Abstract
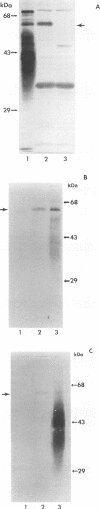
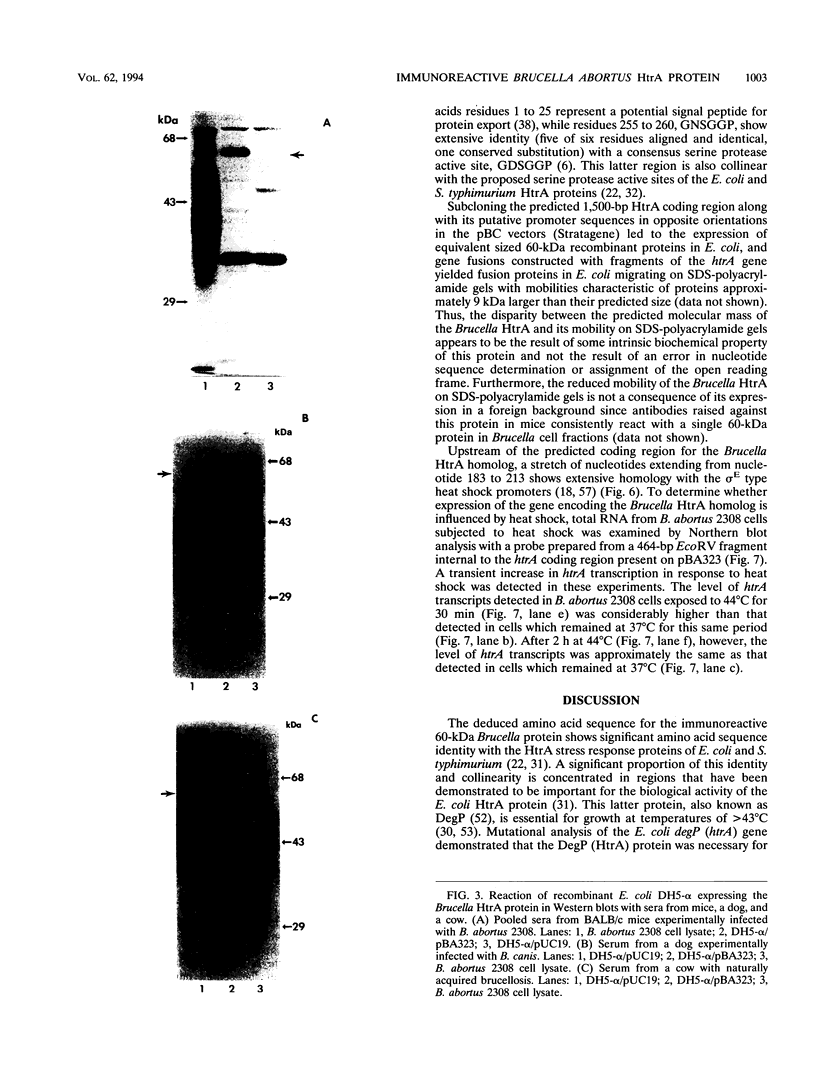
An 11-kb fragment of Brucella abortus genomic DNA cloned into the BamHI site of pUC9 expressed a 60-kDa protein in Escherichia coli DH5-alpha. Antibodies reactive with this 60-kDa protein were detected by Western blot (immunoblot) analysis in sera from mice, cattle, and goats experimentally infected with B. abortus, in sera from mice experimentally infected with Brucella melitensis, and in serum from a dog experimentally infected with Brucella canis. Similar results were seen with sera obtained from cattle and dogs with naturally acquired brucellosis. The gene encoding the 60-kDa Brucella protein was localized to a 2-kb EcoRI fragment which was also reactive in Southern blots with genomic DNA from other strains of B. abortus as well as with genomic DNA from B. melitensis and B. canis. Nucleotide sequence analysis of the cloned EcoRI fragment revealed an open reading frame encoding a protein with a predicted molecular mass of 51,847 Da and an isoelectric point of 5.15. Comparison of the deduced amino acid sequence of the immunoreactive Brucella protein with the SWISS-PROT protein sequence data base revealed that it shares > 40% amino acid sequence identity with the E. coli and Salmonella typhimurium HtrA stress response proteins. Computer-assisted analysis of this amino acid sequence also predicted that the putative Brucella HtrA homolog contains an export signal sequence and a serine protease active site, two structural features characteristic of previously described HtrA proteins. A potential sigma E type heat shock promoter sequence was detected upstream of the cloned Brucella htrA gene, and Northern (RNA) blot analysis demonstrated that exposure of B. abortus 2308 to heat shock conditions resulted in a transient elevation of htrA transcription. These results strongly suggest that the immunoreactive 60-kDa Brucella protein is a member of the HtrA class of stress response proteins.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abu Kwaik Y., Eisenstein B. I., Engleberg N. C. Phenotypic modulation by Legionella pneumophila upon infection of macrophages. Infect Immun. 1993 Apr;61(4):1320–1329. doi: 10.1128/iai.61.4.1320-1329.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araya L. N., Elzer P. H., Rowe G. E., Enright F. M., Winter A. J. Temporal development of protective cell-mediated and humoral immunity in BALB/c mice infected with Brucella abortus. J Immunol. 1989 Nov 15;143(10):3330–3337. [PubMed] [Google Scholar]
- Brenner S. The molecular evolution of genes and proteins: a tale of two serines. Nature. 1988 Aug 11;334(6182):528–530. doi: 10.1038/334528a0. [DOI] [PubMed] [Google Scholar]
- Buchmeier N. A., Heffron F. Induction of Salmonella stress proteins upon infection of macrophages. Science. 1990 May 11;248(4956):730–732. doi: 10.1126/science.1970672. [DOI] [PubMed] [Google Scholar]
