., Iglewski B. H. Characterization of a gene that regulates toxin A synthesis in Pseudomonas aeruginosa. Nucleic Acids Res. 1988 Jun 24;16(12):5699–5699. doi: 10.1093/nar/16.12.5699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindahl M. S., Frank D. W., Iglewski B. H. Molecular studies of a positive regulator of toxin A synthesis in Pseudomonas aeruginosa. Antibiot Chemother (1971) 1987;39:279–289. doi: 10.1159/000414353. [DOI] [PubMed] [Google Scholar]
- Iglewski B. H., Kabat D. NAD-dependent inhibition of protein synthesis by Pseudomonas aeruginosa toxin,. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2284–2288. doi: 10.1073/pnas.72.6.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishimoto K. S., Lory S. Formation of pilin in Pseudomonas aeruginosa requires the alternative sigma factor (RpoN) of RNA polymerase. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1954–1957. doi: 10.1073/pnas.86.6.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato J., Misra T. K., Chakrabarty A. M. AlgR3, a protein resembling eukaryotic histone H1, regulates alginate synthesis in Pseudomonas aeruginosa. Proc Natl Acad Sci U S A. 1990 Apr;87(8):2887–2891. doi: 10.1073/pnas.87.8.2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P. V. Exotoxins of Pseudomonas aeruginosa. I. Factors that influence the production of exotoxin A. J Infect Dis. 1973 Oct;128(4):506–513. doi: 10.1093/infdis/128.4.506. [DOI] [PubMed] [Google Scholar]
- Martin D. W., Holloway B. W., Deretic V. Characterization of a locus determining the mucoid status of Pseudomonas aeruginosa: AlgU shows sequence similarities with a Bacillus sigma factor. J Bacteriol. 1993 Feb;175(4):1153–1164. doi: 10.1128/jb.175.4.1153-1164.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. F., Mekalanos J. J., Falkow S. Coordinate regulation and sensory transduction in the control of bacterial virulence. Science. 1989 Feb 17;243(4893):916–922. doi: 10.1126/science.2537530. [DOI] [PubMed] [Google Scholar]
- Ohman D. E., Sadoff J. C., Iglewski B. H. Toxin A-deficient mutants of Pseudomonas aeruginosa PA103: isolation and characterization. Infect Immun. 1980 Jun;28(3):899–908. doi: 10.1128/iai.28.3.899-908.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen R. H., DeBusscher G., McCombie W. R. Development of broad-host-range vectors and gene banks: self-cloning of the Pseudomonas aeruginosa PAO chromosome. J Bacteriol. 1982 Apr;150(1):60–69. doi: 10.1128/jb.150.1.60-69.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raibaud O. Nucleoprotein structures at positively regulated bacterial promoters: homology with replication origins and some hypotheses on the quaternary structure of the activator proteins in these complexes. Mol Microbiol. 1989 Mar;3(3):455–458. doi: 10.1111/j.1365-2958.1989.tb00191.x. [DOI] [PubMed] [Google Scholar]
- Smith A. W., Iglewski B. H. Transformation of Pseudomonas aeruginosa by electroporation. Nucleic Acids Res. 1989 Dec 25;17(24):10509–10509. doi: 10.1093/nar/17.24.10509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starnbach M. N., Lory S. The fliA (rpoF) gene of Pseudomonas aeruginosa encodes an alternative sigma factor required for flagellin synthesis. Mol Microbiol. 1992 Feb;6(4):459–469. doi: 10.1111/j.1365-2958.1992.tb01490.x. [DOI] [PubMed] [Google Scholar]
- Storey D. G., Frank D. W., Farinha M. A., Kropinski A. M., Iglewski B. H. Multiple promoters control the regulation of the Pseudomonas aeruginosa regA gene. Mol Microbiol. 1990 Mar;4(3):499–503. doi: 10.1111/j.1365-2958.1990.tb00616.x. [DOI] [PubMed] [Google Scholar]
- Storey D. G., Raivio T. L., Frank D. W., Wick M. J., Kaye S., Iglewski B. H. Effect of regB on expression from the P1 and P2 promoters of the Pseudomonas aeruginosa regAB operon. J Bacteriol. 1991 Oct;173(19):6088–6094. doi: 10.1128/jb.173.19.6088-6094.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Totten P. A., Lara J. C., Lory S. The rpoN gene product of Pseudomonas aeruginosa is required for expression of diverse genes, including the flagellin gene. J Bacteriol. 1990 Jan;172(1):389–396. doi: 10.1128/jb.172.1.389-396.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsaur M. L., Clowes R. C. Localization of the control region for expression of exotoxin A in Pseudomonas aeruginosa. J Bacteriol. 1989 May;171(5):2599–2604. doi: 10.1128/jb.171.5.2599-2604.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasil M. L., Grant C. C., Prince R. W. Regulation of exotoxin A synthesis in Pseudomonas aeruginosa: characterization of toxA-lacZ fusions in wild-type and mutant strains. Mol Microbiol. 1989 Mar;3(3):371–381. doi: 10.1111/j.1365-2958.1989.tb00182.x. [DOI] [PubMed] [Google Scholar]
- Wick M. J., Frank D. W., Storey D. G., Iglewski B. H. Identification of regB, a gene required for optimal exotoxin A yields in Pseudomonas aeruginosa. Mol Microbiol. 1990 Mar;4(3):489–497. doi: 10.1111/j.1365-2958.1990.tb00615.x. [DOI] [PubMed] [Google Scholar]
- Zimniak L., Dayn A., Iglewski B. H. Identification of RegA protein from Pseudomonas aeruginosa using anti-RegA antibody. Biochem Biophys Res Commun. 1989 Sep 29;163(3):1312–1318. doi: 10.1016/0006-291x(89)91121-2. [DOI] [PubMed] [Google Scholar]



