Abstract
Hypothalamic neuropeptide Y (NPY) has been implicated in the regulation of energy balance and reproduction, and chronically elevated NPY levels in the hypothalamus are associated with obesity and reduced reproductive function. However, it is not known which one of the five cloned Y receptors mediates these effects. Here we show that crossing the Y4 receptor knockout mouse (Y4−/−) onto the ob/ob background restores the reduced plasma testosterone levels of ob/ob mice as well as the reduced testis and seminal vesicle size and morphology to control values. Fertility in the sterile ob/ob mice was greatly improved by Y4 receptor deletion, with 100% of male and 50% of female Y4−/−,ob/ob double knockout mice producing live offspring. Development of the mammary ducts and lobuloalveoli was significantly enhanced in pregnant Y4−/− and Y4−/−,ob/ob females. Consistent with the improved fertility and enhanced mammary gland development, gonadotropin releasing hormone (GnRH) expression was significantly increased in Y4−/− and Y4−/−,ob/ob animals. Y4−/− mice displayed lower body weight and reduced white adipose tissue mass accompanied by increased plasma levels of pancreatic polypeptide (PP). However, Y4 deficiency had no beneficial effects to reduce body weight or excessive adiposity of ob/ob mice. These data suggest that central Y4 receptor signaling specifically inhibits reproductive function under conditions of elevated central NPY-ergic tonus.
Keywords: Neuropeptide Y, Y4 receptor knockout, fertility, ob/ob mouse, gonadotropin releasing hormone, adiposity
Hypothalamic neuropeptide Y (NPY) is a major central regulator of sexual behavior and reproductive functions. Intracerebroventricular (ICV) administration of NPY to sex steroid-primed ovariectomized (OVX) rats increases secretion of luteinizing hormone (LH), and stimulates secretion of gonadotropin releasing hormone (GnRH) from the median eminence in vitro, functions that probably mediate the preovulatory surge of LH release (Sabatino et al. 1990; Urban et al. 1996; Jain et al. 1999). In contrast, when centrally administered to sex steroid-deficient OVX rats, or to intact male and female rats, NPY markedly inhibits reproductive function (Clark et al. 1985; Reznikov and McCann 1993; Xu et al. 1993; Pierroz et al. 1996). This includes decreased GnRH receptor concentration in the pituitary gland, reduced pituitary weight, and decreased plasma concentrations of prolactin, LH, follicle stimulating hormone (FSH), and testosterone. Testicular and seminal vesicle or ovarian weights are also reduced, and sexual maturation and estrous cyclicity in female rats is disrupted, leading to drastic suppression of male and female copulatory behavior (Clark et al. 1985; Reznikov and McCann 1993; Xu et al. 1993; Pierroz et al. 1996). These inhibitory effects of NPY on reproductive function probably contribute to the decreased fertility observed under conditions of negative energy balance, such as food restriction, heavy exercise, lactation, and insulin-dependent diabetes mellitus, all of which are associated with elevated hypothalamic NPY expression (Aubert et al. 1998; Krysiak et al. 1999). In this way NPY coordinates energy availability with reproduction, inhibiting procreation during unfavorable metabolic conditions.
Interestingly, obesity is also associated with reproductive defects and reduced fertility (Caprio et al. 2001). Genetically obese ob/ob mice lack functional leptin, the hormone produced mainly by white adipose tissue. This mutation not only leads to hyperphagia, massive obesity, and the associated hormonometabolic defects (hypercorticosteronemia, hyperinsulinemia, hyperglycemia, and insulin resistance), but also leads to infertility due to insufficient hypothalamo-pituitary-gonadal drive, underdevelopment of reproductive organs, and impaired spermatogenesis (Caprio et al. 2001). In ob/ob mice, the lack of leptin-mediated inhibition of NPY expression and secretion in the hypothalamus (Stephens et al. 1995; Schwartz et al. 1996; Widdowson and Wilding 2000) leads to chronically elevated hypothalamic NPY-ergic activity. This secondary effect of leptin deficiency contributes to many of the associated defects. Indeed, treatment of ob/ob mice with leptin reduces NPY mRNA expression and peptide levels in the hypothalamus, reduces the hyperphagic, obese phenotype, and also restores fertility of male and female mice by improved function of the hypothalamo-pituitary-gonadal axis (Stephens et al. 1995; Chehab et al. 1996; Mounzih et al. 1997). Importantly, food restriction that produced a degree of weight loss similar to that produced by leptin treatment did not reduce central NPY expression and did not restore fertility in ob/ob mice (Mounzih et al. 1997). Further evidence that elevated central NPY-ergic tonus mediates the pathology of leptin deficiency is that most of the defects of ob/ob mice, including infertility, are attenuated or normalized when crossed onto NPY knockout mice (Erickson et al. 1996).
