Abstract
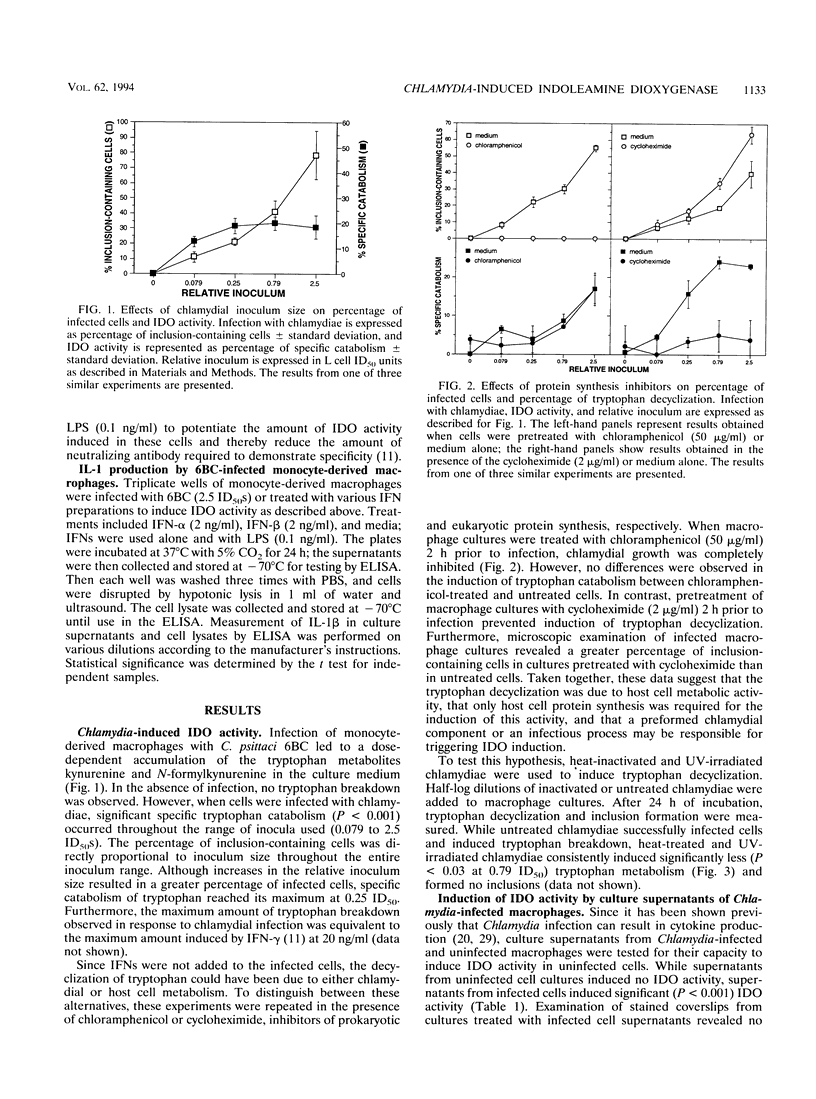
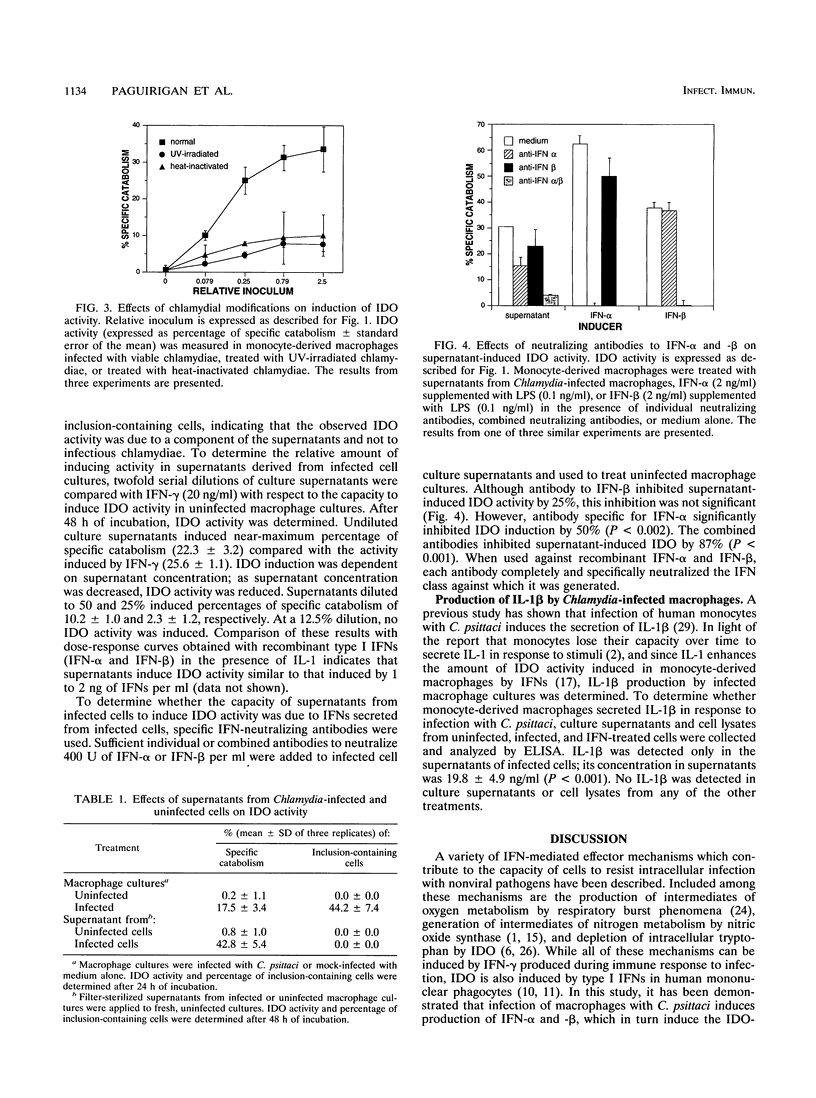
The purpose of this study was to characterize further the events leading to the metabolic degradation of tryptophan in Chlamydia-infected cultures in the absence of added interferon (IFN). Macrophages on coverslips were infected with Chlamydia psittaci, and tryptophan decyclization was determined 24 h later by reverse-phase high-performance liquid chromatography. Tryptophan metabolites cochromatographed with kynurenine and N-formylkynurenine, the end products of tryptophan decyclization by the IFN-inducible enzyme indoleamine 2,3-dioxygenase (IDO). Although chloramphenicol pretreatment completely inhibited chlamydial replication, IDO was stimulated to an extent similar to that in untreated, infected cells. No IDO induction was observed in cells pretreated with cycloheximide even though chlamydial growth was slightly greater than in untreated cells. These results indicate that enhanced tryptophan decyclization was due to induction of IDO. IDO induction was dependent on the size of the chlamydial inoculum. Heat- or UV-inactivated chlamydiae induced significantly less IDO activity than viable chlamydiae. Culture supernatants from Chlamydia-infected macrophages induced IDO activity in a dose-dependent manner, suggesting that a secreted product of infected cells was responsible for IDO induction. A combination of neutralizing antibodies to IFN-alpha and IFN-beta inhibited induction of IDO activity by infected cell culture supernatants. Furthermore, IL-1 enzyme-linked immunosorbent assay results indicated the accumulation of IL-1 beta in the culture medium. Thus, induction of IDO in Chlamydia-infected macrophages reflects the production of cytokines in response to infection and may represent a normal host cell response to control intracellular infection.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams L. B., Hibbs J. B., Jr, Taintor R. R., Krahenbuhl J. L. Microbiostatic effect of murine-activated macrophages for Toxoplasma gondii. Role for synthesis of inorganic nitrogen oxides from L-arginine. J Immunol. 1990 Apr 1;144(7):2725–2729. [PubMed] [Google Scholar]
