Abstract
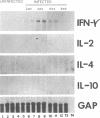
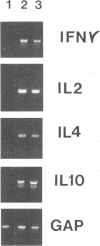
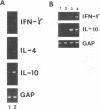
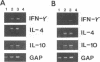
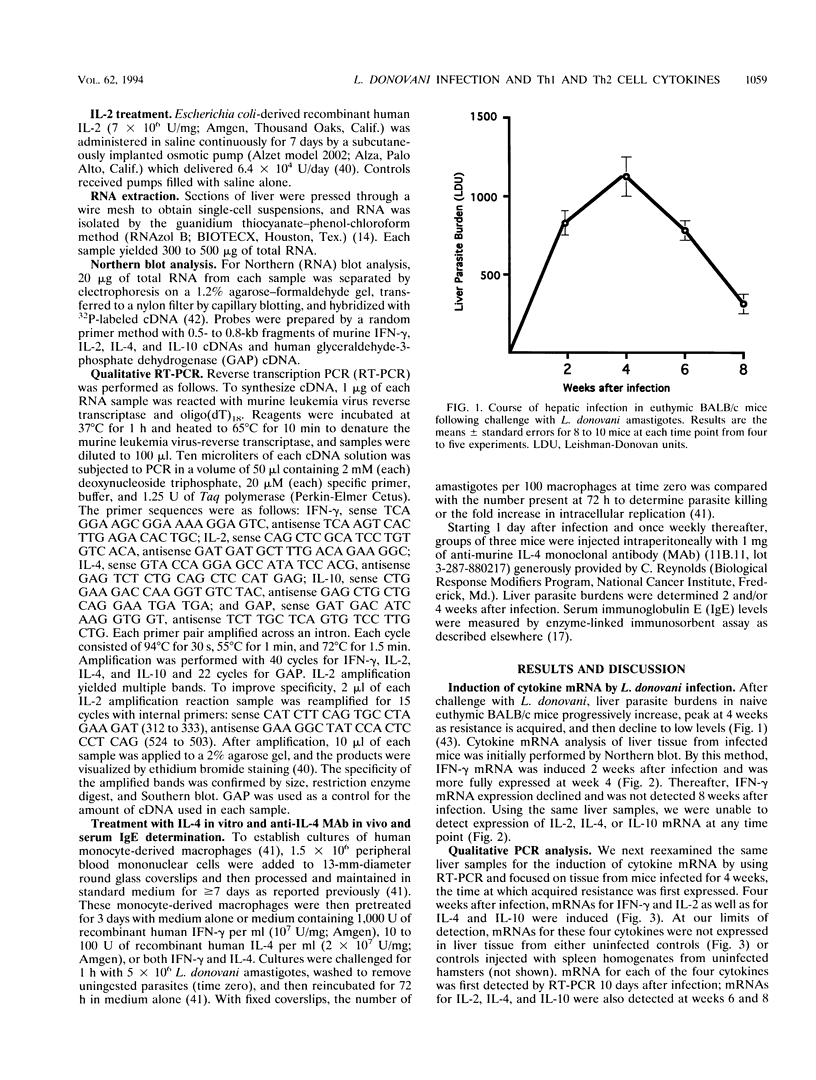
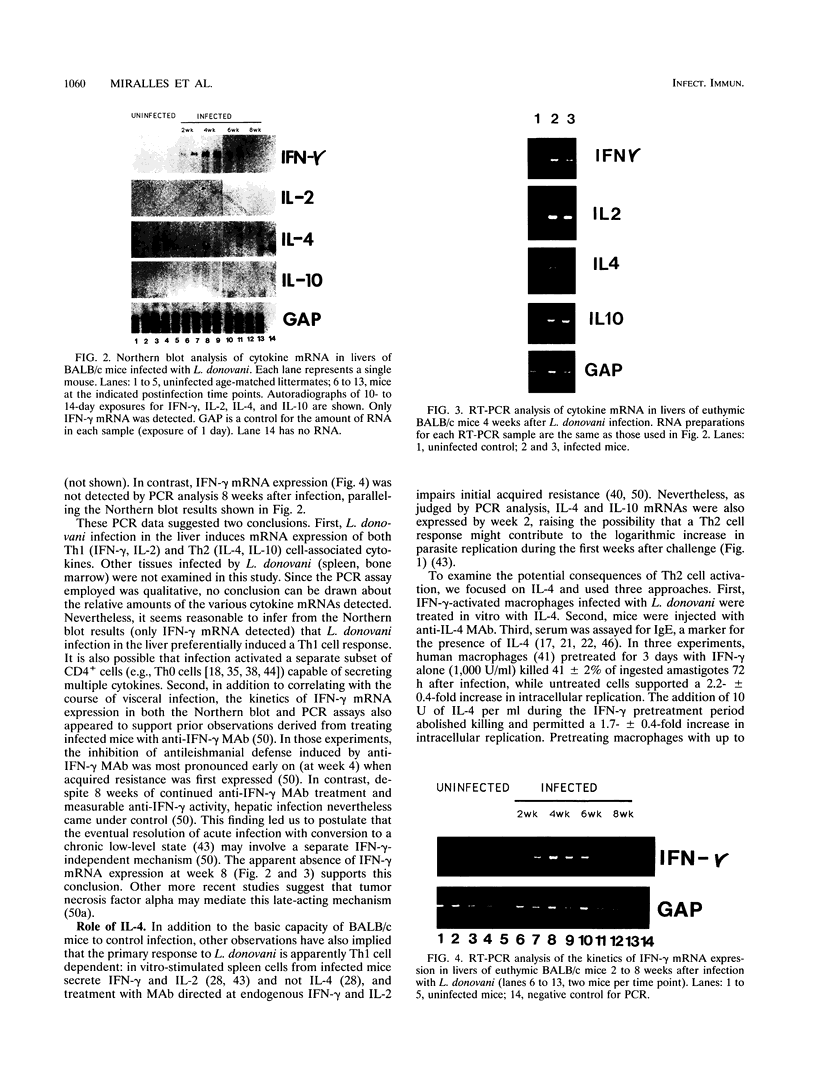
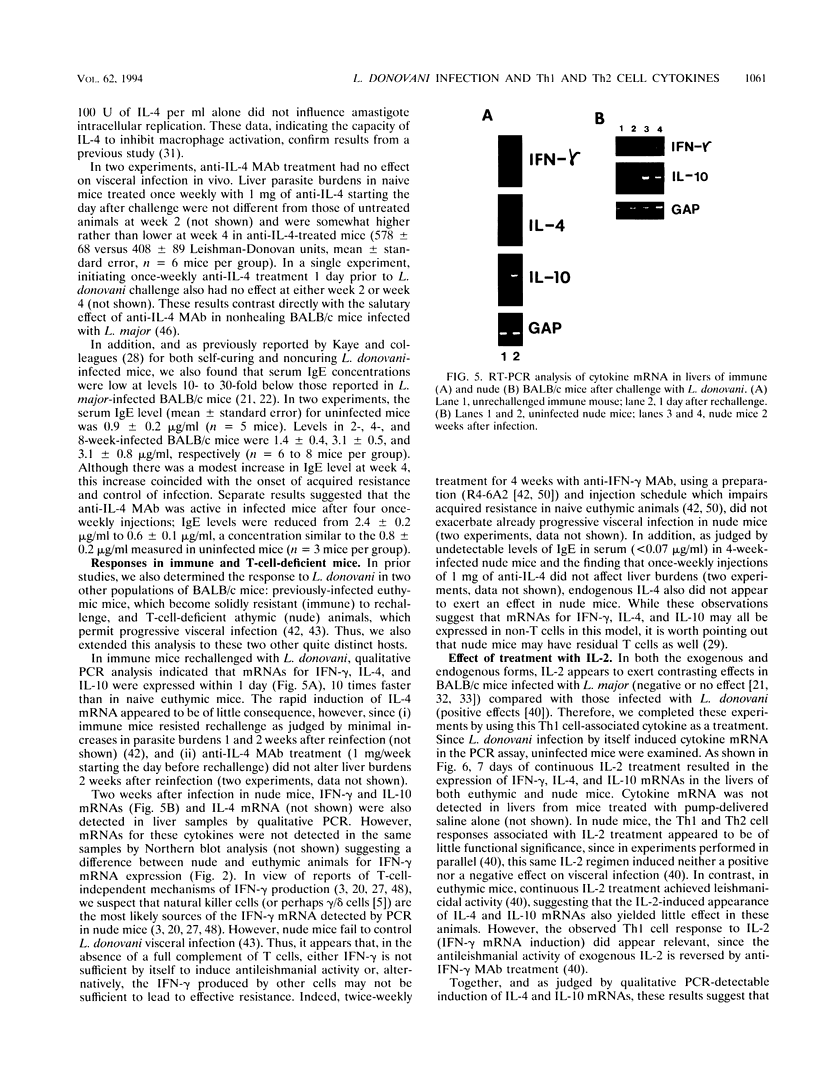
In experimental Leishmania donovani infection in BALB/c mice, initial susceptibility gives way to T-cell-dependent acquired resistance and eventual control over visceral infection. Since various cytokines appear to underlie the host response to Leishmania infection, we examined infected liver tissue for gene expression of cytokines associated with Th1 (gamma interferon [IFN-gamma] and interleukin-2 [IL-2]) and Th2 cells (IL-4 and IL-10). By Northern (RNA) blot analysis, only IFN-gamma mRNA expression was detected in livers of infected euthymic mice. To determine whether activation of Th1 cells develops selectively in this model, qualitative PCR analysis was used. These results indicated that mRNAs for IFN-gamma, IL-2, IL-4, and IL-10 were all induced by L. donovani infection. The potentially negative Th2 cell-associated response did not appear to play a functional role, however, since resistance was acquired, anti-IL-4 monoclonal antibody treatment did not accelerate control over visceral infection, and serum immunoglobulin E levels remained low. As judged by PCR analysis, IL-4 and IL-10 mRNAs were also expressed under three other conditions without apparent effect: in naive euthymic mice treated with IL-2, which induces leishmanicidal activity; in rechallenged immune mice, which resist reinfection; and in nude mice, which fail to control L. donovani. These results suggest that, like other Leishmania species, L. donovani infection may trigger a potentially suppressive Th2 cell-associated cytokine response. However, in T-cell-intact mice able to control L. donovani, this response either is insufficient to influence outcome or more likely is overshadowed by the Th1 cell response.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Afonso L. C., Scott P. Immune responses associated with susceptibility of C57BL/10 mice to Leishmania amazonensis. Infect Immun. 1993 Jul;61(7):2952–2959. doi: 10.1128/iai.61.7.2952-2959.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badaro R., Jones T. C., Carvalho E. M., Sampaio D., Reed S. G., Barral A., Teixeira R., Johnson W. D., Jr New perspectives on a subclinical form of visceral leishmaniasis. J Infect Dis. 1986 Dec;154(6):1003–1011. doi: 10.1093/infdis/154.6.1003. [DOI] [PubMed] [Google Scholar]
