Abstract
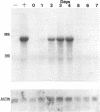
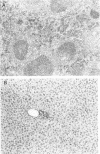
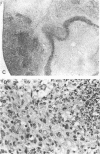
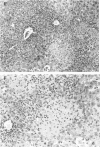
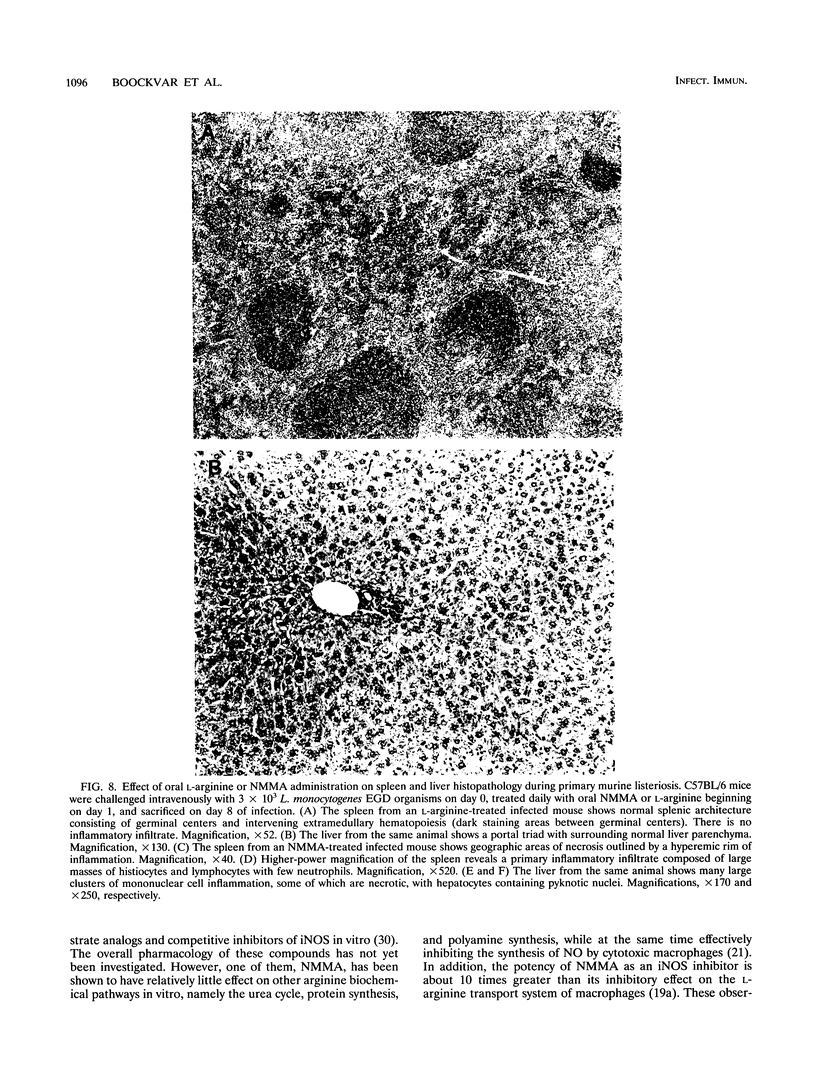
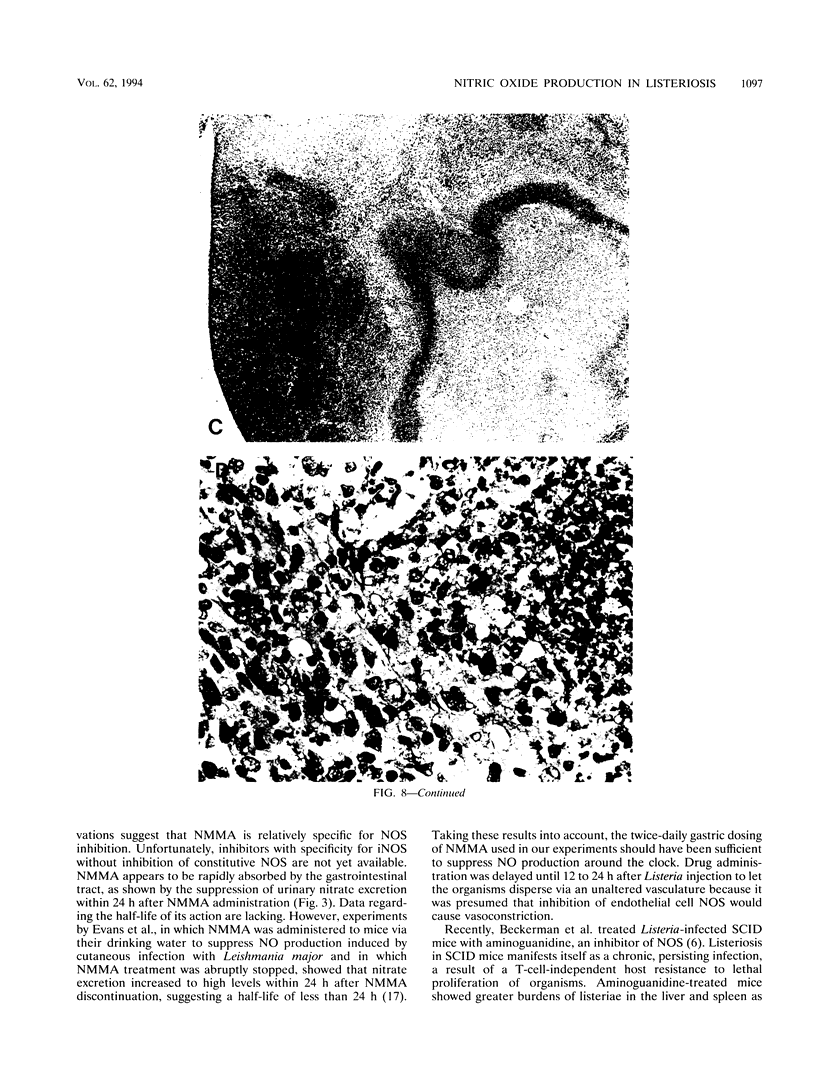
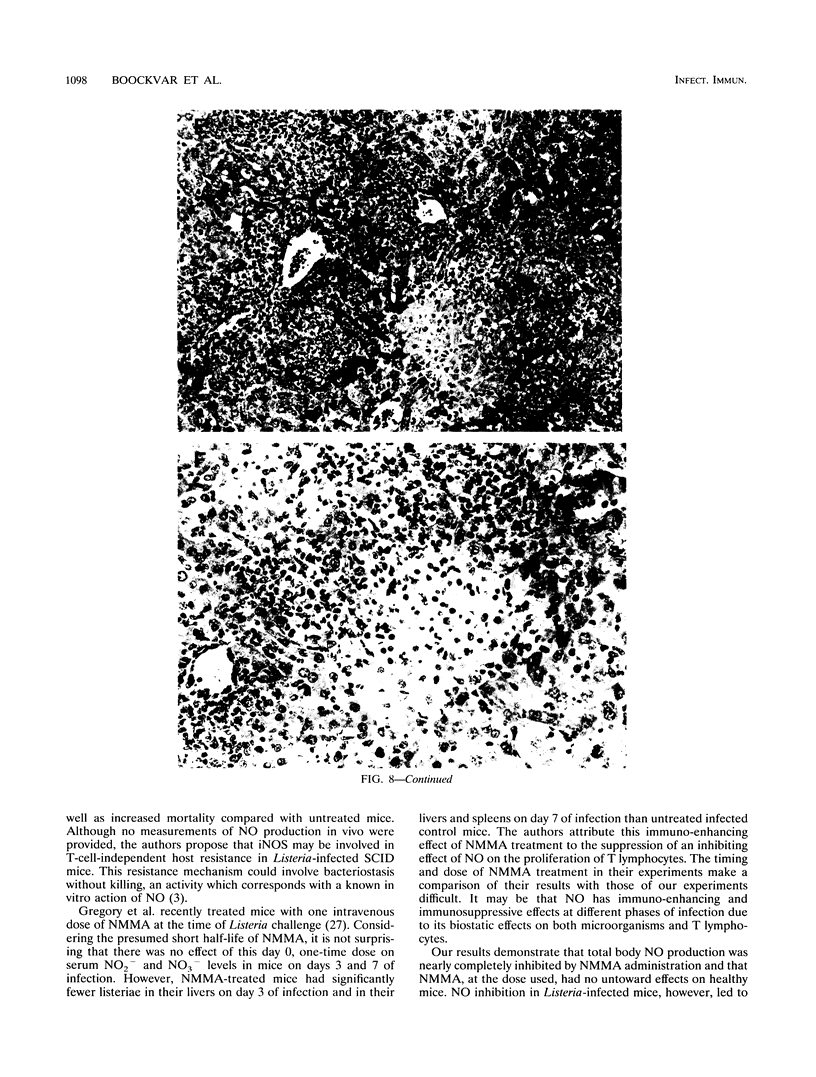
Nitric oxide (NO) has been shown to be important for intracellular microbiostasis in vitro. To determine the role of NO in immune function in vivo, groups of C57BL/6 mice were given a sublethal intravenous inoculum of Listeria monocytogenes EGD, and their urine was monitored daily for nitrate, the mammalian end product of NO metabolism. Urinary nitrate levels peaked at 5 to 10 times the basal level on days 5 to 6, when spleen and liver Listeria counts declined most steeply, and decreased thereafter, when spleens and livers were nearly sterile. Peritoneal macrophages explanted from Listeria-infected mice produced nitrite spontaneously, whereas macrophages from uninfected mice did not. The inducible NO synthase mRNA was detectable in the spleens of infected mice on days 1 to 4 of infection. When Listeria-infected mice were treated orally throughout the infection with NG-monomethyl-L-arginine (NMMA), a specific NO synthase inhibitor they showed no detectable rise in urinary nitrate excretion. Mean Listeria counts in the livers and spleens NMMA-treated mice were 1 to 3 orders of magnitude greater than counts in control mice on days 4 through 8 of infection. Compared with control mice, NMMA-treated mice also showed worse clinical signs of infection, namely, weight loss, hypothermia, decreased food and water intake, and decreased urine output. Histologically NMMA-treated mice had many more inflammatory foci in their livers and spleens than control mice. The histologic observation that mononuclear cells are