The various functions of NPY are mediated by the Y receptor gene family, consisting of at least five distinct members (Y1, Y2, Y4, Y5, and y6) (Blomqvist and Herzog 1997). In addition to their involvement in reproduction and energy homeostasis, these Y-receptors when activated by their ligands, NPY, peptide YY (PYY), and pancreatic polypeptide (PP), can also modulate other important physiological functions, including circadian rhythms, gastrointestinal motility, memory, anxiety, nociception, and blood pressure (Hokfelt et al. 1998; Gehlert 1999; Kalra et al. 1999). Messenger RNAs for Y1, Y2, and Y5 are widely distributed throughout the brain (Naveilhan et al. 1998; Parker and Herzog 1999). In contrast, the Y4 receptor is predominantly expressed in the periphery including tissues such as the pancreas, intestine, colon, heart, and liver (Bard et al. 1995; Lundell et al. 1995). However, significant amounts of Y4 mRNA and specific binding sites have also been found in key areas of the hypothalamus such as the paraventricular nucleus and in certain brainstem nuclei including the area postrema and the nucleus tractus solitarius (Parker and Herzog 1999; Larsen and Kristensen 2000). Y receptors show very low primary amino acid sequence identity, yet surprisingly exhibit very similar pharmacology, with NPY and PYY being equipotent at all receptor subtypes. PP has high affinity only for the Y4 receptor, and in some species it also has moderate affinity for the Y1 and Y5 receptors.
Although NPY is known to be involved in numerous physiological and pathophysiological processes, the clarification of the functions of specific Y receptor subtypes has been severely hampered by the lack of subtype-selective agonists and antagonists. The functions of the Y4 receptor subtype and its high-affinity agonist PP are among the less well understood of the Y receptor family. However, it was recently demonstrated that ICV administration of the Y1 antagonist and Y4 agonist 1229U91 (Schober et al. 1998) to estrogen-primed OVX or intact male rats rapidly increased FSH and/or LH secretion (Jain et al. 1999; Raposinho et al. 2000). These effects were attributed to Y4 activation by pharmacological analysis of other partial Y4 agonists (Raposinho et al. 2000), and also because rat PP, which is specific for the rat Y4 receptor, induced a similar profile of LH secretion in estrogen-primed OVX rats (Jain et al. 1999). Since PP is not expressed within the brain and does not cross the blood–brain barrier, it is possible that instead NPY acts as a central, albeit lower-affinity ligand for the hypothalamic Y4 receptor when expression levels are sufficiently high to agonize this receptor. We therefore hypothesized that under conditions of high hypothalamic NPY levels, as in energy deficit or leptin-deficient obesity, NPY could modulate reproductive functions through activation of Y4 receptors. To test this hypothesis we generated Y4 receptor knockout mice and crossed them with the obese ob/ob mutant strain and analyzed the effects on energy homeostasis and fertility.
Results
Generation of Y4 receptor knockout mice
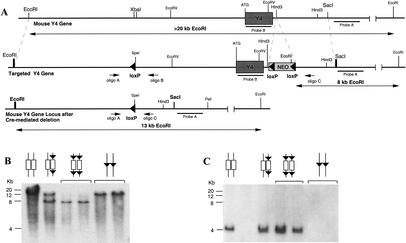
A targeting vector for the Y4 receptor gene was designed which allowed the production of both germ-line (Y4−/−) and conditional (Y4lox/lox) knockout mice (Fig. 1A). A cassette containing the neomycin resistance gene (Neo) flanked on either side by a 34 bp-long Cre-recombinase recognition (loxP) site oriented in the same direction was placed downstream of the Y4 receptor gene. A third loxP sequence was inserted into intron I of the Y4 receptor gene (Fig. 1A). Mouse embryonic stem (ES) cells from the strain 129/SvJ were transfected and selected under standard conditions. Positively targeted clones were identified by Southern analysis. Hybridization with the Neo gene was used to verify single integration (data not shown).
