Abstract
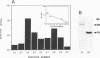
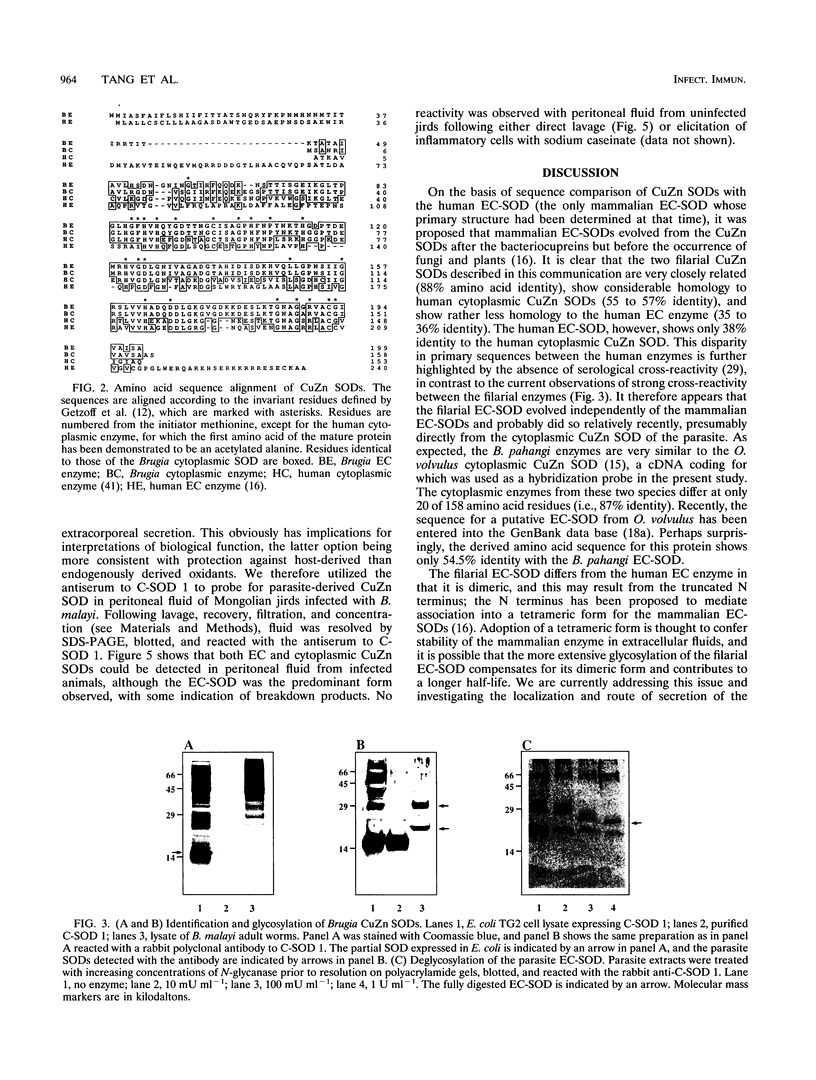
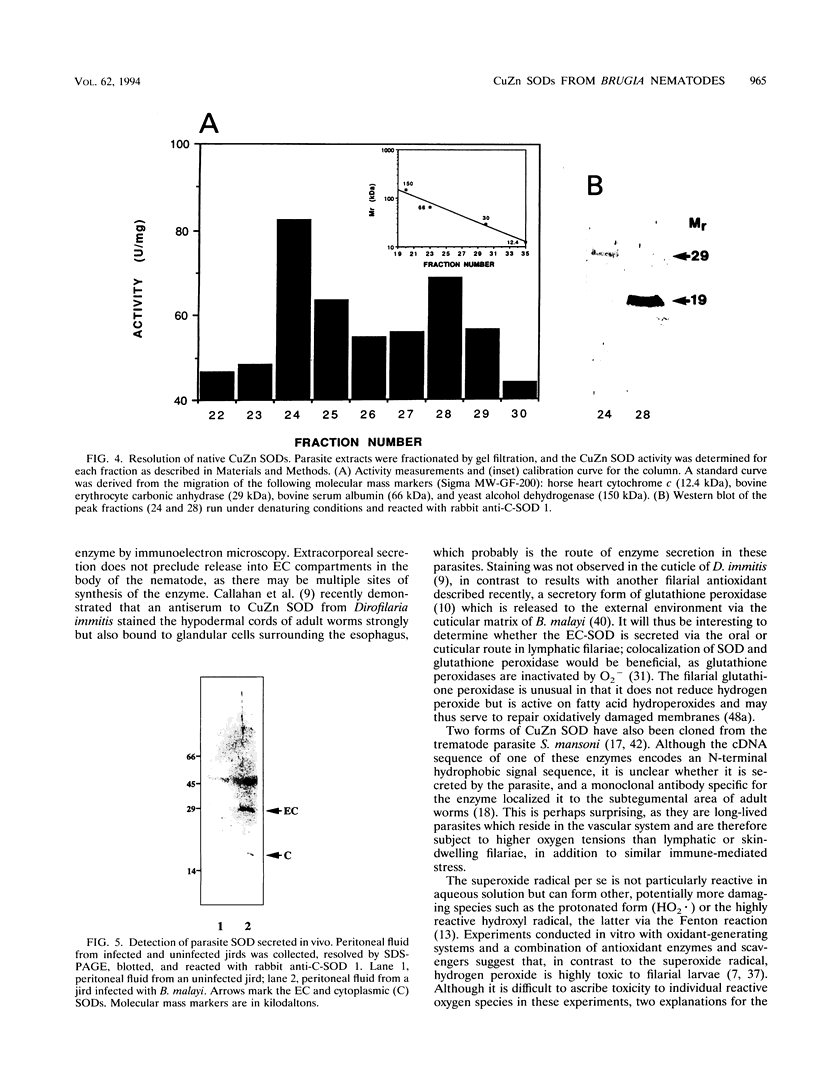
We have isolated full-length cDNAs encoding two distinct types of CuZn superoxide dismutases (SODs) from the filarial nematode parasite Brugia pahangi. The derived amino acid sequences suggested that one class of cDNAs represented a cytoplasmic form of SOD and the second class represented an extracellular (EC) variant. The predicted proteins were highly homologous to each other, but the sequence of the latter contained an additional 43 residues at the N terminus, the first 16 of which were markedly hydrophobic, and four potential sites for N-linked glycosylation. Western blotting (immunoblotting) with an antiserum to a partial SOD expressed in Escherichia coli revealed two proteins with estimated molecular masses of 19 and 29 kDa. Digestion with N-glycanase indicated that the latter protein corresponded to the EC form, as it possessed N-linked oligosaccharide chains at three sites, leaving a peptide backbone with an estimated molecular mass of 22 kDa, which was consistent with the additional 27 amino acids predicted from the cDNA sequence. Gel filtration indicated that both enzymes were dimeric in their native forms, in contrast to the human EC-SOD, which is tetrameric. Comparison of the primary structure of the parasite EC-SOD with that of the human EC enzyme revealed two major differences: the N-terminal extension of the parasite enzyme was shorter by 25 residues, and it also lacked the C-terminal charged extension which mediates binding to cell surface sulfated proteoglycans. Lavage of Mongolian jirds infected intraperitoneally with Brugia malayi resulted in the recovery of filarial CuZn SODs, principally the EC form, indicating that this form of SOD is secreted in vivo. This EC enzyme may contribute to parasite persistence by neutralizing superoxide generated by activated leukocytes, thus acting as both an antioxidant and an anti-inflammatory factor.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adachi T., Marklund S. L. Interactions between human extracellular superoxide dismutase C and sulfated polysaccharides. J Biol Chem. 1989 May 25;264(15):8537–8541. [PubMed] [Google Scholar]
- Andersen P., Askgaard D., Ljungqvist L., Bennedsen J., Heron I. Proteins released from Mycobacterium tuberculosis during growth. Infect Immun. 1991 Jun;59(6):1905–1910. doi: 10.1128/iai.59.6.1905-1910.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaman B. L., Black C. M., Doughty F., Beaman L. Role of superoxide dismutase and catalase as determinants of pathogenicity of Nocardia asteroides: importance in resistance to microbicidal activities of human polymorphonuclear neutrophils. Infect Immun. 