- Carmichael L. E., Joubert J. C., Jones L. Characterization of Brucella canis protein antigens and polypeptide antibody responses of infected dogs. Vet Microbiol. 1989 Apr;19(4):373–387. doi: 10.1016/0378-1135(89)90102-8. [DOI] [PubMed] [Google Scholar]
- Cellier M. F., Teyssier J., Nicolas M., Liautard J. P., Marti J., Sri Widada J. Cloning and characterization of the Brucella ovis heat shock protein DnaK functionally expressed in Escherichia coli. J Bacteriol. 1992 Dec;174(24):8036–8042. doi: 10.1128/jb.174.24.8036-8042.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatfield S. N., Strahan K., Pickard D., Charles I. G., Hormaeche C. E., Dougan G. Evaluation of Salmonella typhimurium strains harbouring defined mutations in htrA and aroA in the murine salmonellosis model. Microb Pathog. 1992 Feb;12(2):145–151. doi: 10.1016/0882-4010(92)90117-7. [DOI] [PubMed] [Google Scholar]
- Chatfield S. N., Strugnell R. A., Dougan G. Live Salmonella as vaccines and carriers of foreign antigenic determinants. Vaccine. 1989 Dec;7(6):495–498. doi: 10.1016/0264-410x(89)90271-5. [DOI] [PubMed] [Google Scholar]
- Cheers C. Pathogenesis and cellular immunity in experimental murine brucellosis. Dev Biol Stand. 1984;56:237–246. [PubMed] [Google Scholar]
- Cohen I. R. Autoimmunity to chaperonins in the pathogenesis of arthritis and diabetes. Annu Rev Immunol. 1991;9:567–589. doi: 10.1146/annurev.iy.09.040191.003031. [DOI] [PubMed] [Google Scholar]
- Erickson J. W., Gross C. A. Identification of the sigma E subunit of Escherichia coli RNA polymerase: a second alternate sigma factor involved in high-temperature gene expression. Genes Dev. 1989 Sep;3(9):1462–1471. doi: 10.1101/gad.3.9.1462. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Harboe M., Quayle A. J. Heat shock proteins: friend and foe? Clin Exp Immunol. 1991 Oct;86(1):2–5. doi: 10.1111/j.1365-2249.1991.tb05764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X., Baldwin C. L. Effects of cytokines on intracellular growth of Brucella abortus. Infect Immun. 1993 Jan;61(1):124–134. doi: 10.1128/iai.61.1.124-134.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K., Charles I., Dougan G., Pickard D., O'Gaora P., Costa G., Ali T., Miller I., Hormaeche C. The role of a stress-response protein in Salmonella typhimurium virulence. Mol Microbiol. 1991 Feb;5(2):401–407. doi: 10.1111/j.1365-2958.1991.tb02122.x. [DOI] [PubMed] [Google Scholar]
- Kaufmann S. H. Heat shock proteins and the immune response. Immunol Today. 1990 Apr;11(4):129–136. doi: 10.1016/0167-5699(90)90050-j. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Limet J. N., Bosseray N., Garin-Bastuji B., Dubray G., Plommet M. Humoral immunity in mice mediated by monoclonal antibodies against the A and M antigens of Brucella. J Med Microbiol. 1989 Sep;30(1):37–43. doi: 10.1099/00222615-30-1-37. [DOI] [PubMed] [Google Scholar]