Figure 1.
Generation of Y4 receptor knockout mice. (A) Targeting vector design and screening strategy. Small arrows indicate the position and orientation of oligonucleotides used in the PCR analysis, and bars indicate the position of probes used for Southern analysis of genomic DNA from targeted ES cells as well as knockout animals. (B,C) Southern analysis of genomic DNA isolated from conditional and germ line Y4 receptor knockout mice cut with EcoRI or EcoRV, using probes A and B, respectively.
Two positive ES cell clones for the Y4 receptor construct were injected into blastocysts originating from C57BL/6 mice and implanted into pseudopregnant mice. Offspring with the highest percentage of agouti coat color were crossed with X chromosome-linked, oocyte-specific Cre-recombinase-expressing C57BL/6 mice (Schwenk et al. 1995) to obtain either heterozygotes carrying the floxed gene (conditional, Y4+/lox), or heterozygotes carrying the Cre-recombinase gene and having the floxed gene already deleted (germ line Y4+/−). DNA isolated from tail tips was used in Southern blot analysis (EcoRI) to confirm correct integration and/or modification of the targeted allele (Fig. 1B). Absence of the Y4 gene in Y4−/− mice was confirmed by Southern analysis (EcoRV) using a DNA fragment specific for the Y4 receptor coding sequence (Fig. 1C). All further mice generated were maintained on this 50% C57BL/6 − 50% 129/SvJ background.
Breeding of heterozygous germ-line knockout animals produced no significant deviation from the expected Mendelian ratio of genotypes (25:50:25). Y4−/− animals breed normally; however, the gender ratio in Y4−/− offspring is significantly shifted to a greater proportion of females (56%, n = 112, P < 0.01). Interestingly, male Y4−/− mice show very aggressive behavior with increased incidences of fighting between littermates causing injuries. Female Y4−/− mice behave normally in early life and adulthood, but show aggressive behavior and occasionally kill littermates after 20–24 wk of age
Reduced body weight and increased plasma pancreatic polypeptide levels in Y4−/− mice
At 4 wk of age, the body weights of female Y4−/− mice were significantly less than that of Y4+/+ controls. The body weights of male Y4−/− mice at that age were not significantly different from control values. Both genders of Y4−/− mice, particularly males, gained significantly less weight over the next 12 wk of monitoring (Fig. 2). Consistent with this is the finding that the 24-h food intake of male but not female Y4−/− mice was significantly less than that of wild-type controls (4.42 ± 0.10 vs. 5.64 ± 0.30 g at 8 wk, P < 0.001, and 4.18 ± 0.10 vs. 4.70 ± 0.28 g at 12 wk, P < 0.05, means ± SEM of 9–27 mice per group).
Figure 2.
Body weight of Y4 receptor knockout mice. Body weight of male Y4−/− (▪) and female Y4−/− (●) mice compared with combined wild-type (Y4+/+) and heterozygous (Y4+/−) controls (males □, females ○). Values are means ± SEM of 20–32 mice per group. *P < 0.05 and **P < 0.01 versus curve for same-sex controls.
Plasma levels of PP (the endogenous high-affinity ligand for Y4 receptors) were increased by two- to threefold in both male and female Y4−/− mice compared to wild-type controls (Fig. 3A), with male mice having significantly greater basal plasma PP concentrations than females (Fig. 3A, P < 0.0001). The combined mass of white adipose tissue (WAT) deposits was significantly lower in Y4−/− mice than in wild types, significantly so in male mice (Fig. 3B). Interestingly, when individual WAT deposits were investigated, mesenteric WAT was the major contributor to decreased WAT weight in male and female Y4−/− mice (0.65 ± 0.06 vs. 0.84 ± 0.05 % of body weight in male mice, P < 0.01, and 0.53 ± 0.06 vs. 0.76 ± 0.05 % of body weight in female mice, P < 0.05, means ± SEM of 28–37 mice per group), with no significant effect of Y4 deletion on the weight of epididymal or periovarian WAT, nor on inguinal or retroperitoneal WAT (data not shown). Y4−/− mice showed no significant difference from wild types with respect to plasma concentrations of leptin, insulin, glucose, or testosterone but had a tendency to reduced levels of corticosterone (P = 0.055; Table 1). The weights (as a percentage of body weight) of the pancreas, stomach, small intestine, liver, kidney, and testis or ovary of Y4−/− mice were not significantly different from those of wild-type controls (Table 1).