- Beatty W. L., Byrne G. I., Morrison R. P. Morphologic and antigenic characterization of interferon gamma-mediated persistent Chlamydia trachomatis infection in vitro. Proc Natl Acad Sci U S A. 1993 May 1;90(9):3998–4002. doi: 10.1073/pnas.90.9.3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne G. I., Faubion C. L. Lymphokine-mediated microbistatic mechanisms restrict Chlamydia psittaci growth in macrophages. J Immunol. 1982 Jan;128(1):469–474. [PubMed] [Google Scholar]
- Byrne G. I., Lehmann L. K., Landry G. J. Induction of tryptophan catabolism is the mechanism for gamma-interferon-mediated inhibition of intracellular Chlamydia psittaci replication in T24 cells. Infect Immun. 1986 Aug;53(2):347–351. doi: 10.1128/iai.53.2.347-351.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne G. I., Moulder J. W. Parasite-specified phagocytosis of Chlamydia psittaci and Chlamydia trachomatis by L and HeLa cells. Infect Immun. 1978 Feb;19(2):598–606. doi: 10.1128/iai.19.2.598-606.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne G. I. Requirements for ingestion of Chlamydia psittaci by mouse fibroblasts (L cells). Infect Immun. 1976 Sep;14(3):645–651. doi: 10.1128/iai.14.3.645-651.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell H. D., Hitchcock P. J. Monoclonal antibody against a genus-specific antigen of Chlamydia species: location of the epitope on chlamydial lipopolysaccharide. Infect Immun. 1984 May;44(2):306–314. doi: 10.1128/iai.44.2.306-314.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlin J. M., Borden E. C., Byrne G. I. Interferon-induced indoleamine 2,3-dioxygenase activity inhibits Chlamydia psittaci replication in human macrophages. J Interferon Res. 1989 Jun;9(3):329–337. doi: 10.1089/jir.1989.9.329. [DOI] [PubMed] [Google Scholar]
- Carlin J. M., Borden E. C., Sondel P. M., Byrne G. I. Biologic-response-modifier-induced indoleamine 2,3-dioxygenase activity in human peripheral blood mononuclear cell cultures. J Immunol. 1987 Oct 1;139(7):2414–2418. [PubMed] [Google Scholar]
- Carlin J. M., Borden E. C., Sondel P. M., Byrne G. I. Interferon-induced indoleamine 2,3-dioxygenase activity in human mononuclear phagocytes. J Leukoc Biol. 1989 Jan;45(1):29–34. doi: 10.1002/jlb.45.1.29. [DOI] [PubMed] [Google Scholar]
- Eissenberg L. G., Wyrick P. B., Davis C. H., Rumpp J. W. Chlamydia psittaci elementary body envelopes: ingestion and inhibition of phagolysosome fusion. Infect Immun. 1983 May;40(2):741–751. doi: 10.1128/iai.40.2.741-751.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friis R. R. Interaction of L cells and Chlamydia psittaci: entry of the parasite and host responses to its development. J Bacteriol. 1972 May;110(2):706–721. doi: 10.1128/jb.110.2.706-721.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerrard T. L., Siegel J. P., Dyer D. R., Zoon K. C. Differential effects of interferon-alpha and interferon-gamma on interleukin 1 secretion by monocytes. J Immunol. 1987 Apr 15;138(8):2535–2540. [PubMed] [Google Scholar]