1985 Jan;47(1):135–141. doi: 10.1128/iai.47.1.135-141.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaman L., Beaman B. L. Monoclonal antibodies demonstrate that superoxide dismutase contributes to protection of Nocardia asteroides within the intact host. Infect Immun. 1990 Sep;58(9):3122–3128. doi: 10.1128/iai.58.9.3122-3128.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck B. L., Tabatabai L. B., Mayfield J. E. A protein isolated from Brucella abortus is a Cu-Zn superoxide dismutase. Biochemistry. 1990 Jan 16;29(2):372–376. doi: 10.1021/bi00454a010. [DOI] [PubMed] [Google Scholar]
- Callahan H. L., Crouch R. K., James E. R. Dirofilaria immitis superoxide dismutase: purification and characterization. Mol Biochem Parasitol. 1991 Dec;49(2):245–251. doi: 10.1016/0166-6851(91)90068-h. [DOI] [PubMed] [Google Scholar]
- Callahan H. L., Crouch R. K., James E. R. Helminth anti-oxidant enzymes: a protective mechanism against host oxidants? Parasitol Today. 1988 Aug;4(8):218–225. doi: 10.1016/0169-4758(88)90162-7. [DOI] [PubMed] [Google Scholar]
- Callahan H. L., Crouch R. K., James E. R. Hydrogen peroxide is the most toxic oxygen species for Onchocerca cervicalis microfilariae. Parasitology. 1990 Jun;100(Pt 3):407–415. doi: 10.1017/s0031182000078690. [DOI] [PubMed] [Google Scholar]
- Callahan H. L., Hazen-Martin D., Crouch R. K., James E. R. Immunolocalization of superoxide dismutase in Dirofilaria immitis adult worms. Infect Immun. 1993 Mar;61(3):1157–1163. doi: 10.1128/iai.61.3.1157-1163.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cookson E., Blaxter M. L., Selkirk M. E. Identification of the major soluble cuticular glycoprotein of lymphatic filarial nematode parasites (gp29) as a secretory homolog of glutathione peroxidase. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):5837–5841. doi: 10.1073/pnas.89.13.5837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalbey R. E., Von Heijne G. Signal peptidases in prokaryotes and eukaryotes--a new protease family. Trends Biochem Sci. 1992 Nov;17(11):474–478. doi: 10.1016/0968-0004(92)90492-r. [DOI] [PubMed] [Google Scholar]
- Getzoff E. D., Tainer J. A., Stempien M. M., Bell G. I., Hallewell R. A. Evolution of CuZn superoxide dismutase and the Greek key beta-barrel structural motif. Proteins. 1989;5(4):322–336. doi: 10.1002/prot.340050408. [DOI] [PubMed] [Google Scholar]
- Hamann K. J., Gleich G. J., Checkel J. L., Loegering D. A., McCall J. W., Barker R. L. In vitro killing of microfilariae of Brugia pahangi and Brugia malayi by eosinophil granule proteins. J Immunol. 1990 Apr 15;144(8):3166–3173. [PubMed] [Google Scholar]