present at sites of infection suggests that inhibiting NO production did not block the flux of macrophages into infected viscera. As controls for possible drug toxicity, a group of uninfected mice given NMMA orally showed no detrimental effects on weight, temperature, and food and water intake. These experiments demonstrate that inhibition of NO production in Listeria-infected mice results in an exacerbated infection and thus that NO synthesis is important for immune defense against Listeria infection in mice.
Full text
PDF

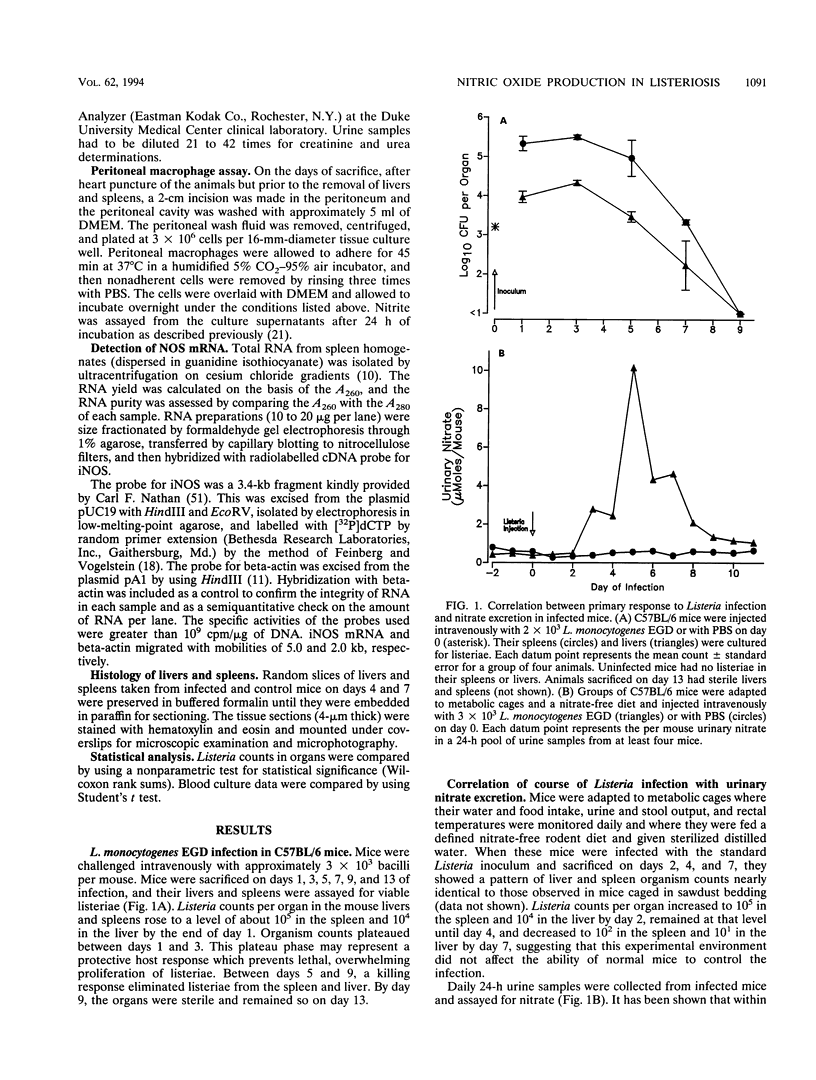

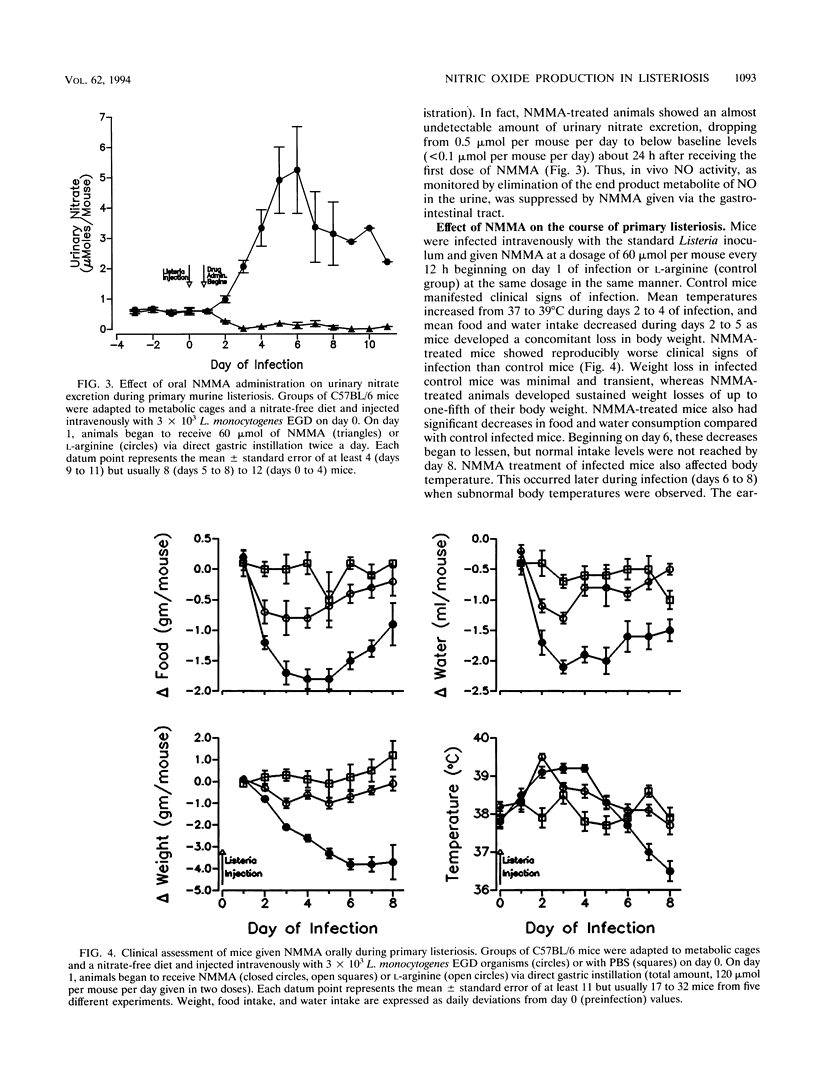
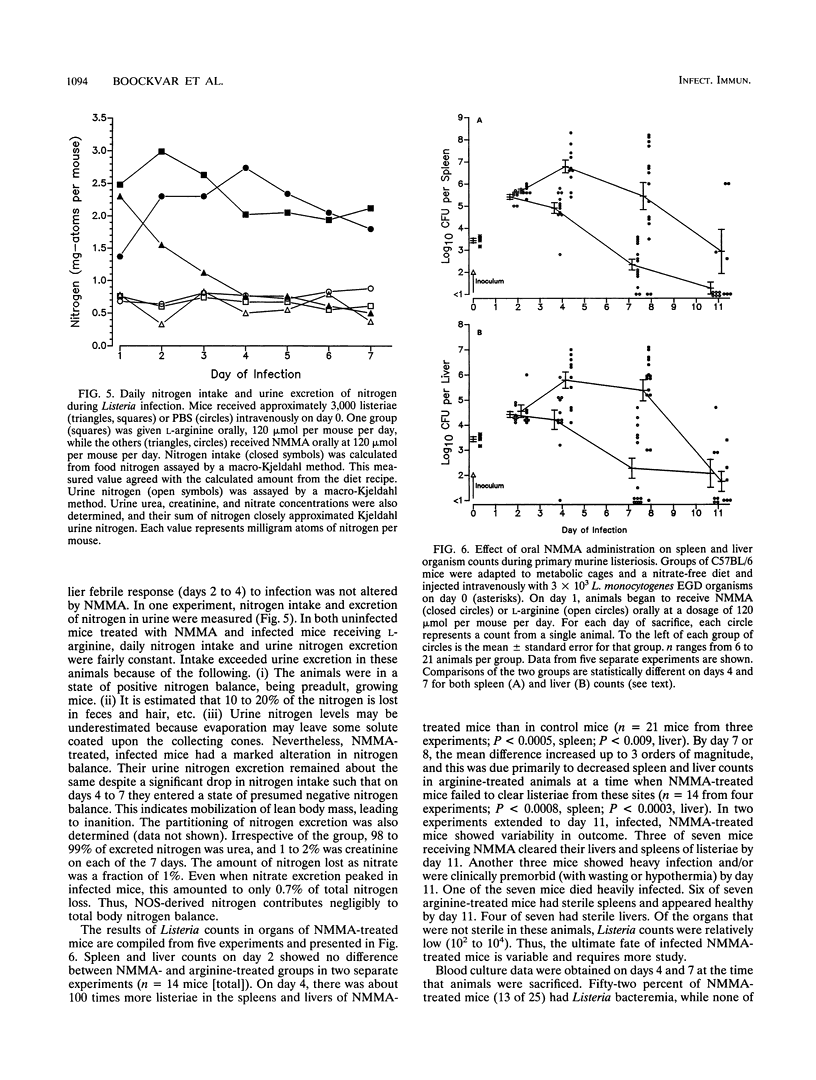
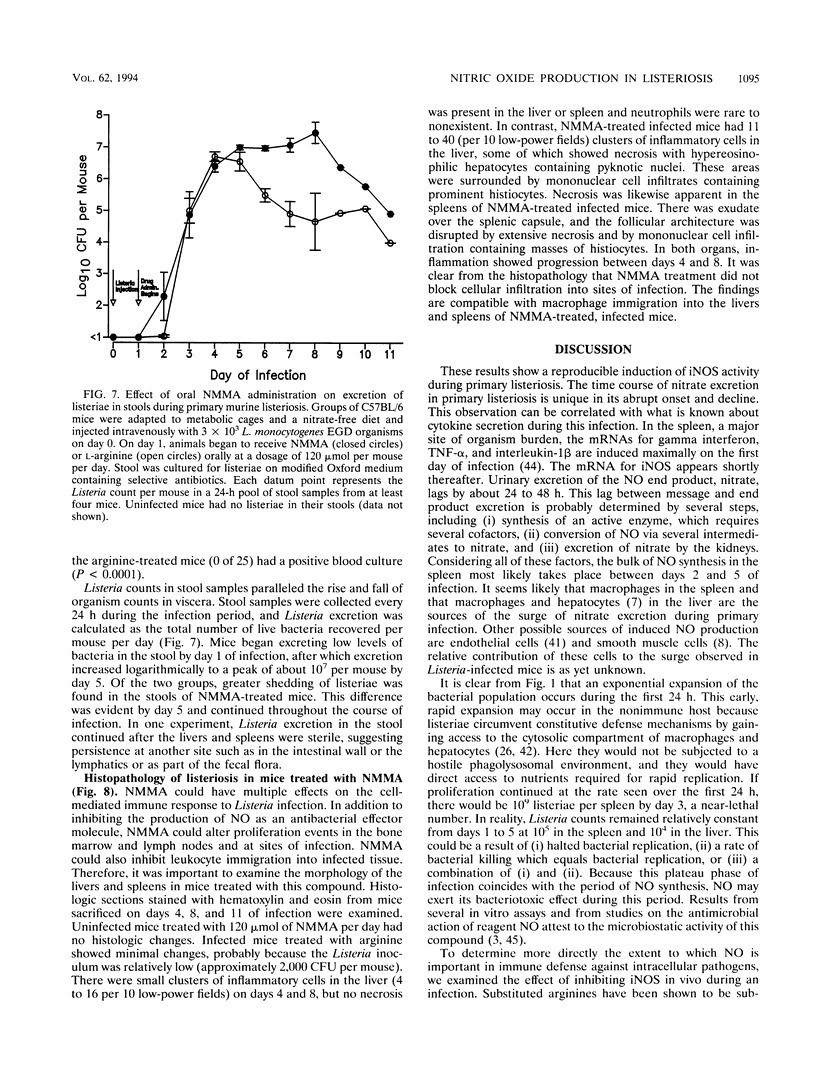


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams L. B., Franzblau S. G., Vavrin Z., Hibbs J. B., Jr, Krahenbuhl J. L. L-arginine-dependent macrophage effector functions inhibit metabolic activity of Mycobacterium leprae. J Immunol. 1991 Sep 1;147(5):1642–1646. [PubMed] [Google Scholar]
- Adams L. B., Hibbs J. B., Jr, Taintor R. R., Krahenbuhl J. L. Microbiostatic effect of murine-activated macrophages for Toxoplasma gondii. Role for synthesis of inorganic nitrogen oxides from L-arginine. J Immunol. 1990 Apr 1;144(7):2725–2729. [PubMed] [Google Scholar]
- Alspaugh J. A., Granger D. L. Inhibition of Cryptococcus neoformans replication by nitrogen oxides supports the role of these molecules as effectors of macrophage-mediated cytostasis. Infect Immun. 1991 Jul;59(7):2291–2296. doi: 10.1128/iai.59.7.2291-2296.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony L. S., Morrissey P. J., Nano F. E. Growth inhibition of Francisella tularensis live vaccine strain by IFN-gamma-activated macrophages is mediated by reactive nitrogen intermediates derived from L-arginine metabolism. J Immunol. 1992 Mar 15;148(6):1829–1834. [PubMed] [Google Scholar]
