Abstract
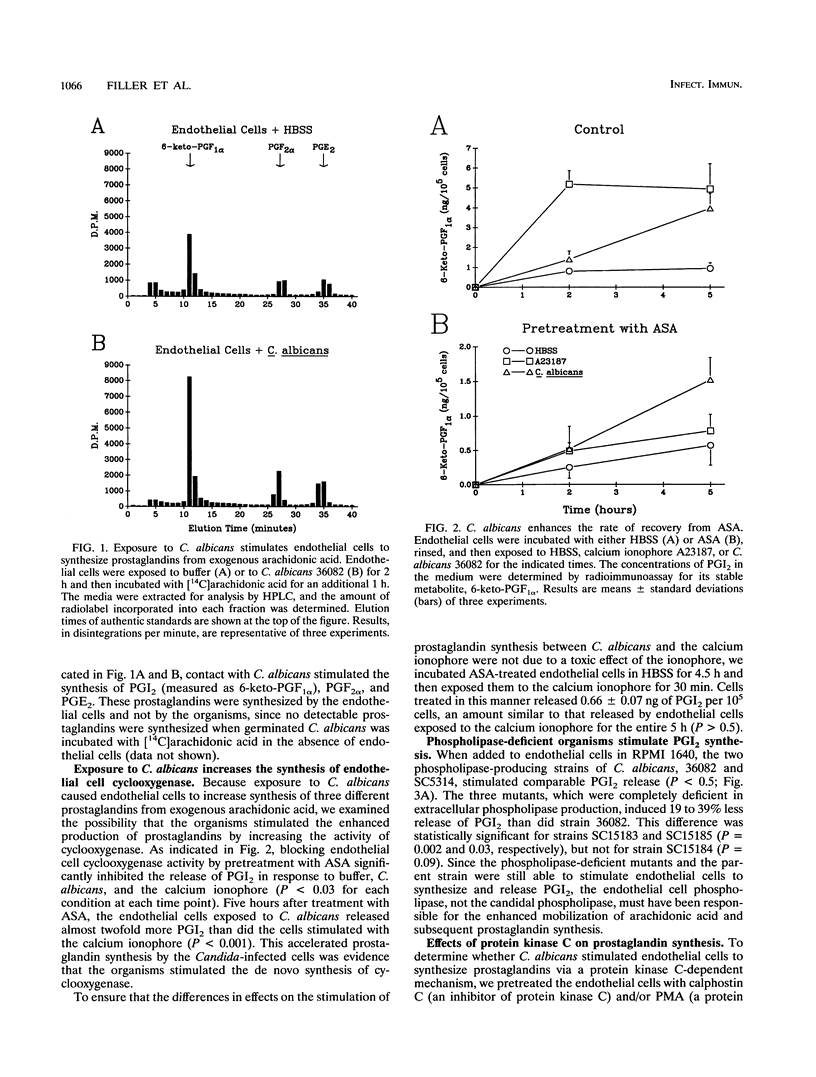
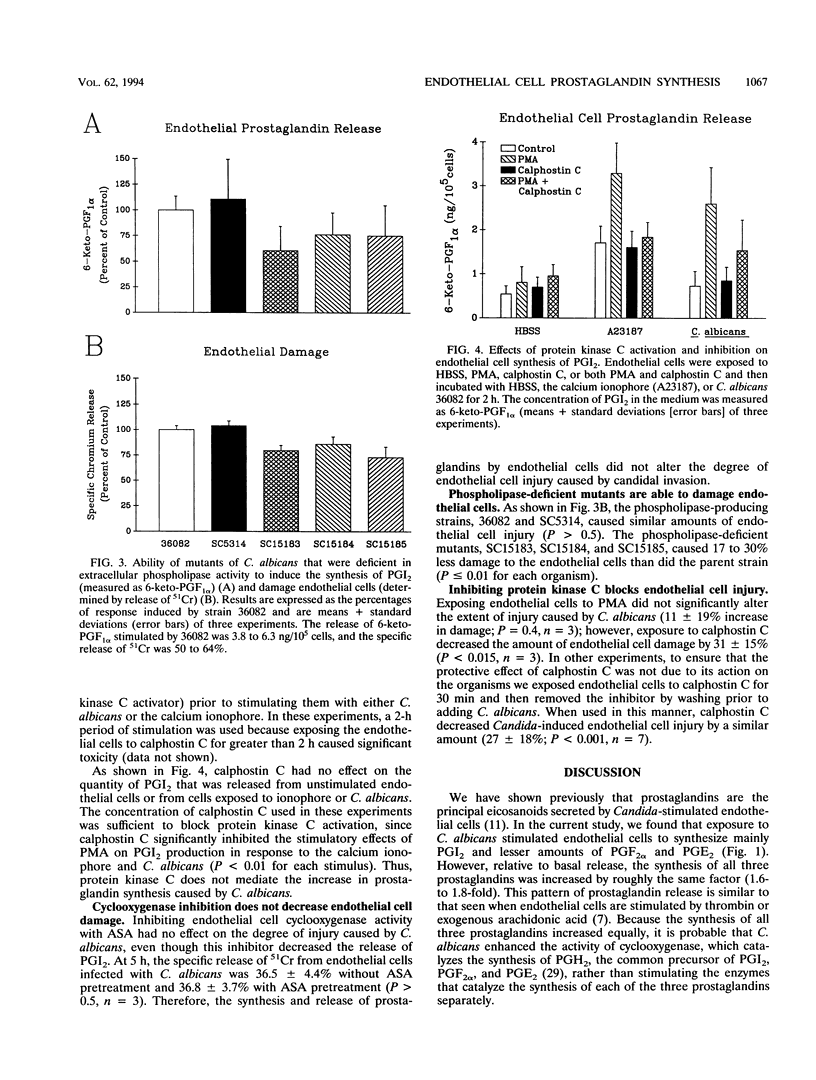
One strategy for improving resistance to opportunistic pathogens is to determine host cellular responses during the invasion process and upregulate those responses that are relevant to host defense mechanisms. Within this context, we have shown previously that invasion of endothelial cells by Candida albicans in vitro causes increased production of prostaglandins. As a prerequisite for modulating endothelial cell prostaglandin production, we now characterize the mechanisms through which this process occurs. Endothelial cell invasion by C. albicans appeared to stimulate the conversion of arachidonic acid into prostaglandins by upregulating the synthesis of endothelial cell cyclooxygenase and increasing the activity of the endothelial cell phospholipase. The enhanced activities of these two enzymes were independent of calphostin C-sensitive protein kinase C and resulted in the increased production and extracellular secretion of prostaglandin I2 (PGI2), PGF2 alpha, and PGE2. The secretion of these prostaglandins had no effect on the amount of endothelial cell injury induced by C. albicans. The role of the increased prostaglandin secretion by endothelial cells is likely related to modulation of the leukocyte response at the candida-leukocyte-endothelial cell interface.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Breviario F., Proserpio P., Bertocchi F., Lampugnani M. G., Mantovani A., Dejana E. Interleukin-1 stimulates prostacyclin production by cultured human endothelial cells by increasing arachidonic acid mobilization and conversion. Arteriosclerosis. 1990 Jan-Feb;10(1):129–134. doi: 10.1161/01.atv.10.1.129. [DOI] [PubMed] [Google Scholar]
- Bruns R. F., Miller F. D., Merriman R. L., Howbert J. J., Heath W. F., Kobayashi E., Takahashi I., Tamaoki T., Nakano H. Inhibition of protein kinase C by calphostin C is light-dependent. Biochem Biophys Res Commun. 1991 Apr 15;176(1):288–293. doi: 10.1016/0006-291x(91)90922-t. [DOI] [PubMed] [Google Scholar]
- Campbell W. B., Ojeda S. R. Measurement of prostaglandins by radioimmunoassay. Methods Enzymol. 1987;141:323–341. doi: 10.1016/0076-6879(87)41080-x. [DOI] [PubMed] [Google Scholar]