- Henkle K. J., Liebau E., Müller S., Bergmann B., Walter R. D. Characterization and molecular cloning of a Cu/Zn superoxide dismutase from the human parasite Onchocerca volvulus. Infect Immun. 1991 Jun;59(6):2063–2069. doi: 10.1128/iai.59.6.2063-2069.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjalmarsson K., Marklund S. L., Engström A., Edlund T. Isolation and sequence of complementary DNA encoding human extracellular superoxide dismutase. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6340–6344. doi: 10.1073/pnas.84.18.6340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong Z., LoVerde P. T., Hammarskjöld M. L., Rekosh D. Schistosoma mansoni: cloning of a complementary DNA encoding a cytosolic Cu/Zn superoxide dismutase and high-yield expression of the enzymatically active gene product in Escherichia coli. Exp Parasitol. 1992 Nov;75(3):308–322. doi: 10.1016/0014-4894(92)90216-w. [DOI] [PubMed] [Google Scholar]
- Hong Z., LoVerde P. T., Thakur A., Hammarskjöld M. L., Rekosh D. Schistosoma mansoni: a Cu/Zn superoxide dismutase is glycosylated when expressed in mammalian cells and localizes to a subtegumental region in adult schistosomes. Exp Parasitol. 1993 Mar;76(2):101–114. doi: 10.1006/expr.1993.1012. [DOI] [PubMed] [Google Scholar]
- Karlsson K., Marklund S. L. Heparin-induced release of extracellular superoxide dismutase to human blood plasma. Biochem J. 1987 Feb 15;242(1):55–59. doi: 10.1042/bj2420055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause M., Hirsh D. A trans-spliced leader sequence on actin mRNA in C. elegans. Cell. 1987 Jun 19;49(6):753–761. doi: 10.1016/0092-8674(87)90613-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroll J. S., Langford P. R., Loynds B. M. Copper-zinc superoxide dismutase of Haemophilus influenzae and H. parainfluenzae. J Bacteriol. 1991 Dec;173(23):7449–7457. doi: 10.1128/jb.173.23.7449-7457.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusunose E., Ichihara K., Noda Y., Kusunose M. Superoxide dismutase from Mycobacterium tuberculosis. J Biochem. 1976 Dec;80(6):1343–1352. doi: 10.1093/oxfordjournals.jbchem.a131407. [DOI] [PubMed] [Google Scholar]
- Lynch R. E., Fridovich I. Permeation of the erythrocyte stroma by superoxide radical. J Biol Chem. 1978 Jul 10;253(13):4697–4699. [PubMed] [Google Scholar]
- Maizels R. M., Bundy D. A., Selkirk M. E., Smith D. F., Anderson R. M. Immunological modulation and evasion by helminth parasites in human populations. Nature. 1993 Oct 28;365(6449):797–805. doi: 10.1038/365797a0. [DOI] [PubMed] [Google Scholar]
- Maizels R. M., Gregory W. F., Kwan-Lim G. E., Selkirk M. E. Filarial surface antigens: the major 29 kilodalton glycoprotein and a novel 17-200 kilodalton complex from adult Brugia malayi parasites. Mol Biochem Parasitol. 1989 Jan 15;32(2-3):213–227. doi: 10.1016/0166-6851(89)90072-8. [DOI] [PubMed] [Google Scholar]