- Bauss F., Dröge W., Männel D. N. Tumor necrosis factor mediates endotoxic effects in mice. Infect Immun. 1987 Jul;55(7):1622–1625. doi: 10.1128/iai.55.7.1622-1625.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckerman K. P., Rogers H. W., Corbett J. A., Schreiber R. D., McDaniel M. L., Unanue E. R. Release of nitric oxide during the T cell-independent pathway of macrophage activation. Its role in resistance to Listeria monocytogenes. J Immunol. 1993 Feb 1;150(3):888–895. [PubMed] [Google Scholar]
- Billiar T. R., Curran R. D., Stuehr D. J., Stadler J., Simmons R. L., Murray S. A. Inducible cytosolic enzyme activity for the production of nitrogen oxides from L-arginine in hepatocytes. Biochem Biophys Res Commun. 1990 May 16;168(3):1034–1040. doi: 10.1016/0006-291x(90)91133-d. [DOI] [PubMed] [Google Scholar]
- Busse R., Mülsch A. Induction of nitric oxide synthase by cytokines in vascular smooth muscle cells. FEBS Lett. 1990 Nov 26;275(1-2):87–90. doi: 10.1016/0014-5793(90)81445-t. [DOI] [PubMed] [Google Scholar]
- Cameron M. L., Granger D. L., Weinberg J. B., Kozumbo W. J., Koren H. S. Human alveolar and peritoneal macrophages mediate fungistasis independently of L-arginine oxidation to nitrite or nitrate. Am Rev Respir Dis. 1990 Dec;142(6 Pt 1):1313–1319. doi: 10.1164/ajrccm/142.6_Pt_1.1313. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Lopata M. A., MacDonald R. J., Cowan N. J., Rutter W. J., Kirschner M. W. Number and evolutionary conservation of alpha- and beta-tubulin and cytoplasmic beta- and gamma-actin genes using specific cloned cDNA probes. Cell. 1980 May;20(1):95–105. doi: 10.1016/0092-8674(80)90238-x. [DOI] [PubMed] [Google Scholar]