- Green S. J., Meltzer M. S., Hibbs J. B., Jr, Nacy C. A. Activated macrophages destroy intracellular Leishmania major amastigotes by an L-arginine-dependent killing mechanism. J Immunol. 1990 Jan 1;144(1):278–283. [PubMed] [Google Scholar]
- Hatch T. P. Competition between Chlamydia psittaci and L cells for host isoleucine pools: a limiting factor in chlamydial multiplication. Infect Immun. 1975 Jul;12(1):211–220. doi: 10.1128/iai.12.1.211-220.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X., Baldwin C. L. Effects of cytokines on intracellular growth of Brucella abortus. Infect Immun. 1993 Jan;61(1):124–134. doi: 10.1128/iai.61.1.124-134.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee D. M., Smith J. G., Bleicker C. A., Carter C. J., Bonewald L. F., Schachter J., Williams D. M. Chlamydia trachomatis pneumonia induces in vivo production of interleukin-1 and -6. Infect Immun. 1992 Mar;60(3):1217–1220. doi: 10.1128/iai.60.3.1217-1220.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merigan T. C., Hanna L. Characteristics of interferon induced in vitro and in vivo by a TRIC agent. Proc Soc Exp Biol Med. 1966 Jun;122(2):421–424. doi: 10.3181/00379727-122-31151. [DOI] [PubMed] [Google Scholar]
- Meyer K. C., Cornwell R., Carlin J. M., Powers C., Irizarry A., Byrne G. I., Borden E. C. Effects of interferons beta or gamma on neopterin biosynthesis and tryptophan degradation by human alveolar macrophages in vitro: synergy with lipopolysaccharide. Am J Respir Cell Mol Biol. 1992 Jun;6(6):639–646. doi: 10.1165/ajrcmb/6.6.639. [DOI] [PubMed] [Google Scholar]
- Moulder J. W. Interaction of chlamydiae and host cells in vitro. Microbiol Rev. 1991 Mar;55(1):143–190. doi: 10.1128/mr.55.1.143-190.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray H. W., Rubin B. Y., Rothermel C. D. Killing of intracellular Leishmania donovani by lymphokine-stimulated human mononuclear phagocytes. Evidence that interferon-gamma is the activating lymphokine. J Clin Invest. 