- Bancroft G. J., Schreiber R. D., Bosma G. C., Bosma M. J., Unanue E. R. A T cell-independent mechanism of macrophage activation by interferon-gamma. J Immunol. 1987 Aug 15;139(4):1104–1107. [PubMed] [Google Scholar]
- Belosevic M., Davis C. E., Meltzer M. S., Nacy C. A. Regulation of activated macrophage antimicrobial activities. Identification of lymphokines that cooperate with IFN-gamma for induction of resistance to infection. J Immunol. 1988 Aug 1;141(3):890–896. [PubMed] [Google Scholar]
- Bluestone J. A., Matis L. A. TCR gamma delta cells--minor redundant T cell subset or specialized immune system component? J Immunol. 1989 Mar 15;142(6):1785–1788. [PubMed] [Google Scholar]
- Bogdan C., Stenger S., Röllinghoff M., Solbach W. Cytokine interactions in experimental cutaneous leishmaniasis. Interleukin 4 synergizes with interferon-gamma to activate murine macrophages for killing of Leishmania major amastigotes. Eur J Immunol. 1991 Feb;21(2):327–333. doi: 10.1002/eji.1830210213. [DOI] [PubMed] [Google Scholar]
- Bretscher P. A., Wei G., Menon J. N., Bielefeldt-Ohmann H. Establishment of stable, cell-mediated immunity that makes "susceptible" mice resistant to Leishmania major. Science. 1992 Jul 24;257(5069):539–542. doi: 10.1126/science.1636090. [DOI] [PubMed] [Google Scholar]
- Carter K. C., Gallagher G., Baillie A. J., Alexander J. The induction of protective immunity to Leishmania major in the BALB/c mouse by interleukin 4 treatment. Eur J Immunol. 1989 Apr;19(4):779–782. doi: 10.1002/eji.1830190432. [DOI] [PubMed] [Google Scholar]
- Carvalho E. M., Badaró R., Reed S. G., Jones T. C., Johnson W. D., Jr Absence of gamma interferon and interleukin 2 production during active visceral leishmaniasis. J Clin Invest. 1985 Dec;76(6):2066–2069. doi: 10.1172/JCI112209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho E. M., Barral A., Pedral-Sampaio D., Barral-Netto M., Badaró R., Rocha H., Johnson W. D., Jr Immunologic markers of clinical evolution in children recently infected with Leishmania donovani chagasi. J Infect Dis. 1992 Mar;165(3):535–540. doi: 10.1093/infdis/165.3.535. [DOI] [PubMed] [Google Scholar]
- Chatelain R., Varkila K., Coffman R. L. IL-4 induces a Th2 response in Leishmania major-infected mice. J Immunol. 1992 Feb 15;148(4):1182–1187. [PubMed] [Google Scholar]
- Cherwinski H. M., Schumacher J. H., Brown K. D., Mosmann T. R. Two types of mouse helper T cell clone. III. Further differences in lymphokine synthesis between Th1 and Th2 clones revealed by RNA hybridization, functionally monospecific bioassays, and monoclonal antibodies. J Exp Med. 1987 Nov 1;166(5):1229–1244. doi: 10.1084/jem.166.5.