- Curran R. D., Billiar T. R., Stuehr D. J., Ochoa J. B., Harbrecht B. G., Flint S. G., Simmons R. L. Multiple cytokines are required to induce hepatocyte nitric oxide production and inhibit total protein synthesis. Ann Surg. 1990 Oct;212(4):462–471. doi: 10.1097/00000658-199010000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denis M. Interferon-gamma-treated murine macrophages inhibit growth of tubercle bacilli via the generation of reactive nitrogen intermediates. Cell Immunol. 1991 Jan;132(1):150–157. doi: 10.1016/0008-8749(91)90014-3. [DOI] [PubMed] [Google Scholar]
- Ding A. H., Nathan C. F., Stuehr D. J. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J Immunol. 1988 Oct 1;141(7):2407–2412. [PubMed] [Google Scholar]
- Drapier J. C., Wietzerbin J., Hibbs J. B., Jr Interferon-gamma and tumor necrosis factor induce the L-arginine-dependent cytotoxic effector mechanism in murine macrophages. Eur J Immunol. 1988 Oct;18(10):1587–1592. doi: 10.1002/eji.1830181018. [DOI] [PubMed] [Google Scholar]
- Evans T. G., Thai L., Granger D. L., Hibbs J. B., Jr Effect of in vivo inhibition of nitric oxide production in murine leishmaniasis. J Immunol. 1993 Jul 15;151(2):907–915. [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Garthwaite J., Charles S. L., Chess-Williams R. Endothelium-derived relaxing factor release on activation of NMDA receptors suggests role as intercellular messenger in the brain. Nature. 1988 Nov 24;336(6197):385–388. doi: 10.1038/336385a0. [DOI] [PubMed] [Google Scholar]
- Granger D. L., Hibbs J. B., Jr, Broadnax L. M. Urinary nitrate excretion in relation to murine macrophage activation. Influence of dietary L-arginine and oral NG-monomethyl-L-arginine. J Immunol. 1991 Feb 15;146(4):1294–1302. [PubMed] [Google Scholar]
