Abstract
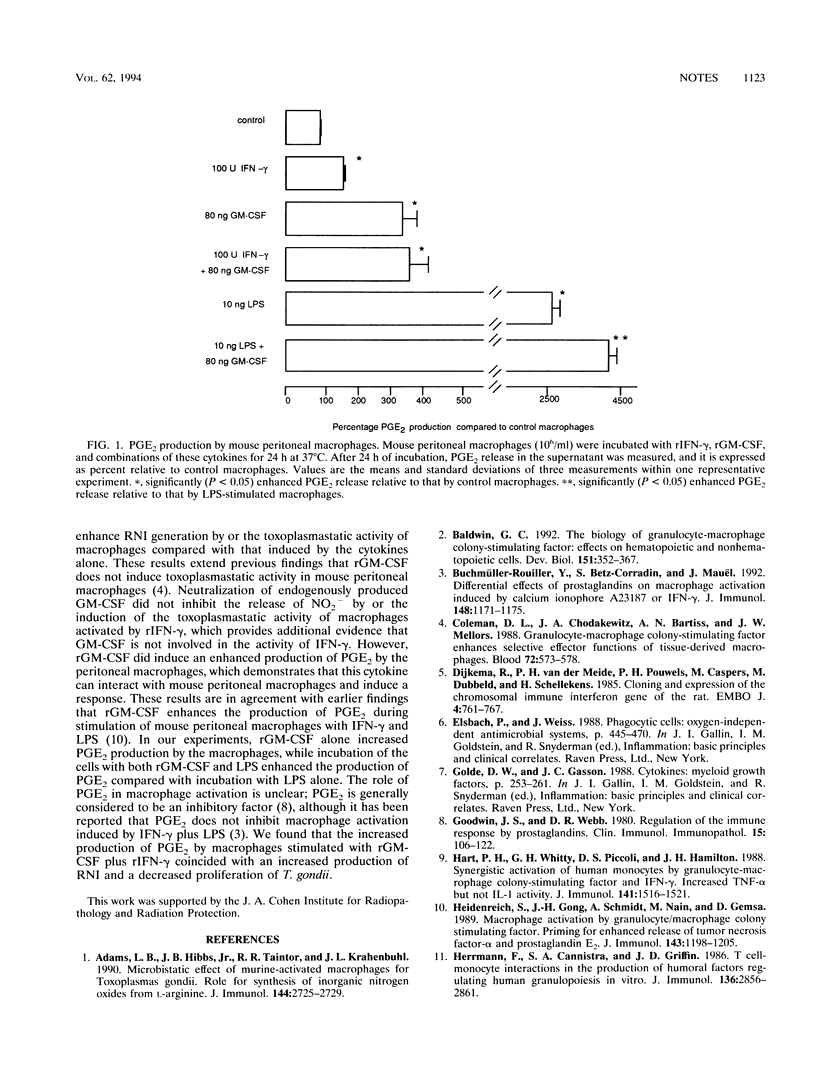
The induction of reactive nitrogen intermediates (RNI) and toxoplasmastatic activity of murine macrophages by recombinant gamma interferon (rIFN-gamma) is mediated by an autocrine pathway involving tumor necrosis factor alpha (TNF-alpha). To investigate whether cytokines other than TNF-alpha play a role in the activation of these effector functions, granulocyte-macrophage colony-stimulating factor (GM-CSF) was studied. Recombinant GM-CSF (rGM-CSF) could stimulate peritoneal macrophages, since this cytokine stimulated the production of prostaglandin E2 by these cells. However, rGM-CSF did not induce either the release of RNI by or the toxoplasmastatic activity of macrophages. rGM-CSF in combination with various concentrations of rIFN-gamma did not enhance these effector functions more than rIFN-gamma alone. Furthermore, neutralization of endogenously produced GM-CSF by monoclonal antibodies did not affect the release of RNI by or the toxoplasmastatic activity of rIFN-gamma-activated macrophages. Together these results indicate that GM-CSF is not involved in RNI production by and toxoplasmastatic activity of IFN-gamma-activated murine macrophages.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams L. B., Hibbs J. B., Jr, Taintor R. R., Krahenbuhl J. L. Microbiostatic effect of murine-activated macrophages for Toxoplasma gondii. Role for synthesis of inorganic nitrogen oxides from L-arginine. J Immunol. 1990 Apr 1;144(7):2725–2729. [PubMed] [Google Scholar]
- Baldwin G. C. The biology of granulocyte-macrophage colony-stimulating factor: effects on hematopoietic and nonhematopoietic cells. Dev Biol. 1992 Jun;151(2):352–367. doi: 10.1016/0012-1606(92)90175-g. [DOI] [PubMed] [Google Scholar]
- Buchmüller-Rouiller Y., Betz-Corradin S., Mauël J. Differential effects of prostaglandins on macrophage activation induced by calcium ionophore A23187 or IFN-gamma. J Immunol. 1992 Feb 15;148(4):1171–1175. [PubMed] [Google Scholar]
- Coleman D. L., Chodakewitz J. A., Bartiss A. H., Mellors J. W. Granulocyte-macrophage colony-stimulating factor enhances selective effector functions of tissue-derived macrophages. Blood. 1988 Aug;72(2):573–578. [PubMed] [Google Scholar]
- Dijkema R., van der Meide P. H., Pouwels P. H., Caspers M., Dubbeld M., Schellekens H. Cloning and expression of the chromosomal immune interferon gene of the rat. EMBO J. 1985 Mar;4(3):761–767. doi: 10.1002/j.1460-2075.1985.tb03694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin J. S., Webb D. R. Regulation of the immune response by prostaglandins. Clin Immunol Immunopathol. 1980 Jan;15(1):106–122. doi: 10.1016/0090-1229(80)90024-0. [DOI] [PubMed] [Google Scholar]
- Hart P. H., Whitty G. A., Piccoli D. S., Hamilton J. A. Synergistic activation of human monocytes by granulocyte-macrophage colony-stimulating factor and IFN-gamma. Increased TNF-alpha but not IL-1 activity. J Immunol. 1988 Sep 1;141(5):1516–1521. [PubMed] [Google Scholar]
- Heidenreich S., Gong J. H., Schmidt A., Nain M., Gemsa D. Macrophage activation by granulocyte/macrophage colony-stimulating factor. Priming for enhanced release of tumor necrosis factor-alpha and prostaglandin E2. J Immunol. 1989 Aug 15;143(4):1198–1205. [PubMed] [Google Scholar]
- Herrmann F., Cannistra S. A., Griffin J. D. T cell-monocyte interactions in the production of humoral factors regulating human granulopoiesis in vitro. J Immunol. 1986 Apr 15;136(8):2856–2861. [PubMed] [Google Scholar]