1983 Oct;72(4):1506–1510. doi: 10.1172/JCI111107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F., Murray H. W., Wiebe M. E., Rubin B. Y. Identification of interferon-gamma as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J Exp Med. 1983 Sep 1;158(3):670–689. doi: 10.1084/jem.158.3.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferkorn E. R., Guyre P. M. Inhibition of growth of Toxoplasma gondii in cultured fibroblasts by human recombinant gamma interferon. Infect Immun. 1984 May;44(2):211–216. doi: 10.1128/iai.44.2.211-216.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferkorn E. R., Rebhun S., Eckel M. Characterization of an indoleamine 2,3-dioxygenase induced by gamma-interferon in cultured human fibroblasts. J Interferon Res. 1986 Jun;6(3):267–279. doi: 10.1089/jir.1986.6.267. [DOI] [PubMed] [Google Scholar]
- Rapoza P. A., Tahija S. G., Carlin J. P., Miller S. L., Padilla M. L., Byrne G. I. Effect of interferon on a primary conjunctival epithelial cell model of trachoma. Invest Ophthalmol Vis Sci. 1991 Oct;32(11):2919–2923. [PubMed] [Google Scholar]
- Rothermel C. D., Rubin B. Y., Murray H. W. Gamma-interferon is the factor in lymphokine that activates human macrophages to inhibit intracellular Chlamydia psittaci replication. J Immunol. 1983 Nov;131(5):2542–2544. [PubMed] [Google Scholar]
- Rothermel C. D., Schachter J., Lavrich P., Lipsitz E. C., Francus T. Chlamydia trachomatis-induced production of interleukin-1 by human monocytes. Infect Immun. 1989 Sep;57(9):2705–2711. doi: 10.1128/iai.57.9.2705-2711.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shemer Y., Sarov I. Inhibition of growth of Chlamydia trachomatis by human gamma interferon. Infect Immun. 1985 May;48(2):592–596. doi: 10.1128/iai.48.2.592-596.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu T., Nomiyama S., Hirata F., Hayaishi O. Indoleamine 2,3-dioxygenase. Purification and some properties. J Biol Chem. 1978 Jul 10;253(13):4700–4706. [PubMed] [Google Scholar]
- Turco J., Winkler H. H. Cloned mouse interferon-gamma inhibits the growth of Rickettsia prowazekii in cultured mouse fibroblasts. J Exp Med. 1983 Dec 1;158(6):2159–2164. doi: 10.1084/jem.158.6.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong S., Lau S. Rapid separation of tryptophan, kynurenines, and indoles using reversed-phase high-performance liquid chromatography. J Chromatogr. 1979 Jul 13;175(2):343–346. doi: 10.1016/s0021-9673(00)89443-1. [DOI] [PubMed] [Google Scholar]