- Lin J., Adams L. G., Ficht T. A. Characterization of the heat shock response in Brucella abortus and isolation of the genes encoding the GroE heat shock proteins. Infect Immun. 1992 Jun;60(6):2425–2431. doi: 10.1128/iai.60.6.2425-2431.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipinska B., Fayet O., Baird L., Georgopoulos C. Identification, characterization, and mapping of the Escherichia coli htrA gene, whose product is essential for bacterial growth only at elevated temperatures. J Bacteriol. 1989 Mar;171(3):1574–1584. doi: 10.1128/jb.171.3.1574-1584.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipinska B., Sharma S., Georgopoulos C. Sequence analysis and regulation of the htrA gene of Escherichia coli: a sigma 32-independent mechanism of heat-inducible transcription. Nucleic Acids Res. 1988 Nov 11;16(21):10053–10067. doi: 10.1093/nar/16.21.10053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipinska B., Zylicz M., Georgopoulos C. The HtrA (DegP) protein, essential for Escherichia coli survival at high temperatures, is an endopeptidase. J Bacteriol. 1990 Apr;172(4):1791–1797. doi: 10.1128/jb.172.4.1791-1797.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller I., Maskell D., Hormaeche C., Johnson K., Pickard D., Dougan G. Isolation of orally attenuated Salmonella typhimurium following TnphoA mutagenesis. Infect Immun. 1989 Sep;57(9):2758–2763. doi: 10.1128/iai.57.9.2758-2763.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison R. P., Belland R. J., Lyng K., Caldwell H. D. Chlamydial disease pathogenesis. The 57-kD chlamydial hypersensitivity antigen is a stress response protein. J Exp Med. 1989 Oct 1;170(4):1271–1283. doi: 10.1084/jem.170.4.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss B. Vaccinia virus: a tool for research and vaccine development. Science. 1991 Jun 21;252(5013):1662–1667. doi: 10.1126/science.2047875. [DOI] [PubMed] [Google Scholar]
- Oliver D. Protein secretion in Escherichia coli. Annu Rev Microbiol. 1985;39:615–648. doi: 10.1146/annurev.mi.39.100185.003151. [DOI] [PubMed] [Google Scholar]
- Roop R. M., 2nd, Jeffers G., Bagchi T., Walker J., Enright F. M., Schurig G. G. Experimental infection of goat fetuses in utero with a stable, rough mutant of Brucella abortus. Res Vet Sci. 1991 Sep;51(2):123–127. doi: 10.1016/0034-5288(91)90001-5. [DOI] [PubMed] [Google Scholar]
- Roop R. M., 2nd, Preston-Moore D., Bagchi T., Schurig G. G. Rapid identification of smooth Brucella species with a monoclonal antibody. J Clin Microbiol. 1987 Nov;25(11):2090–2093. doi: 10.1128/jcm.25.11.2090-2093.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roop R. M., 2nd, Price M. L., Dunn B. E., Boyle S. M., Sriranganathan N., Schurig G. G. Molecular cloning and nucleotide sequence analysis of the gene encoding the immunoreactive Brucella abortus Hsp60 protein, BA60K. Microb Pathog. 1992 Jan;12(1):47–62. doi: 10.1016/0882-4010(92)90065-v. [DOI] [PubMed] [Google Scholar]