Figure 3.
Plasma pancreatic polypeptide concentrations and adiposity of Y4 receptor knockout mice. (A) Plasma levels of pancreatic polypeptide, and (B) weight (as percent of body weight) of combined right inguinal, right epididymal or periovarian, right retroperitoneal, and mesenteric white adipose tissue (WAT) of germ line Y4−/− mice (filled bars) versus Y4+/+ control mice (open bars). Values are means ± SEM of 15–31 mice per group. (*) P < 0.05 versus same-sex Y4+/+ controls.
Table 1.
Plasma hormone and metabolite concentrations, and organ weights in Y4−/− mice
|
|
|
Y4+/+
|
Y4−/−
|
|---|---|---|---|
| Leptin (ng/mL) | male | 5.3 ± 0.8 | 4.5 ± 0.8 |
| female | 5.7 ± 0.6 | 5.1 ± 0.7 | |
| Insulin (pM) | male | 110 ± 14 | 89 ± 10 |
| female | 58 ± 7 | 50 ± 5 | |
| Glucose (mM) | male | 9.9 ± 0.3 | 10.5 ± 0.5 |
| female | 10.0 ± 0.3 | 10.7 ± 0.3 | |
| Corticosterone (ng/mL) | male | 76 ± 16 | 57 ± 16 |
| female | 165 ± 25 | 111 ± 15 | |
| Testosterone (nM) | male | 10.4 ± 3.3 | 15.9 ± 4.3 |
| female | ND | ND | |
| Gonad weight (% BWt) | male | 0.75 ± 0.03 | 0.80 ± 0.03 |
| female | 0.08 ± 0.01 | 0.07 ± 0.01 |
Data are means ± SEM of 7–21 mice per group. ND, Not determined.
Decreased corticosteronemia in Y4−/−,ob/ob double knockout mice
Male and female heterozygous (OB/ob) mice on a C57BL/6 background were crossed with Y4−/− animals. Double heterozygous (Y4+/−,OB/ob) animals were crossed again to subsequently obtain all of the nine possible genotypes. The food intake of male and female Y4−/−,ob/ob double knockout mice was not significantly different from that of the ob/ob mice (data not shown), suggesting that Y4 receptors are not directly involved in the regulation of feeding behavior in these animals. At 4 wk of age, male and female Y4−/−,ob/ob double knockout mice had body weights similar to those of the ob/ob mice, but male double knockouts gained significantly more weight in the following 12 wk of monitoring compared to ob/ob controls (Fig. 4). A tendency for increased body weight was also apparent in Y4−/−,OB/ob males compared to OB/ob or wild-type controls (Fig. 4). Female Y4−/−,ob/ob double knockout mice showed no significant difference from ob/ob mice with respect to body weight over the 12 wk of monitoring (data not shown).
Figure 4.
Body weight of Y4−/−,ob/ob double knockout mice. Body weight of male Y4−/−,ob/ob (♦) compared with ob/ob (⋄), Y4−/−,ob/ob (▴), OB/ob (▵) and wild-type (□) mice. Values are means ± SEM of 20–32 mice per group. *P < 0.05 versus curve for ob/ob mice.
There was a significant interaction effect between Y4 and leptin deficiency on WAT mass (P < 0.01). Whereas Y4 deficiency significantly increased the WAT mass of lean OB/ob mice, the increased WAT mass of obese ob/ob mice was not further increased in Y4−/−,ob/ob double knockout mice (Table 2). Insulinemia and glycemia of ob/ob mice were significantly elevated over wild-type values, and the absence of Y4 receptors in Y4−/−,ob/ob double knockout mice had no significant effect on these parameters (Table 2). Plasma levels of PP in ob/ob mice were strongly reduced compared to wild-type mice (Fig. 3A). Y4 deletion restored plasma PP levels in Y4−/−,ob/ob mice to values not significantly different from those of control mice (Fig. 3A; Table 2). Furthermore, the hypercorticosteronemia of ob/ob mice was significantly attenuated in Y4−/−,ob/ob double knockout mice (Fig. 5A).