- Mao G. D., Thomas P. D., Lopaschuk G. D., Poznansky M. J. Superoxide dismutase (SOD)-catalase conjugates. Role of hydrogen peroxide and the Fenton reaction in SOD toxicity. J Biol Chem. 1993 Jan 5;268(1):416–420. [PubMed] [Google Scholar]
- Marklund S. L. Human copper-containing superoxide dismutase of high molecular weight. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7634–7638. doi: 10.1073/pnas.79.24.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marklund S. L. Properties of extracellular superoxide dismutase from human lung. Biochem J. 1984 May 15;220(1):269–272. doi: 10.1042/bj2200269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- Meister A., Anderson M. E. Glutathione. Annu Rev Biochem. 1983;52:711–760. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- Paxton W. A., Yazdanbakhsh M., Kurniawan A., Partono F., Maizels R. M., Selkirk M. E. Primary structure of and immunoglobulin E response to the repeat subunit of gp15/400 from human lymphatic filarial parasites. Infect Immun. 1993 Jul;61(7):2827–2833. doi: 10.1128/iai.61.7.2827-2833.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry A. C., Jones R., Hall L. Isolation and characterization of a rat cDNA clone encoding a secreted superoxide dismutase reveals the epididymis to be a major site of its expression. Biochem J. 1993 Jul 1;293(Pt 1):21–25. doi: 10.1042/bj2930021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry A. C., Jones R., Niang L. S., Jackson R. M., Hall L. Genetic evidence for an androgen-regulated epididymal secretory glutathione peroxidase whose transcript does not contain a selenocysteine codon. Biochem J. 1992 Aug 1;285(Pt 3):863–870. doi: 10.1042/bj2850863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrone W. F., English D. K., Wong K., McCord J. M. Free radicals and inflammation: superoxide-dependent activation of a neutrophil chemotactic factor in plasma. Proc Natl Acad Sci U S A. 1980 Feb;77(2):1159–1163. doi: 10.1073/pnas.77.2.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhoads M. L. Trichinella spiralis: identification and purification of superoxide dismutase. Exp Parasitol. 1983 Aug;56(1):41–54. doi: 10.1016/0014-4894(83)90095-4. [DOI] [PubMed] [Google Scholar]
- Rzepczyk C. M., Bishop C. J. Immunological and ultrastructural aspects of the cell-mediated killing of Dirofilaria immitis microfilariae. Parasite Immunol. 1984 Sep;6(5):443–457. doi: 10.1111/j.1365-3024.1984.tb00815.x. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkirk M. E., Denham D. A., Partono F., Sutanto I., Maizels R. M. Molecular characterization of antigens of lymphatic filarial parasites. Parasitology. 1986;92 (Suppl):S15–S38. doi: 10.1017/s003118200008567x. [DOI] [PubMed] [Google Scholar]
