Abstract
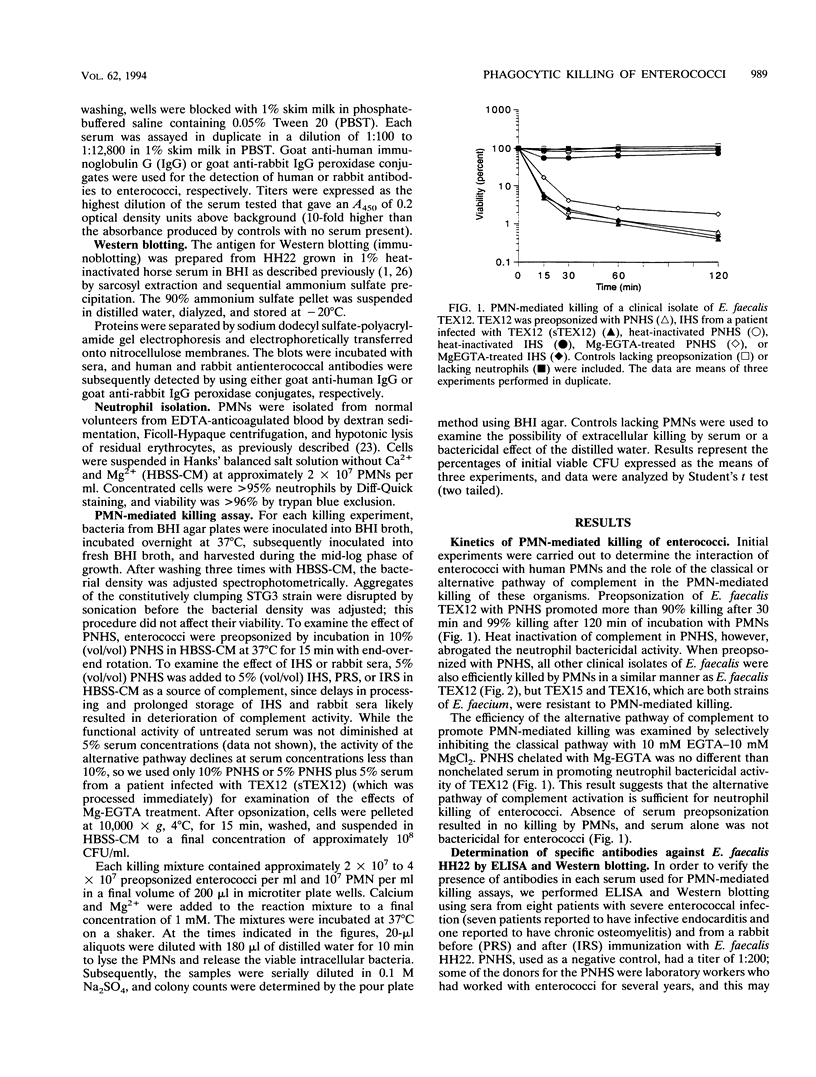
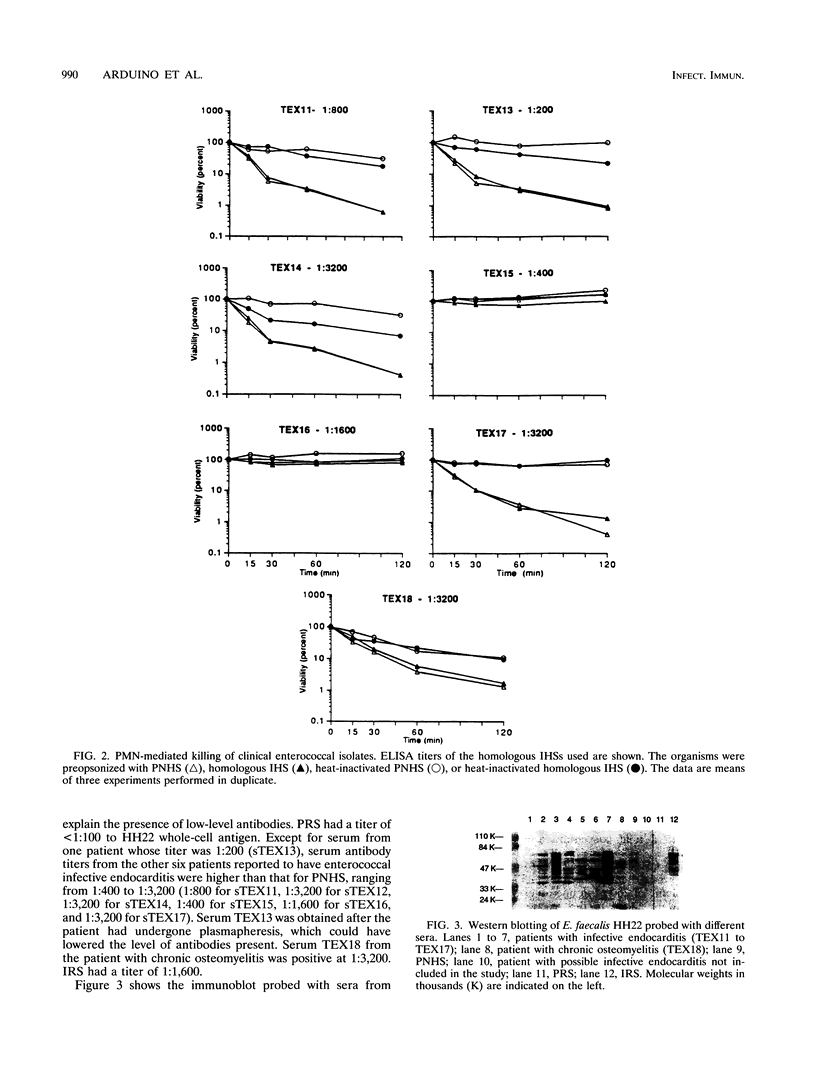
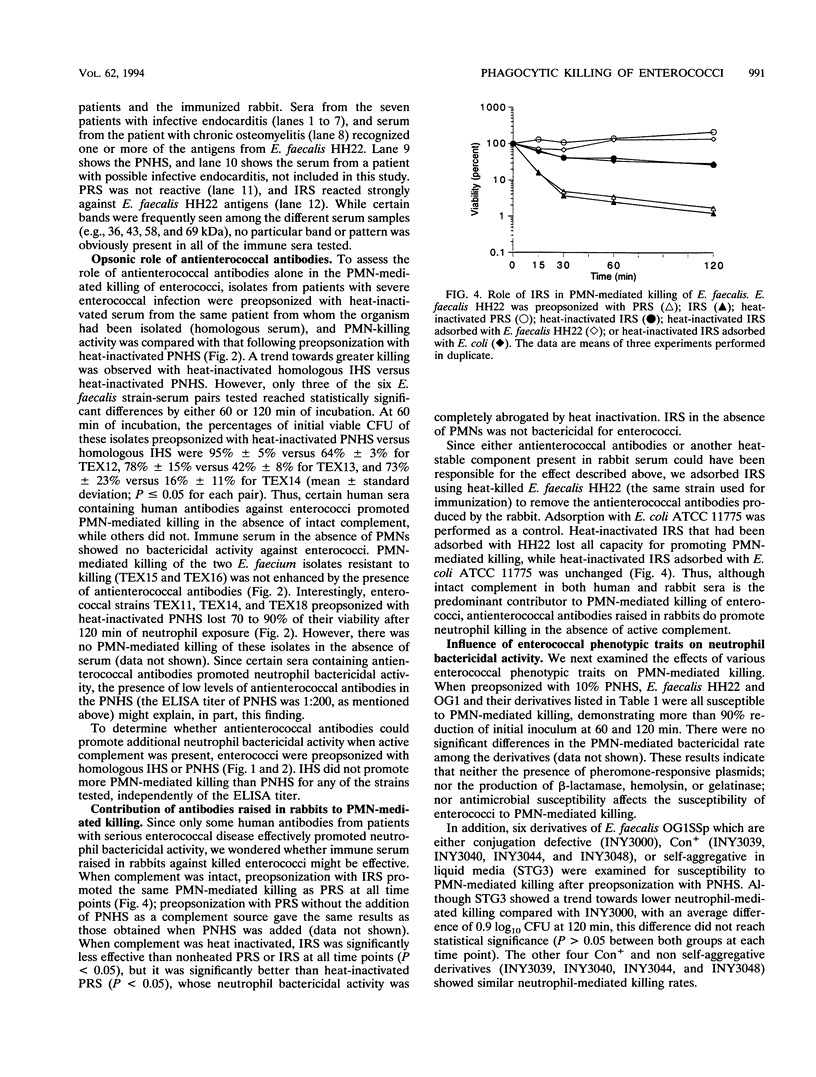
The contributions of complement and antibodies to polymorphonuclear leukocyte (PMN)-mediated killing of enterococci were investigated with pooled normal human serum (PNHS) or immune human sera (IHS) from patients with serious enterococcal infections. Each IHS containing antienterococcal antibodies demonstrated by enzyme-linked immunosorbent assay and Western blotting (immunoblotting) was examined with the enterococcus strain isolated from the same patient. PNHS promoted PMN-mediated killing of enterococci similar to that for IHS. PMN-mediated killing was consistently abrogated after preopsonization with heat-inactivated PNHS, but some heat-inactivated IHS supported neutrophil bactericidal activity. Inhibition of the classical pathway of complement by chelation of either PNHS or IHS with Mg-EGTA [Mg-ethylene glycol-bis(beta-aminoethyl ether)-N,N,N',N'-tetraacetic acid] did not alter PMN-mediated killing, suggesting that activation of the alternative pathway of complement is sufficient to promote killing of enterococci by PMNs. PMN-mediated killing assays were also performed with normal rabbit serum and immune rabbit serum against enterococci. Preopsonization with heat-inactivated immune rabbit serum resulted in PMN-mediated killing of enterococci, which was ablated after adsorption of the serum with the same isolate used for immunization. The influence of different phenotypic enterococcal traits on neutrophil-mediated killing was also investigated. Similar kinetics of killing were observed for derivatives of Enterococcus faecalis strains regardless of resistance to antimicrobial agents or production of beta-lactamase, hemolysin, gelatinase, or surface proteins involved in the aggregative response to pheromones. In summary, PMN-mediated killing of enterococci appears to depend primarily on complement activation by either the classical or the alternative pathway. Human antienterococcal antibodies generated during infection variably promoted neutrophil bactericidal activity, while antibody raised in a rabbit supported PMN-mediated killing of the organism examined. Finally, the different phenotypic properties of E. faecalis examined did not influence the neutrophil-mediated killing of these organisms.