Table 2.
Weight of white adipose tissue deposits, and plasma hormone and metabolite concentrations in male Y4−/−,ob/ob double knockout mice and controls
|
|
OB/ob
|
Y4−/−OB/ob
|
ob/ob
|
Y4−/−ob/ob
|
|---|---|---|---|---|
| Sum of WAT (%) | 2.48 ± 0.22 | 3.51 ± 0.33a | 8.19 ± 0.29c | 7.51 ± 0.30c |
| Insulin (pmol/L) | 89 ± 16 | 201 ± 41 | 7420 ± 2230c | 9800 ± 2300b |
| Glucose (mmol/L) | 10.5 ± 0.5 | 12.8 ± 0.7 | 18.3 ± 4.0b | 13.7 ± 2.0 |
| PP (nmol/L) | ND | ND | 1.18 ± 1.04 | 6.45 ± 1.70d |
Data are means ± SEM of 6–18 mice per group. Sum of WAT, combined weight of right inguinal, right epididymal or pcriovarian, right retroperitoneal, and mesenteric white adipose tissue as a percent of body weight; PP, pancreatic polypeptide; ND, not determined.
P < 0.05, bP < 0.01, cP < 0.001 versus OB/ob control mice. dP < 0.05 versus ob/ob mice.
Figure 5.
Effect of Y4 deletion on plasma corticosterone and testosterone levels, and testis and seminal vesicle weight in mice with an ob/ob background. Plasma concentrations of (A) corticosterone and (B) testosterone (C) testis weight and (D) seminal vesicle weight of Y4+/+ mice (open bars) and Y4−/− mice (filled bars) on an OB/ob or ob/ob background. Values are means ± SEM of 6–17 mice per group. *P < 0.05, **P < 0.01, and ***P < 0.001 for the comparison indicated by horizontal bars.
Increased fertility in Y4−/−,ob/ob double knockout mice
The most profound effects of Y4 deletion on ob/ob mice were observed upon reproductive functions. Figure 5B–D shows plasma testosterone levels, and the absolute weight of testis and seminal vesicle of heterozygous OB/ob and homozygous ob/ob mice with or without Y4 receptor deletion. OB/ob mice that were Y4+/+ showed no significant difference from wild-type mice with respect to any of the parameters shown in Figure 5, nor in any other of the parameters measured (see Materials and Methods), with the exception of a significant increase in relative and absolute hepatic weight. In ob/ob mice, plasma testosterone concentrations were markedly depressed compared to wild-type or heterozygous OB/ob animals, in keeping with the significantly reduced weight of testis and seminal vesicle in these animals (Fig. 5B–D). Notably, crossing the Y4 receptor knockout onto ob/ob mice significantly increased the plasma testosterone levels of males compared to ob/ob animals, and significantly increased absolute testis and seminal vesicle weights to values not significantly different from those of OB/ob or wild-type control mice (Fig. 5B–D). The observed increase in body weight of male Y4−/−,ob/ob compared to ob/ob mice (Fig. 4) is probably in part due to this increase in plasma testosterone levels. Figure 6 shows the improvement in testis and seminal vesicle size in ob/ob mice with Y4 receptor deficiency, since the organs of Y4−/−,ob/ob double knockout mice resembled those of wild types. Under light microscopy, the morphology of testis and seminal vesicle from Y4−/−,ob/ob double knockout mice was similar to that of wild-type organs. Leydig cell and mature sperm densities were increased over ob/ob levels (Fig. 6).
Figure 6.
Effects of Y4 receptor deletion on testis and seminal vesicle morphology in Y4−/− and Y4−/−,ob/ob double knockout mice. Lower panels show hematoxylin/eosin-stained sections from testis of the indicated genotypes. Three seminiferous tubules were chosen that displayed different stages of sperm production. Leydig cells are seen at the center of the panels. Original magnification, 200×.
