Abstract
The pathological manifestations of schistosomiasis mansoni are primarily induced by circumovum hepatic granuloma formation and fibrosis. The growth and modulation of the granulomas are regulated by T lymphocytes and their products. In the present study, we isolated T-lymphocyte clones from lesions at the acute and chronic stages of murine infection. All of the T-cell clones were characterized by immunofluorescence as CD4+CD8- helper cells. Three T-cell clones derived from vigorous granulomas produced interleukin 2 (IL-2), IL-4, and gamma interferon (IFN-gamma) and were therefore classified as TH0-type T lymphocytes. Of the three clones derived from immunomodulated lesions, two produced IL-2 or IL-4 and/or IFN-gamma (TH0 type) and the other one did not produce IFN-gamma but did produce IL-4 and was therefore characterized as a TH2-type helper clone. The clones were further characterized by their responsiveness to soluble egg antigen fractions. The acute infection-derived clones responded to the lower-molecular-mass (60- to 66-kDa and 25- to 30-kDa) fractions, whereas the immunomodulated-granuloma-derived clones responded to the 60- to 66-kDa and higher-molecular-mass (70- to 90-, 93- to 125-, and > 200-kDa) fractions. Upon adoptive transfer into naive mice, both the TH0- and TH2-type clones were capable of inducing granuloma formation of similar magnitude around antigen-coated beads and/or freshly isolated parasite eggs. The present study revealed the presence of TH0-type precursor helper cells within the liver granulomas. The findings underscore the complexity of the granulomatous response at the T-cell level and demonstrate that both TH0- and TH2-type granuloma lymphocytes play a role in parasite egg-induced granuloma formation.
Full text
PDF

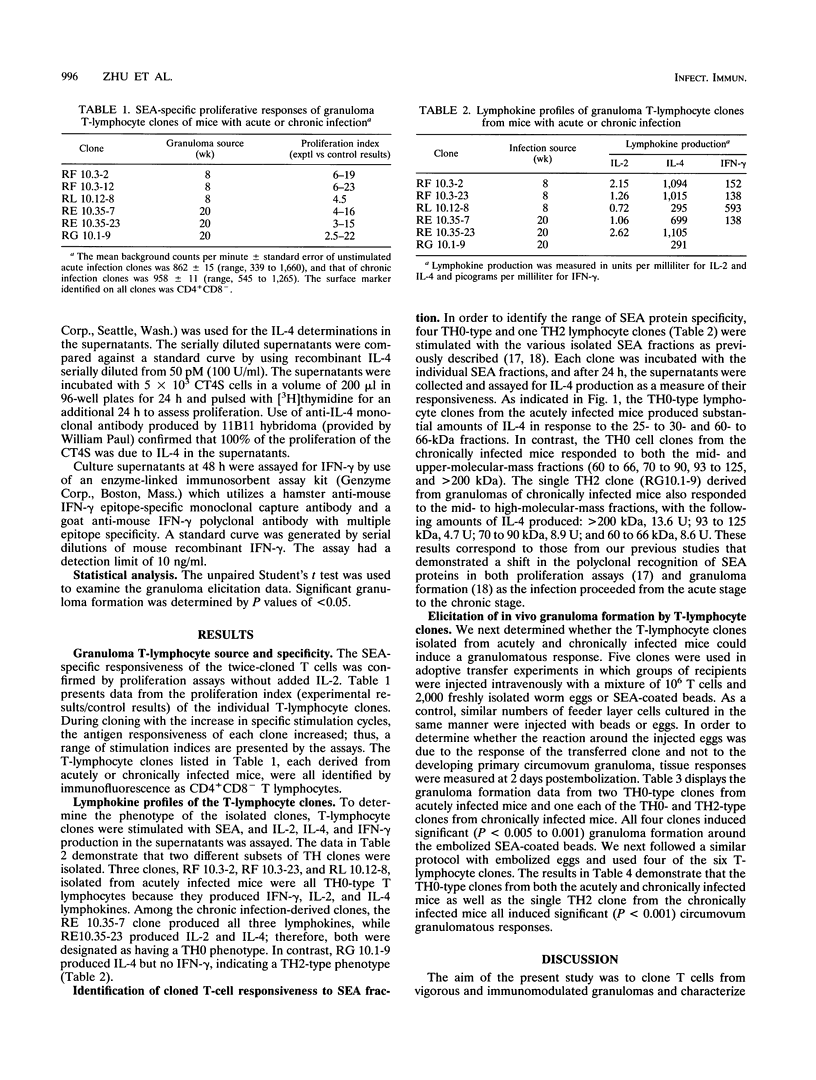
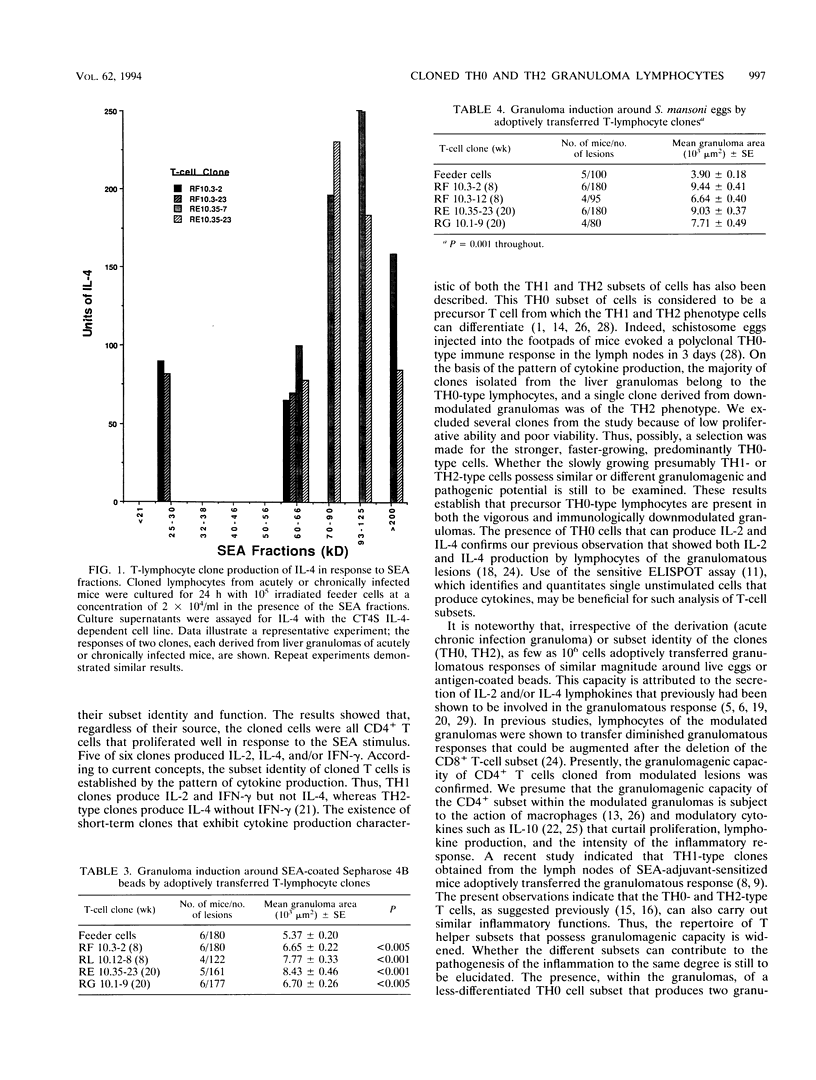


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abehsira-Amar O., Gibert M., Joliy M., Thèze J., Jankovic D. L. IL-4 plays a dominant role in the differential development of Tho into Th1 and Th2 cells. J Immunol. 1992 Jun 15;148(12):3820–3829. [PubMed] [Google Scholar]
- Boros D. L. Immunopathology of Schistosoma mansoni infection. Clin Microbiol Rev. 1989 Jul;2(3):250–269. doi: 10.1128/cmr.2.3.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boros D. L., Pelley R. P., Warren K. S. Spontaneous modulation of granulomatous hypersensitivity in schistosomiasis mansoni. J Immunol. 1975 May;114(5):1437–1441. [PubMed] [Google Scholar]