- Chakraborti S., Gurtner G. H., Michael J. R. Oxidant-mediated activation of phospholipase A2 in pulmonary endothelium. Am J Physiol. 1989 Dec;257(6 Pt 1):L430–L437. doi: 10.1152/ajplung.1989.257.6.L430. [DOI] [PubMed] [Google Scholar]
- Chakraborti S., Michael J. R., Sanyal T. Defining the role of protein kinase c in calcium-ionophore-(A23187)-mediated activation of phospholipase A2 in pulmonary endothelium. Eur J Biochem. 1992 Jun 15;206(3):965–972. doi: 10.1111/j.1432-1033.1992.tb17007.x. [DOI] [PubMed] [Google Scholar]
- Clark M. A., Littlejohn D., Conway T. M., Mong S., Steiner S., Crooke S. T. Leukotriene D4 treatment of bovine aortic endothelial cells and murine smooth muscle cells in culture results in an increase in phospholipase A2 activity. J Biol Chem. 1986 Aug 15;261(23):10713–10718. [PubMed] [Google Scholar]
- Downey G. P., Chan C. K., Lea P., Takai A., Grinstein S. Phorbol ester-induced actin assembly in neutrophils: role of protein kinase C. J Cell Biol. 1992 Feb;116(3):695–706. doi: 10.1083/jcb.116.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake T. A., Pang M. Staphylococcus aureus induces tissue factor expression in cultured human cardiac valve endothelium. J Infect Dis. 1988 Apr;157(4):749–756. doi: 10.1093/infdis/157.4.749. [DOI] [PubMed] [Google Scholar]
- Edwards J. E., Jr, Rotrosen D., Fontaine J. W., Haudenschild C. C., Diamond R. D. Neutrophil-mediated protection of cultured human vascular endothelial cells from damage by growing Candida albicans hyphae. Blood. 1987 May;69(5):1450–1457. [PubMed] [Google Scholar]
- Filler S. G., Ibe B. O., Luckett P. M., Raj J. U., Edwards J. E., Jr Candida albicans stimulates endothelial cell eicosanoid production. J Infect Dis. 1991 Nov;164(5):928–935. doi: 10.1093/infdis/164.5.928. [DOI] [PubMed] [Google Scholar]
- Garcia J. G., Stasek J., Natarajan V., Patterson C. E., Dominguez J. Role of protein kinase C in the regulation of prostaglandin synthesis in human endothelium. Am J Respir Cell Mol Biol. 1992 Mar;6(3):315–325. doi: 10.1165/ajrcmb/6.3.315. [DOI] [PubMed] [Google Scholar]
- Ghannoum M. A., Filler S. G., Ibrahim A. S., Fu Y., Edwards J. E., Jr Modulation of interactions of Candida albicans and endothelial cells by fluconazole and amphotericin B. Antimicrob Agents Chemother. 1992 Oct;36(10):2239–2244. doi: 10.1128/aac.36.10.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ham E. A., Soderman D. D., Zanetti M. E., Dougherty H. W., McCauley E., Kuehl F. A., Jr Inhibition by prostaglandins of leukotriene B4 release from activated neutrophils. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4349–4353. doi: 10.1073/pnas.80.14.4349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hla T., Neilson K. Human cyclooxygenase-2 cDNA. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7384–7388. doi: 10.1073/pnas.89.16.7384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe E. A., Grulich J., Weksler B. B., Hampel G., Watanabe K. Correlation between thrombin-induced prostacyclin production and inositol trisphosphate and cytosolic free calcium levels in cultured human endothelial cells. J Biol Chem. 1987 Jun 25;262(18):8557–8565. [PubMed] [Google Scholar]
- Jaffe E. A., Nachman R. L., Becker C. G., Minick C. R. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973 Nov;52(11):2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe E. A. Physiologic functions of normal endothelial cells. Ann N Y Acad Sci. 1985;454:279–291. doi: 10.1111/j.1749-6632.1985.tb11868.x. [DOI] [PubMed] [Google Scholar]
- Lyman C. A., Simons E. R., Melnick D. A., Diamond R. D. Unopsonized Candida albicans hyphae stimulate a neutrophil respiratory burst and a cytosolic calcium flux without membrane depolarization. J Infect Dis. 1987 Nov;156(5):770–776. doi: 10.1093/infdis/156.5.770. [DOI] [PubMed] [Google Scholar]
- Mantovani A., Dejana E. Cytokines as communication signals between leukocytes and endothelial cells. Immunol Today. 1989 Nov;10(11):370–375. doi: 10.1016/0167-5699(89)90270-3. [DOI] [PubMed] [Google Scholar]
- McIntyre T. M., Zimmerman G. A., Prescott S. M. Leukotrienes C4 and D4 stimulate human endothelial cells to synthesize platelet-activating factor and bind neutrophils. Proc Natl Acad Sci U S A. 1986 Apr;83(7):2204–2208. doi: 10.1073/pnas.83.7.2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogletree M. L. Pharmacology of prostaglandins in the pulmonary microcirculation. Ann N Y Acad Sci. 1982;384:191–206. doi: 10.1111/j.1749-6632.1982.tb21372.x. [DOI] [PubMed] [Google Scholar]
- Price M. F., Wilkinson I. D., Gentry L. O. Plate method for detection of phospholipase activity in Candida albicans. Sabouraudia. 1982 Mar;20(1):7–14. doi: 10.1080/00362178285380031. [DOI] [PubMed] [Google Scholar]