- Selkirk M. E., Gregory W. F., Yazdanbakhsh M., Jenkins R. E., Maizels R. M. Cuticular localisation and turnover of the major surface glycoprotein (gp29) of adult Brugia malayi. Mol Biochem Parasitol. 1990 Aug;42(1):31–43. doi: 10.1016/0166-6851(90)90110-8. [DOI] [PubMed] [Google Scholar]
- Sherman L., Dafni N., Lieman-Hurwitz J., Groner Y. Nucleotide sequence and expression of human chromosome 21-encoded superoxide dismutase mRNA. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5465–5469. doi: 10.1073/pnas.80.18.5465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simurda M. C., van Keulen H., Rekosh D. M., LoVerde P. T. Schistosoma mansoni: identification and analysis of an mRNA and a gene encoding superoxide dismutase (Cu/Zn). Exp Parasitol. 1988 Oct;67(1):73–84. doi: 10.1016/0014-4894(88)90010-0. [DOI] [PubMed] [Google Scholar]
- Steinman H. M. Bacteriocuprein superoxide dismutase of Photobacterium leiognathi. Isolation and sequence of the gene and evidence for a precursor form. J Biol Chem. 1987 Feb 5;262(4):1882–1887. [PubMed] [Google Scholar]
- Steinman H. M. Bacteriocuprein superoxide dismutases in pseudomonads. J Bacteriol. 1985 Jun;162(3):1255–1260. doi: 10.1128/jb.162.3.1255-1260.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman H. M. Copper-zinc superoxide dismutase from Caulobacter crescentus CB15. A novel bacteriocuprein form of the enzyme. J Biol Chem. 1982 Sep 10;257(17):10283–10293. [PubMed] [Google Scholar]
- Steinman H. M., Ely B. Copper-zinc superoxide dismutase of Caulobacter crescentus: cloning, sequencing, and mapping of the gene and periplasmic location of the enzyme. J Bacteriol. 1990 Jun;172(6):2901–2910. doi: 10.1128/jb.172.6.2901-2910.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi M. A., Asada K. Superoxide anion permeability of phospholipid membranes and chloroplast thylakoids. Arch Biochem Biophys. 1983 Oct 15;226(2):558–566. doi: 10.1016/0003-9861(83)90325-9. [DOI] [PubMed] [Google Scholar]
- Tatum F. M., Detilleux P. G., Sacks J. M., Halling S. M. Construction of Cu-Zn superoxide dismutase deletion mutants of Brucella abortus: analysis of survival in vitro in epithelial and phagocytic cells and in vivo in mice. Infect Immun. 1992 Jul;60(7):2863–2869. doi: 10.1128/iai.60.7.2863-2869.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignais P. M., Terech A., Meyer C. M., Henry M. F. Isolation and characterization of a protein with cyanide-sensitive superoxide dismutase activity from the prokaryote, Paracoccus denitrificans. Biochim Biophys Acta. 1982 Mar 4;701(3):305–317. doi: 10.1016/0167-4838(82)90233-3. [DOI] [PubMed] [Google Scholar]