- Boros D. L., Warren K. S. Delayed hypersensitivity-type granuloma formation and dermal reaction induced and elicited by a soluble factor isolated from Schistosoma mansoni eggs. J Exp Med. 1970 Sep 1;132(3):488–507. doi: 10.1084/jem.132.3.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheever A. W., Finkelman F. D., Caspar P., Heiny S., Macedonia J. G., Sher A. Treatment with anti-IL-2 antibodies reduces hepatic pathology and eosinophilia in Schistosoma mansoni-infected mice while selectively inhibiting T cell IL-5 production. J Immunol. 1992 May 15;148(10):3244–3248. [PubMed] [Google Scholar]
- Chensue S. W., Terebuh P. D., Warmington K. S., Hershey S. D., Evanoff H. L., Kunkel S. L., Higashi G. I. Role of IL-4 and IFN-gamma in Schistosoma mansoni egg-induced hypersensitivity granuloma formation. Orchestration, relative contribution, and relationship to macrophage function. J Immunol. 1992 Feb 1;148(3):900–906. [PubMed] [Google Scholar]
- Chikunguwo S. M., Harris T. S., Brodeur P. H., Harn D. A., Stadecker M. J. The cell-mediated response to schistosomal antigens at the clonal level: development and characterization of a panel of egg antigen-specific murine T cell clones. Eur J Immunol. 1992 Apr;22(4):917–922. doi: 10.1002/eji.1830220406. [DOI] [PubMed] [Google Scholar]
- Chikunguwo S. M., Kanazawa T., Dayal Y., Stadecker M. J. The cell-mediated response to schistosomal antigens at the clonal level. In vivo functions of cloned murine egg antigen-specific CD4+ T helper type 1 lymphocytes. J Immunol. 1991 Dec 1;147(11):3921–3925. [PubMed] [Google Scholar]
- Chikunguwo S. M., Quinn J. J., Harn D. A., Stadecker M. J. The cell-mediated response to schistosomal antigens at the clonal level. III. Identification of soluble egg antigens recognized by cloned specific granulomagenic murine CD4+ Th1-type lymphocytes. J Immunol. 1993 Feb 15;150(4):1413–1421. [PubMed] [Google Scholar]
- Colley D. G. Immune responses to a soluble schistosomal egg antigen preparation during chronic primary infection with Schistosoma mansoni. J Immunol. 1975 Jul;115(1):150–156. [PubMed] [Google Scholar]