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aitchison E. J., Lambert P. A., Smith E. G., Farrell I. D. Serodiagnosis of Streptococcus faecalis endocarditis by immunoblotting of surface protein antigens. J Clin Microbiol. 1987 Feb;25(2):211–215. doi: 10.1128/jcm.25.2.211-215.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnie J. P., Clark I. Diagnosing endocarditis with the cloned 112 kDa antigen of Enterococcus faecalis. J Immunol Methods. 1989 Oct 24;123(2):217–225. doi: 10.1016/0022-1759(89)90225-1. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Weaver K. E. Sex pheromones and plasmid transfer in Enterococcus faecalis. Plasmid. 1989 May;21(3):175–184. doi: 10.1016/0147-619x(89)90041-3. [DOI] [PubMed] [Google Scholar]
- Ember J. A., Hugli T. E. Characterization of the human neutrophil response to sex pheromones from Streptococcus faecalis. Am J Pathol. 1989 Apr;134(4):797–805. [PMC free article] [PubMed] [Google Scholar]
- Facklam R. R., Collins M. D. Identification of Enterococcus species isolated from human infections by a conventional test scheme. J Clin Microbiol. 1989 Apr;27(4):731–734. doi: 10.1128/jcm.27.4.731-734.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsgren A., Quie P. G. Opsonic activity in human serum chelated with ethylene glycoltetra-acetic acid. Immunology. 1974 Jun;26(6):1251–1256. [PMC free article] [PubMed] [Google Scholar]
- Harvey B. S., Baker C. J., Edwards M. S. Contributions of complement and immunoglobulin to neutrophil-mediated killing of enterococci. Infect Immun. 1992 Sep;60(9):3635–3640. doi: 10.1128/iai.60.9.3635-3640.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodel-Christian S. L., Murray B. E. Characterization of the gentamicin resistance transposon Tn5281 from Enterococcus faecalis and comparison to staphylococcal transposons Tn4001 and Tn4031. Antimicrob Agents Chemother. 1991 Jun;35(6):1147–1152. doi: 10.1128/aac.35.6.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ike Y., Hashimoto H., Clewell D. B. Hemolysin of Streptococcus faecalis subspecies zymogenes contributes to virulence in mice. Infect Immun. 