- SPINK W. W., HALL J. W., 3rd, FINSTAD J., MALLET E. Immunization with viable Brucella organisms. Results of a safety test in humans. Bull World Health Organ. 1962;26:409–419. [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schurig G. G., Roop R. M., 2nd, Bagchi T., Boyle S., Buhrman D., Sriranganathan N. Biological properties of RB51; a stable rough strain of Brucella abortus. Vet Microbiol. 1991 Jul;28(2):171–188. doi: 10.1016/0378-1135(91)90091-s. [DOI] [PubMed] [Google Scholar]
- Segal G., Ron E. Z. Heat shock transcription of the groESL operon of Agrobacterium tumefaciens may involve a hairpin-loop structure. J Bacteriol. 1993 May;175(10):3083–3088. doi: 10.1128/jb.175.10.3083-3088.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seol J. H., Woo S. K., Jung E. M., Yoo S. J., Lee C. S., Kim K. J., Tanaka K., Ichihara A., Ha D. B., Chung C. H. Protease Do is essential for survival of Escherichia coli at high temperatures: its identity with the htrA gene product. Biochem Biophys Res Commun. 1991 Apr 30;176(2):730–736. doi: 10.1016/s0006-291x(05)80245-1. [DOI] [PubMed] [Google Scholar]
- Strahan K., Chatfield S. N., Tite J., Dougan G., Hormaeche C. E. Impaired resistance to infection does not increase the virulence of Salmonella htrA live vaccines for mice. Microb Pathog. 1992 Apr;12(4):311–317. doi: 10.1016/0882-4010(92)90049-t. [DOI] [PubMed] [Google Scholar]
- Strauch K. L., Beckwith J. An Escherichia coli mutation preventing degradation of abnormal periplasmic proteins. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1576–1580. doi: 10.1073/pnas.85.5.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauch K. L., Johnson K., Beckwith J. Characterization of degP, a gene required for proteolysis in the cell envelope and essential for growth of Escherichia coli at high temperature. J Bacteriol. 1989 May;171(5):2689–2696. doi: 10.1128/jb.171.5.2689-2696.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swamy K. H., Chung C. H., Goldberg A. L. Isolation and characterization of protease do from Escherichia coli, a large serine protease containing multiple subunits. Arch Biochem Biophys. 1983 Jul 15;224(2):543–554. doi: 10.1016/0003-9861(83)90242-4. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q. P., Kaguni J. M. A novel sigma factor is involved in expression of the rpoH gene of Escherichia coli. J Bacteriol. 1989 Aug;171(8):4248–4253. doi: 10.1128/jb.171.8.4248-4253.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young D. B. Heat-shock proteins: immunity and autoimmunity. Curr Opin Immunol. 1992 Aug;4(4):396–400. doi: 10.1016/s0952-7915(06)80029-4. [DOI] [PubMed] [Google Scholar]
- Zhong G., Brunham R. C. Antigenic analysis of the chlamydial 75-kilodalton protein. Infect Immun. 1992 Mar;60(3):1221–1224. doi: 10.1128/iai.60.3.1221-1224.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]