These changes were also reflected in fertility. To test the fertility of male Y4−/−,ob/ob double knockouts, they were paired with female Y4−/−,OB/ob mice which were previously proven to be fertile by their ability to produce live offspring with male wild-type mice. The same strategy was applied to test fertility in female Y4−/−,ob/ob mice. For comparison, male or female ob/ob mice were paired with fertile OB/ob animals of the opposite sex. All eight breeding pairs consisting of Y4−/−,ob/ob males and Y4−/−,OB/ob females produced live offspring. In contrast, only one of the eight ob/ob males was able to produce offspring. Interestingly, four of the eight Y4−/−,ob/ob females were able to establish pregnancies and deliver live pups, two of them twice, whereas none of the eight ob/ob females tested were capable of doing so.
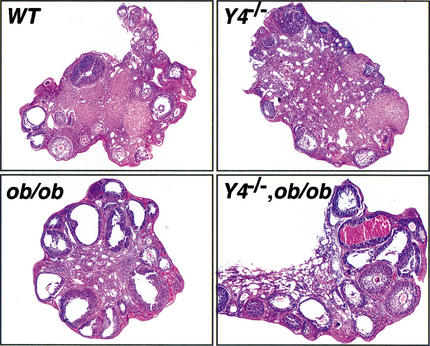
To identify possible abnormalities in estrous cycles, estrous cyclicity was assessed by daily vaginal smear cytology in Y4−/−, ob/ob, and Y4−/−,ob/ob mice. Y4−/− animals exhibited normal vaginal opening and onset of normal estrous cyclicity, with proestrous, estrous, metestrous, and diestrous stages all present, and a cycle length of 4–5 d. In ob/ob animals, the vaginal opening was very small and a diestrous-like swab was observed until 9 wk of age, when cycles lasting from 11 to 15 d commenced. An estrous-like cytology lasted 6–8 d and was characterized by a small number of poorly clumped superficial epithelial cells, large quantities of cellular debris, and increased mucous. In fertile Y4−/−,ob/ob animals, the vaginal opening was complete by 9 wk of age but the total cycle length remained extended, from 11 to 16 d with an estrous lasting up to 7 d. This extended estrous showed plentiful clumped superficial epithelial cells, and the female was receptive in this state and mating could produce a pregnancy. Infertile Y4−/−,ob/ob animals showed a persistent small vaginal orifice and vaginal cytology similar to ob/ob animals, characterized by an absence of large numbers of clumped superficial epithelial cells during an extended estrous. Ovarian morphology of the four different genotypes was also assessed (Fig. 7). Hematoxylin/eosin-stained sections show normal histology in Y4−/− and wild-type (WT) mice, characterized by the presence of multiple primary and secondary follicles and well developed corpora lutea. Ovaries from ob/ob mice, taken during their extended estrous, showed very few primary or secondary follicles. Many large empty follicles were seen and no proper developed corpora lutea were present. Loss of Y4 on the ob/ob background resulted in an increase in the number of corpora lutea and primary and secondary follicles, the extent of which was variable among individuals, consistent with the partial rescue of fertility in this genotype. Large empty follicles, characteristic of the ob/ob genotype, continued to be present in all animals.
Figure 7.
Effects of Y4 receptor deletion on ovary morphology. Hematoxylin/eosin-stained sections of ovaries at estrous from wild-type (WT) and Y4−/− animals, or extended estrous in ob/ob and Y4−/−, ob/ob animals. Original magnification, 100×.