- Granger D. L., Hibbs J. B., Jr, Perfect J. R., Durack D. T. Metabolic fate of L-arginine in relation to microbiostatic capability of murine macrophages. J Clin Invest. 1990 Jan;85(1):264–273. doi: 10.1172/JCI114422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green L. C., Tannenbaum S. R., Goldman P. Nitrate synthesis in the germfree and conventional rat. Science. 1981 Apr 3;212(4490):56–58. doi: 10.1126/science.6451927. [DOI] [PubMed] [Google Scholar]
- Green S. J., Meltzer M. S., Hibbs J. B., Jr, Nacy C. A. Activated macrophages destroy intracellular Leishmania major amastigotes by an L-arginine-dependent killing mechanism. J Immunol. 1990 Jan 1;144(1):278–283. [PubMed] [Google Scholar]
- Green S. J., Nacy C. A., Schreiber R. D., Granger D. L., Crawford R. M., Meltzer M. S., Fortier A. H. Neutralization of gamma interferon and tumor necrosis factor alpha blocks in vivo synthesis of nitrogen oxides from L-arginine and protection against Francisella tularensis infection in Mycobacterium bovis BCG-treated mice. Infect Immun. 1993 Feb;61(2):689–698. doi: 10.1128/iai.61.2.689-698.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory S. H., Barczynski L. K., Wing E. J. Effector function of hepatocytes and Kupffer cells in the resolution of systemic bacterial infections. J Leukoc Biol. 1992 Apr;51(4):421–424. doi: 10.1002/jlb.51.4.421. [DOI] [PubMed] [Google Scholar]
- Gregory S. H., Wing E. J., Hoffman R. A., Simmons R. L. Reactive nitrogen intermediates suppress the primary immunologic response to Listeria. J Immunol. 1993 Apr 1;150(7):2901–2909. [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Taintor R. R., Vavrin Z. Macrophage cytotoxicity: role for L-arginine deiminase and imino nitrogen oxidation to nitrite. Science. 1987 Jan 23;235(4787):473–476. doi: 10.1126/science.2432665. [DOI] [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Taintor R. R., Vavrin Z., Rachlin E. M. Nitric oxide: a cytotoxic activated macrophage effector molecule. Biochem Biophys Res Commun. 