- Langermans J. A., Van der Hulst M. E., Nibbering P. H., Hiemstra P. S., Fransen L., Van Furth R. IFN-gamma-induced L-arginine-dependent toxoplasmastatic activity in murine peritoneal macrophages is mediated by endogenous tumor necrosis factor-alpha. J Immunol. 1992 Jan 15;148(2):568–574. [PubMed] [Google Scholar]
- Langermans J. A., van der Hulst M. E., Nibbering P. H., van Furth R. Activation of mouse peritoneal macrophages during infection with Salmonella typhimurium does not result in enhanced intracellular killing. J Immunol. 1990 Jun 1;144(11):4340–4346. [PubMed] [Google Scholar]
- Langermans J. A., van der Hulst M. E., Nibbering P. H., van Furth R. Endogenous tumor necrosis factor alpha is required for enhanced antimicrobial activity against Toxoplasma gondii and Listeria monocytogenes in recombinant gamma interferon-treated mice. Infect Immun. 1992 Dec;60(12):5107–5112. doi: 10.1128/iai.60.12.5107-5112.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattsson R., Mattsson A., Holmdahl R., Scheynius A., Van der Meide P. H. In vivo treatment with interferon-gamma during early pregnancy in mice induces strong expression of major histocompatibility complex class I and II molecules in uterus and decidua but not in extra-embryonic tissues. Biol Reprod. 1992 Jun;46(6):1176–1186. doi: 10.1095/biolreprod46.6.1176. [DOI] [PubMed] [Google Scholar]
- Morrissey P. J., Grabstein K. H., Reed S. G., Conlon P. J. Granulocyte/macrophage colony stimulating factor. A potent activation signal for mature macrophages and monocytes. Int Arch Allergy Appl Immunol. 1989;88(1-2):40–45. [PubMed] [Google Scholar]
- Nathan C. F., Hibbs J. B., Jr Role of nitric oxide synthesis in macrophage antimicrobial activity. Curr Opin Immunol. 1991 Feb;3(1):65–70. doi: 10.1016/0952-7915(91)90079-g. [DOI] [PubMed] [Google Scholar]
- Reed S. G., Nathan C. F., Pihl D. L., Rodricks P., Shanebeck K., Conlon P. J., Grabstein K. H. Recombinant granulocyte/macrophage colony-stimulating factor activates macrophages to inhibit Trypanosoma cruzi and release hydrogen peroxide. Comparison with interferon gamma. J Exp Med. 1987 Dec 1;166(6):1734–1746. doi: 10.1084/jem.166.6.1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisson S. D., Dinarello C. A. Production of interleukin-1 alpha, interleukin-1 beta and tumor necrosis factor by human mononuclear cells stimulated with granulocyte-macrophage colony-stimulating factor. Blood. 1988 Oct;72(4):1368–1374. [PubMed] [Google Scholar]
- Stuehr D. J., Marletta M. A. Induction of nitrite/nitrate synthesis in murine macrophages by BCG infection, lymphokines, or interferon-gamma. J Immunol. 1987 Jul 15;139(2):518–525. [PubMed] [Google Scholar]
- Suzuki Y., Orellana M. A., Schreiber R. D., Remington J. S. Interferon-gamma: the major mediator of resistance against Toxoplasma gondii. Science. 1988 Apr 22;240(4851):516–518. doi: 10.1126/science.3128869. [DOI] [PubMed] [Google Scholar]
- van Waarde D., Hulsing-Hesselink E., van Furth R. A serum facted by newborn calf serum. Cell Tissue Kinet. 1976 Jan;9(1):51–63. [PubMed] [Google Scholar]
- van der Meide P. H., Dubbeld M., Vijverberg K., Kos T., Schellekens H. The purification and characterization of rat gamma interferon by use of two monoclonal antibodies. J Gen Virol. 1986 Jun;67(Pt 6):1059–1071. doi: 10.1099/0022-1317-67-6-1059. [DOI] [PubMed] [Google Scholar]