- Riley B. S., Oppenheimer-Marks N., Hansen E. J., Radolf J. D., Norgard M. V. Virulent Treponema pallidum activates human vascular endothelial cells. J Infect Dis. 1992 Mar;165(3):484–493. doi: 10.1093/infdis/165.3.484. [DOI] [PubMed] [Google Scholar]
- Samaranayake L. P., Raeside J. M., MacFarlane T. W. Factors affecting the phospholipase activity of Candida species in vitro. Sabouraudia. 1984;22(3):201–207. [PubMed] [Google Scholar]
- Smith W. L. Prostaglandin biosynthesis and its compartmentation in vascular smooth muscle and endothelial cells. Annu Rev Physiol. 1986;48:251–262. doi: 10.1146/annurev.ph.48.030186.001343. [DOI] [PubMed] [Google Scholar]
- Smith W. L. The eicosanoids and their biochemical mechanisms of action. Biochem J. 1989 Apr 15;259(2):315–324. doi: 10.1042/bj2590315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Span A. H., Mullers W., Miltenburg A. M., Bruggeman C. A. Cytomegalovirus induced PMN adherence in relation to an ELAM-1 antigen present on infected endothelial cell monolayers. Immunology. 1991 Mar;72(3):355–360. [PMC free article] [PubMed] [Google Scholar]
- Springer T. A. Adhesion receptors of the immune system. Nature. 1990 Aug 2;346(6283):425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- Walker T. S., Brown J. S., Hoover C. S., Morgan D. A. Endothelial prostaglandin secretion: effects of typhus rickettsiae. J Infect Dis. 1990 Nov;162(5):1136–1144. doi: 10.1093/infdis/162.5.1136. [DOI] [PubMed] [Google Scholar]
- Wu K. K., Hatzakis H., Lo S. S., Seong D. C., Sanduja S. K., Tai H. H. Stimulation of de novo synthesis of prostaglandin G/H synthase in human endothelial cells by phorbol ester. J Biol Chem. 1988 Dec 15;263(35):19043–19047. [PubMed] [Google Scholar]
- Yoshimura K., Tod M. L., Pier K. G., Rubin L. J. Effects of a thromboxane A2 analogue and prostacyclin on lung fluid balance in newborn lambs. Circ Res. 1989 Nov;65(5):1409–1416. doi: 10.1161/01.res.65.5.1409. [DOI] [PubMed] [Google Scholar]
- Zimmerman G. A., Wiseman G. A., Hill H. R. Human endothelial cells modulate granulocyte adherence and chemotaxis. J Immunol. 1985 Mar;134(3):1866–1874. [PubMed] [Google Scholar]
- de Groot P. G., Brinkman H. J., Gonsalves M. D., Van Mourik J. A. The role of thrombin in the regulation of the endothelial prostaglandin production. Biochim Biophys Acta. 1985 Sep 30;846(3):342–349. doi: 10.1016/0167-4889(85)90004-7. [DOI] [PubMed] [Google Scholar]