- Czerkinsky C., Andersson G., Ekre H. P., Nilsson L. A., Klareskog L., Ouchterlony O. Reverse ELISPOT assay for clonal analysis of cytokine production. I. Enumeration of gamma-interferon-secreting cells. J Immunol Methods. 1988 May 25;110(1):29–36. doi: 10.1016/0022-1759(88)90079-8. [DOI] [PubMed] [Google Scholar]
- Doughty B. L., Phillips S. M. Delayed hypersensitivity granuloma formation and modulation around Schistosoma mansoni eggs in vitro. II. Regulatory T cell subsets. J Immunol. 1982 Jan;128(1):37–42. [PubMed] [Google Scholar]
- Elliott D. E., Ragheb S., Wellhausen S. R., Boros D. L. Interactions between adherent mononuclear cells and lymphocytes from granulomas of mice with schistosomiasis mansoni. Infect Immun. 1990 Jun;58(6):1577–1583. doi: 10.1128/iai.58.6.1577-1583.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firestein G. S., Roeder W. D., Laxer J. A., Townsend K. S., Weaver C. T., Hom J. T., Linton J., Torbett B. E., Glasebrook A. L. A new murine CD4+ T cell subset with an unrestricted cytokine profile. J Immunol. 1989 Jul 15;143(2):518–525. [PubMed] [Google Scholar]
- Grzych J. M., Pearce E., Cheever A., Caulada Z. A., Caspar P., Heiny S., Lewis F., Sher A. Egg deposition is the major stimulus for the production of Th2 cytokines in murine schistosomiasis mansoni. J Immunol. 1991 Feb 15;146(4):1322–1327. [PubMed] [Google Scholar]
- Henderson G. S., Conary J. T., Summar M., McCurley T. L., Colley D. G. In vivo molecular analysis of lymphokines involved in the murine immune response during Schistosoma mansoni infection. I. IL-4 mRNA, not IL-2 mRNA, is abundant in the granulomatous livers, mesenteric lymph nodes, and spleens of infected mice. J Immunol. 1991 Aug 1;147(3):992–997. [PubMed] [Google Scholar]
- Lukacs N. W., Boros D. L. Lymphokine regulation of granuloma formation in murine schistosomiasis mansoni. Clin Immunol Immunopathol. 1993 Jul;68(1):57–63. doi: 10.1006/clin.1993.1095. [DOI] [PubMed] [Google Scholar]
- Lukacs N. W., Boros D. L. Splenic and granuloma T-lymphocyte responses to fractionated soluble egg antigens of Schistosoma mansoni-infected mice. Infect Immun. 1991 Mar;59(3):941–948. doi: 10.1128/iai.59.3.941-948.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukacs N. W., Boros D. L. Utilization of fractionated soluble egg antigens reveals selectively modulated granulomatous and lymphokine responses during murine schistosomiasis mansoni. Infect Immun. 1992 Aug;60(8):3209–3216. doi: 10.1128/iai.60.8.3209-3216.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew R. C., Ragheb S., Boros D. L. Recombinant IL-2 therapy reverses diminished granulomatous responsiveness in anti-L3T4-treated, Schistosoma mansoni-infected mice. J Immunol. 1990 Jun 1;144(11):4356–4361. [PubMed] [Google Scholar]