1984 Aug;45(2):528–530. doi: 10.1128/iai.45.2.528-530.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jett B. D., Jensen H. G., Nordquist R. E., Gilmore M. S. Contribution of the pAD1-encoded cytolysin to the severity of experimental Enterococcus faecalis endophthalmitis. Infect Immun. 1992 Jun;60(6):2445–2452. doi: 10.1128/iai.60.6.2445-2452.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreft B., Marre R., Schramm U., Wirth R. Aggregation substance of Enterococcus faecalis mediates adhesion to cultured renal tubular cells. Infect Immun. 1992 Jan;60(1):25–30. doi: 10.1128/iai.60.1.25-30.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogstad D. J., Korfhagen T. R., Moellering R. C., Jr, Wennersten C., Swartz M. N. Aminoglycoside-inactivating enzymes in clinical isolates of Streptococcus faecalis. An explanation for resistance to antibiotic synergism. J Clin Invest. 1978 Aug;62(2):480–486. doi: 10.1172/JCI109149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclercq R., Dutka-Malen S., Brisson-Noël A., Molinas C., Derlot E., Arthur M., Duval J., Courvalin P. Resistance of enterococci to aminoglycosides and glycopeptides. Clin Infect Dis. 1992 Sep;15(3):495–501. doi: 10.1093/clind/15.3.495. [DOI] [PubMed] [Google Scholar]
- Moellering R. C., Jr Emergence of Enterococcus as a significant pathogen. Clin Infect Dis. 1992 Jun;14(6):1173–1176. doi: 10.1093/clinids/14.6.1173. [DOI] [PubMed] [Google Scholar]
- Murray B. E., An F. Y., Clewell D. B. Plasmids and pheromone response of the beta-lactamase producer Streptococcus (Enterococcus) faecalis HH22. Antimicrob Agents Chemother. 1988 Apr;32(4):547–551. doi: 10.1128/aac.32.4.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray B. E. Beta-lactamase-producing enterococci. Antimicrob Agents Chemother. 1992 Nov;36(11):2355–2359. doi: 10.1128/aac.36.11.2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray B. E., Mederski-Samaroj B. Transferable beta-lactamase. A new mechanism for in vitro penicillin resistance in Streptococcus faecalis. J Clin Invest. 