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomarat P., Rissoan M. C., Banchereau J., Miossec P. Interferon gamma inhibits interleukin 10 production by monocytes. J Exp Med. 1993 Feb 1;177(2):523–527. doi: 10.1084/jem.177.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cillari E., Liew F. Y., Lo Campo P., Milano S., Mansueto S., Salerno A. Suppression of IL-2 production by cryopreserved peripheral blood mononuclear cells from patients with active visceral leishmaniasis in Sicily. J Immunol. 1988 Apr 15;140(8):2721–2726. [PubMed] [Google Scholar]
- Evans T. G., Teixeira M. J., McAuliffe I. T., Vasconcelos I., Vasconcelos A. W., Sousa A. de A., Lima J. W., Pearson R. D. Epidemiology of visceral leishmaniasis in northeast Brazil. J Infect Dis. 1992 Nov;166(5):1124–1132. doi: 10.1093/infdis/166.5.1124. [DOI] [PubMed] [Google Scholar]
- Finkelman F. D., Katona I. M., Urban J. F., Jr, Snapper C. M., Ohara J., Paul W. E. Suppression of in vivo polyclonal IgE responses by monoclonal antibody to the lymphokine B-cell stimulatory factor 1. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9675–9678. doi: 10.1073/pnas.83.24.9675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firestein G. S., Roeder W. D., Laxer J. A., Townsend K. S., Weaver C. T., Hom J. T., Linton J., Torbett B. E., Glasebrook A. L. A new murine CD4+ T cell subset with an unrestricted cytokine profile. J Immunol. 1989 Jul 15;143(2):518–525. [PubMed] [Google Scholar]
- Ghalib H. W., Piuvezam M. R., Skeiky Y. A., Siddig M., Hashim F. A., el-Hassan A. M., Russo D. M., Reed S. G. Interleukin 10 production correlates with pathology in human Leishmania donovani infections. J Clin Invest. 1993 Jul;92(1):324–329. doi: 10.1172/JCI116570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handa K., Suzuki R., Matsui H., Shimizu Y., Kumagai K. Natural killer (NK) cells as a responder to interleukin 2 (IL 2). II. IL 2-induced interferon gamma production. J Immunol. 1983 Feb;130(2):988–992. [PubMed] [Google Scholar]
- Heinzel F. P., Rerko R. M., Hatam F., Locksley R. M. IL-2 is necessary for the progression of leishmaniasis in susceptible murine hosts. J Immunol. 1993 May 1;150(9):3924–3931. [PubMed] [Google Scholar]
- Heinzel F. P., Sadick M. D., Holaday B. J., Coffman R. L., Locksley R. M. Reciprocal expression of interferon gamma or interleukin 4 during the resolution or progression of murine leishmaniasis. Evidence for expansion of distinct helper T cell subsets. J Exp Med. 1989 Jan 1;169(1):59–72. doi: 10.1084/jem.169.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinzel F. P., Sadick M. D., Mutha S. S., Locksley R. M. Production of interferon gamma, interleukin 2, interleukin 4, and interleukin 10 by CD4+ lymphocytes in vivo during healing and progressive murine leishmaniasis. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7011–7015. doi: 10.1073/pnas.88.16.7011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holaday B. J., Pompeu M. M., Evans T., Braga D. N., Texeira M. J., Sousa A. de Q., Sadick M. D., Vasconcelos A. W., Abrams J. S., Pearson R. D. Correlates of Leishmania-specific immunity in the clinical spectrum of infection with Leishmania chagasi. J Infect Dis. 1993 Feb;167(2):411–417. doi: 10.1093/infdis/167.2.411. [DOI] [PubMed] [Google Scholar]
- Howard M., O'Garra A. Biological properties of interleukin 10. Immunol Today. 1992 Jun;13(6):198–200. doi: 10.1016/0167-5699(92)90153-X. [DOI] [PubMed] [Google Scholar]
- Karp C. L., el-Safi S. H., Wynn T. A., Satti M. M., Kordofani A. M., Hashim F. A., Hag-Ali M., Neva F. A., Nutman T. B., Sacks D. L. In vivo cytokine profiles in patients with kala-azar. Marked elevation of both interleukin-10 and interferon-gamma. J Clin Invest. 1993 Apr;91(4):1644–1648. doi: 10.1172/JCI116372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karupiah G., Blanden R. V., Ramshaw I. A. Interferon gamma is involved in the recovery of athymic nude mice from recombinant vaccinia virus/interleukin 2 infection. J Exp Med. 1990 Nov 1;172(5):1495–1503. doi: 10.1084/jem.172.5.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye P. M., Curry A. J., Blackwell J. M. Differential production of Th1- and Th2-derived cytokines does not determine the genetically controlled or vaccine-induced rate of cure in murine visceral leishmaniasis. J Immunol. 1991 Apr 15;146(8):2763–2770. [PubMed] [Google Scholar]
- Kung J. T., Thomas C. A., 3rd Athymic nude CD4+8- T cells produce IL-2 but fail to proliferate in response to mitogenic stimuli. J Immunol. 1988 Dec 1;141(11):3691–3696. [PubMed] [Google Scholar]
- Leal L. M., Moss D. W., Kuhn R., Müller W., Liew F. Y. Interleukin-4 transgenic mice of resistant background are susceptible to Leishmania major infection. Eur J Immunol. 1993 Feb;23(2):566–569. doi: 10.1002/eji.1830230241. [DOI] [PubMed] [Google Scholar]
- Lehn M., Weiser W. Y., Engelhorn S., Gillis S., Remold H. G. IL-4 inhibits H2O2 production and antileishmanial capacity of human cultured monocytes mediated by IFN-gamma. J Immunol. 1989 Nov 1;143(9):3020–3024. [PubMed] [Google Scholar]
- Lezama-Davila C. M., Williams D. M., Gallagher G., Alexander J. Cytokine control of Leishmania infection in the BALB/c mouse: enhancement and inhibition of parasite growth by local administration of IL-2 or IL-4 is species and time dependent. Parasite Immunol. 1992 Jan;14(1):37–48. doi: 10.1111/j.1365-3024.1992.tb00004.x. [DOI] [PubMed] [Google Scholar]