- Mosmann T. R., Cherwinski H., Bond M. W., Giedlin M. A., Coffman R. L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986 Apr 1;136(7):2348–2357. [PubMed] [Google Scholar]
- Mosmann T. R., Moore K. W. The role of IL-10 in crossregulation of TH1 and TH2 responses. Immunol Today. 1991 Mar;12(3):A49–A53. doi: 10.1016/S0167-5699(05)80015-5. [DOI] [PubMed] [Google Scholar]
- Quintáns J., Lefkovits I. Precursor cells specific to sheep red cells in nude mice. Estimation of frequency in the microculture system. Eur J Immunol. 1973 Jul;3(7):392–397. doi: 10.1002/eji.1830030704. [DOI] [PubMed] [Google Scholar]
- Ragheb S., Boros D. L. Characterization of granuloma T lymphocyte function from Schistosoma mansoni-infected mice. J Immunol. 1989 May 1;142(9):3239–3246. [PubMed] [Google Scholar]
- Sher A., Fiorentino D., Caspar P., Pearce E., Mosmann T. Production of IL-10 by CD4+ T lymphocytes correlates with down-regulation of Th1 cytokine synthesis in helminth infection. J Immunol. 1991 Oct 15;147(8):2713–2716. [PubMed] [Google Scholar]
- Stadecker M. J., Kamisato J. K., Chikunguwo S. M. Induction of T helper cell unresponsiveness to antigen by macrophages from schistosomal egg granulomas. A basis for immunomodulation in schistosomiasis? J Immunol. 1990 Oct 15;145(8):2697–2700. [PubMed] [Google Scholar]
- Street N. E., Schumacher J. H., Fong T. A., Bass H., Fiorentino D. F., Leverah J. A., Mosmann T. R. Heterogeneity of mouse helper T cells. Evidence from bulk cultures and limiting dilution cloning for precursors of Th1 and Th2 cells. J Immunol. 1990 Mar 1;144(5):1629–1639. [PubMed] [Google Scholar]
- Vella A. T., Pearce E. J. CD4+ Th2 response induced by Schistosoma mansoni eggs develops rapidly, through an early, transient, Th0-like stage. J Immunol. 1992 Apr 1;148(7):2283–2290. [PubMed] [Google Scholar]
- Yamashita T., Boros D. L. IL-4 influences IL-2 production and granulomatous inflammation in murine schistosomiasis mansoni. J Immunol. 1992 Dec 1;149(11):3659–3664. [PubMed] [Google Scholar]