1983 Sep;72(3):1168–1171. doi: 10.1172/JCI111042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray B. E., Mederski-Samoraj B., Foster S. K., Brunton J. L., Harford P. In vitro studies of plasmid-mediated penicillinase from Streptococcus faecalis suggest a staphylococcal origin. J Clin Invest. 1986 Jan;77(1):289–293. doi: 10.1172/JCI112289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray B. E. New aspects of antimicrobial resistance and the resulting therapeutic dilemmas. J Infect Dis. 1991 Jun;163(6):1184–1194. [PubMed] [Google Scholar]
- Newman S. L., Johnston R. B., Jr Role of binding through C3b and IgG in polymorphonuclear neutrophil function: studies with trypsin-generated C3b. J Immunol. 1979 Oct;123(4):1839–1846. [PubMed] [Google Scholar]
- Novak R. M., Holzer T. J., Libertin C. R. Human neutrophil oxidative response and phagocytic killing of clinical and laboratory strains of Enterococcus faecalis. Diagn Microbiol Infect Dis. 1993 Jul;17(1):1–6. doi: 10.1016/0732-8893(93)90061-b. [DOI] [PubMed] [Google Scholar]
- Rosen H., Michel B. R., Chait A. Phagocytosis of opsonized oil droplets by neutrophils. Adaptation to a microtiter plate format. J Immunol Methods. 1991 Nov 5;144(1):117–125. doi: 10.1016/0022-1759(91)90237-a. [DOI] [PubMed] [Google Scholar]
- Ross G. D., Medof M. E. Membrane complement receptors specific for bound fragments of C3. Adv Immunol. 1985;37:217–267. doi: 10.1016/s0065-2776(08)60341-7. [DOI] [PubMed] [Google Scholar]
- Schaberg D. R., Culver D. H., Gaynes R. P. Major trends in the microbial etiology of nosocomial infection. Am J Med. 1991 Sep 16;91(3B):72S–75S. doi: 10.1016/0002-9343(91)90346-y. [DOI] [PubMed] [Google Scholar]
- Shorrock P. J., Lambert P. A., Aitchison E. J., Smith E. G., Farrell I. D., Gutschik E. Serological response in Enterococcus faecalis endocarditis determined by enzyme-linked immunosorbent assay. J Clin Microbiol. 1990 Feb;28(2):195–200. doi: 10.1128/jcm.28.2.195-200.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotter K. M., Dunny G. M. Mutants of Enterococcus faecalis deficient as recipients in mating with donors carrying pheromone-inducible plasmids. Plasmid. 1990 Jul;24(1):57–67. doi: 10.1016/0147-619x(90)90025-8. [DOI] [PubMed] [Google Scholar]