- Mazingue C., Cottrez-Detoeuf F., Louis J., Kweider M., Auriault C., Capron A. In vitro and in vivo effects of interleukin 2 on the protozoan parasite leishmania. Eur J Immunol. 1989 Mar;19(3):487–491. doi: 10.1002/eji.1830190312. [DOI] [PubMed] [Google Scholar]
- Meller-Melloul C., Farnarier C., Dunan S., Faugere B., Franck J., Mary C., Bongrand P., Quilici M., Kaplanski S. Evidence of subjects sensitized to Leishmania infantum on the French Mediterranean coast: differences in gamma interferon production between this population and visceral leishmaniasis patients. Parasite Immunol. 1991 Sep;13(5):531–536. doi: 10.1111/j.1365-3024.1991.tb00549.x. [DOI] [PubMed] [Google Scholar]
- Moll H., Röllinghoff M. Resistance to murine cutaneous leishmaniasis is mediated by TH1 cells, but disease-promoting CD4+ cells are different from TH2 cells. Eur J Immunol. 1990 Sep;20(9):2067–2074. doi: 10.1002/eji.1830200927. [DOI] [PubMed] [Google Scholar]
- Morris L., Troutt A. B., McLeod K. S., Kelso A., Handman E., Aebischer T. Interleukin-4 but not gamma interferon production correlates with the severity of murine cutaneous leishmaniasis. Infect Immun. 1993 Aug;61(8):3459–3465. doi: 10.1128/iai.61.8.3459-3465.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann T. R., Cherwinski H., Bond M. W., Giedlin M. A., Coffman R. L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986 Apr 1;136(7):2348–2357. [PubMed] [Google Scholar]
- Mosmann T. R., Coffman R. L. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- Murray H. W. Effect of continuous administration of interferon-gamma in experimental visceral leishmaniasis. J Infect Dis. 1990 May;161(5):992–994. doi: 10.1093/infdis/161.5.992. [DOI] [PubMed] [Google Scholar]
- Murray H. W., Miralles G. D., Stoeckle M. Y., McDermott D. F. Role and effect of IL-2 in experimental visceral leishmaniasis. J Immunol. 1993 Jul 15;151(2):929–938. [PubMed] [Google Scholar]
- Murray H. W., Rubin B. Y., Rothermel C. D. Killing of intracellular Leishmania donovani by lymphokine-stimulated human mononuclear phagocytes. Evidence that interferon-gamma is the activating lymphokine. J Clin Invest. 1983 Oct;72(4):1506–1510. doi: 10.1172/JCI111107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray H. W., Squires K. E., Miralles C. D., Stoeckle M. Y., Granger A. M., Granelli-Piperno A., Bogdan C. Acquired resistance and granuloma formation in experimental visceral leishmaniasis. Differential T cell and lymphokine roles in initial versus established immunity. J Immunol. 1992 Mar 15;148(6):1858–1863. [PubMed] [Google Scholar]