1988 Nov 30;157(1):87–94. doi: 10.1016/s0006-291x(88)80015-9. [DOI] [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Vavrin Z., Taintor R. R. L-arginine is required for expression of the activated macrophage effector mechanism causing selective metabolic inhibition in target cells. J Immunol. 1987 Jan 15;138(2):550–565. [PubMed] [Google Scholar]
- Hoffman R. A., Langrehr J. M., Billiar T. R., Curran R. D., Simmons R. L. Alloantigen-induced activation of rat splenocytes is regulated by the oxidative metabolism of L-arginine. J Immunol. 1990 Oct 1;145(7):2220–2226. [PubMed] [Google Scholar]
- James S. L., Glaven J. Macrophage cytotoxicity against schistosomula of Schistosoma mansoni involves arginine-dependent production of reactive nitrogen intermediates. J Immunol. 1989 Dec 15;143(12):4208–4212. [PubMed] [Google Scholar]
- Knowles R. G., Palacios M., Palmer R. M., Moncada S. Formation of nitric oxide from L-arginine in the central nervous system: a transduction mechanism for stimulation of the soluble guanylate cyclase. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5159–5162. doi: 10.1073/pnas.86.13.5159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew F. Y., Millott S., Parkinson C., Palmer R. M., Moncada S. Macrophage killing of Leishmania parasite in vivo is mediated by nitric oxide from L-arginine. J Immunol. 1990 Jun 15;144(12):4794–4797. [PubMed] [Google Scholar]
- MACKANESS G. B. Cellular resistance to infection. J Exp Med. 1962 Sep 1;116:381–406. doi: 10.1084/jem.116.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACKANESS G. B. THE IMMUNOLOGICAL BASIS OF ACQUIRED CELLULAR RESISTANCE. J Exp Med. 1964 Jul 1;120:105–120. doi: 10.1084/jem.120.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellion B. T., Ignarro L. J., Ohlstein E. H., Pontecorvo E. G., Hyman A. L., Kadowitz P. J. Evidence for the inhibitory role of guanosine 3', 5'-monophosphate in ADP-induced human platelet aggregation in the presence of nitric oxide and related vasodilators. Blood. 1981 May;57(5):946–955. [PubMed] [Google Scholar]
- Murray H. W., Teitelbaum R. F. L-arginine-dependent reactive nitrogen intermediates and the antimicrobial effect of activated human mononuclear phagocytes. J Infect Dis. 1992 Mar;165(3):513–517. doi: 10.1093/infdis/165.3.513. [DOI] [PubMed] [Google Scholar]
- Nakane A., Minagawa T., Kato K. Endogenous tumor necrosis factor (cachectin) is essential to host resistance against Listeria monocytogenes infection. Infect Immun. 1988 Oct;56(10):2563–2569. doi: 10.1128/iai.56.10.2563-2569.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nüssler A., Drapier J. C., Rénia L., Pied S., Miltgen F., Gentilini M., Mazier D. L-arginine-dependent destruction of intrahepatic malaria parasites in response to tumor necrosis factor and/or interleukin 6 stimulation. Eur J Immunol. 1991 Jan;21(1):227–230. doi: 10.1002/eji.1830210134. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ashton D. S., Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988 Jun 16;333(6174):664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- Portnoy D. A., Chakraborty T., Goebel W., Cossart P. Molecular determinants of Listeria monocytogenes pathogenesis. Infect Immun. 1992 Apr;60(4):1263–1267. doi: 10.1128/iai.60.4.1263-1267.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poston R. M., Kurlander R. J. Analysis of the time course of IFN-gamma mRNA and protein production during primary murine listeriosis. The immune phase of bacterial elimination is not temporally linked to IFN production in vivo. J Immunol. 1991 Jun 15;146(12):4333–4337. [PubMed] [Google Scholar]