- Murray H. W., Stern J. J., Welte K., Rubin B. Y., Carriero S. M., Nathan C. F. Experimental visceral leishmaniasis: production of interleukin 2 and interferon-gamma, tissue immune reaction, and response to treatment with interleukin 2 and interferon-gamma. J Immunol. 1987 Apr 1;138(7):2290–2297. [PubMed] [Google Scholar]
- Paliard X., de Waal Malefijt R., Yssel H., Blanchard D., Chrétien I., Abrams J., de Vries J., Spits H. Simultaneous production of IL-2, IL-4, and IFN-gamma by activated human CD4+ and CD8+ T cell clones. J Immunol. 1988 Aug 1;141(3):849–855. [PubMed] [Google Scholar]
- Sacks D. L., Lal S. L., Shrivastava S. N., Blackwell J., Neva F. A. An analysis of T cell responsiveness in Indian kala-azar. J Immunol. 1987 Feb 1;138(3):908–913. [PubMed] [Google Scholar]
- Sadick M. D., Heinzel F. P., Holaday B. J., Pu R. T., Dawkins R. S., Locksley R. M. Cure of murine leishmaniasis with anti-interleukin 4 monoclonal antibody. Evidence for a T cell-dependent, interferon gamma-independent mechanism. J Exp Med. 1990 Jan 1;171(1):115–127. doi: 10.1084/jem.171.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadick M. D., Street N., Mosmann T. R., Locksley R. M. Cytokine regulation of murine leishmaniasis: interleukin 4 is not sufficient to mediate progressive disease in resistant C57BL/6 mice. Infect Immun. 1991 Dec;59(12):4710–4714. doi: 10.1128/iai.59.12.4710-4714.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salkowski C. A., Balish E. A monoclonal antibody to gamma interferon blocks augmentation of natural killer cell activity induced during systemic cryptococcosis. Infect Immun. 1991 Feb;59(2):486–493. doi: 10.1128/iai.59.2.486-493.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva J. S., Morrissey P. J., Grabstein K. H., Mohler K. M., Anderson D., Reed S. G. Interleukin 10 and interferon gamma regulation of experimental Trypanosoma cruzi infection. J Exp Med. 1992 Jan 1;175(1):169–174. doi: 10.1084/jem.175.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squires K. E., Schreiber R. D., McElrath M. J., Rubin B. Y., Anderson S. L., Murray H. W. Experimental visceral leishmaniasis: role of endogenous IFN-gamma in host defense and tissue granulomatous response. J Immunol. 1989 Dec 15;143(12):4244–4249. [PubMed] [Google Scholar]
- Zwingenberger K., Harms G., Pedrosa C., Omena S., Sandkamp B., Neifer S. Determinants of the immune response in visceral leishmaniasis: evidence for predominance of endogenous interleukin 4 over interferon-gamma production. Clin Immunol Immunopathol. 1990 Nov;57(2):242–249. doi: 10.1016/0090-1229(90)90038-r. [DOI] [PubMed] [Google Scholar]