- Poston R. M., Kurlander R. J. Cytokine expression in vivo during murine listeriosis. Infection with live, virulent bacteria is required for monokine and lymphokine messenger RNA accumulation in the spleen. J Immunol. 1992 Nov 1;149(9):3040–3044. [PubMed] [Google Scholar]
- Rake J. B., Eagon R. G. Inhibition, but not uncoupling, of respiratory energy coupling of three bacterial species by nitrite. J Bacteriol. 1980 Dec;144(3):975–982. doi: 10.1128/jb.144.3.975-982.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneemann M., Schoedon G., Hofer S., Blau N., Guerrero L., Schaffner A. Nitric oxide synthase is not a constituent of the antimicrobial armature of human mononuclear phagocytes. J Infect Dis. 1993 Jun;167(6):1358–1363. doi: 10.1093/infdis/167.6.1358. [DOI] [PubMed] [Google Scholar]
- Sherman M. P., Loro M. L., Wong V. Z., Tashkin D. P. Cytokine- and Pneumocystis carinii- induced L-arginine oxidation by murine and human pulmonary alveolar macrophages. J Protozool. 1991 Nov-Dec;38(6):234S–236S. [PubMed] [Google Scholar]
- Stuehr D. J., Marletta M. A. Mammalian nitrate biosynthesis: mouse macrophages produce nitrite and nitrate in response to Escherichia coli lipopolysaccharide. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7738–7742. doi: 10.1073/pnas.82.22.7738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuehr D. J., Marletta M. A. Synthesis of nitrite and nitrate in murine macrophage cell lines. Cancer Res. 1987 Nov 1;47(21):5590–5594. [PubMed] [Google Scholar]
- Xie Q. W., Cho H. J., Calaycay J., Mumford R. A., Swiderek K. M., Lee T. D., Ding A., Troso T., Nathan C. Cloning and characterization of inducible nitric oxide synthase from mouse macrophages. Science. 1992 Apr 10;256(5054):225–228. doi: 10.1126/science.1373522. [DOI] [PubMed] [Google Scholar]