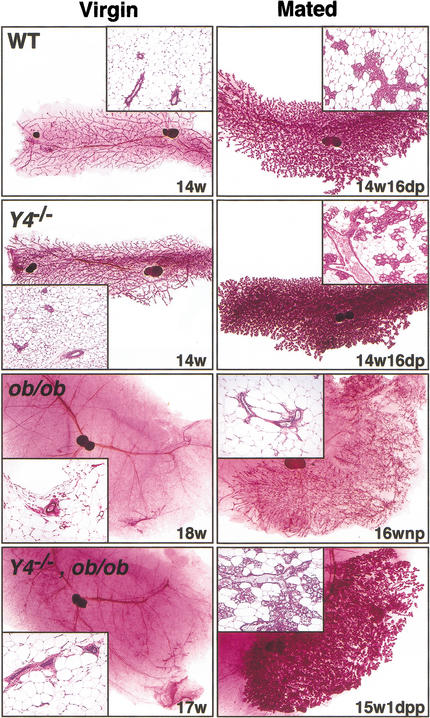
Mammary gland development examined in Y4+/+, Y4−/−, ob/ob, and Y4−/−,ob/ob double knockout females showed significant changes (Fig. 8). In Y4−/− females, ductal development proceeded normally at puberty to produce a branched ductal tree of a complexity seen in mixed 129/SvJ-C57BL/6 animals. During pregnancy, lobuloalveolar development was accelerated as demonstrated by the significantly increased lobuloalveolar content of the glands at day 16 of pregnancy (18.74 ± 0.66% area of 4th gland in Y4−/− animals compared to 15.79 ± 0.86% in wild-type animals, P < 0.05, means ± SEM of 5 mice per group) (Fig. 8). In virgin ob/ob animals, no ductal development occurred, with the exception of one animal from the mated group which showed increased ductal development. However, this development was abnormal, as the gland consisted of only finely branched ducts without the extensive underlying network of major ducts that were seen in wild-type animals (Fig. 8), consistent with development during a prior pseudo- or failed-pregnancy. In nulliparous Y4−/−,ob/ob females, the loss of Y4 failed to rescue the development of the mammary ductal tree and the gland remained in the prepubescent state, consisting of a few short ducts emanating from the sinus beneath the nipple (Fig. 8). In Y4−/−,ob/ob females that experienced a full-term pregnancy, the degree of mammary development varied. Some females showed minimal ductal elongation and produced a few sparse lobules, while others produced large areas of lobules that showed the presence of milk and oil droplets (Fig. 8). However, irrespective of the histological appearance of these glands, the mothers were incapable of ensuring survival of the pups.
Figure 8.
Effect of Y4 receptor deletion on mammary gland development in Y4−/− and Y4−/−,ob/ob double knockout mice. Whole mounts (5× original magnification) and corresponding hematoxylin/eosin-stained histology (insets, 100× original magnification) of the 4th inguinal mammary gland from females of the indicated genotypes, either virgins or after pairing with a fertile male (mated). Animal age is indicated in weeks (w) and reproductive state for the mated group as days of pregnancy (dp), days postpartum (pp), or nulliparous (np).
Altered neuropeptide expression in Y4−/−,ob/ob double knockout mice
To assess possible central mechanisms for changes in energy balance and reproductive function in Y4−/− and Y4−/−,ob/ob mice, we measured central expression of peptides or their precursors known to regulate energy balance [NPY, agouti-related peptide (AgRP), proopio-melanocortin (POMC), and cocaine and amphetamine-regulated transcript (CART)] (Kalra et al. 1999) as well as reproduction (NPY and GnRH) (Pierroz et al. 1996; Jain et al. 1999). In situ hybridization was performed on coronal brain sections obtained from male mice, using specific radiolabeled antisense DNA oligonucleotides for these mRNAs. Background labeling was uniform and never exceeded 5% of specific signal.
Y4−/− mice did not display altered NPY, AgRP, POMC, or CART mRNA levels in any area investigated (Table 3). Typically, increased NPY and AgRP mRNA expression accompanied by decreased POMC and CART mRNA levels were observed in the arcuate nucleus of ob/ob mice. Notably, Y4−/−,ob/ob double knockout mice displayed significantly lower NPY and AgRP levels than ob/ob animals, although the levels were still markedly augmented compared to wild-type animals (Table 3). Most importantly however, Y4−/− mice as well as Y4−/−, ob/ob double knockouts showed significantly increased GnRH mRNA levels in forebrain neurons compared to wild-type values (Table 3; Fig. 9). These data are consistent with the enhanced reproductive function observed in Y4−/−,ob/ob mice, and suggest that the increase in GnRH expression caused by removal of Y4 receptor signaling is a major contributor to the establishment of fertility in male ob/ob mice. This hypothesis is further supported by results obtained from another Y receptor knockout model generated by us (Baldock et al. 2002). In Y2−/− mice, the lack of Y2 receptors did not alter the expression levels of GnRH mRNA in forebrain neurons compared to wild-type values (104 ± 2.11%, n = 5, P < 0.002). More importantly, Y2−/−,ob/ob double knockout animals were completely infertile, as none of the six male or four female mice tested produced any offspring.
Table 3.
Expression levels NPY, AgRP, POMC, and CART mRNAs
| mRNA
|
wild type
|
Y4−/−
|
ob/ob
|
Y4−/−, ob/ob
|
|---|---|---|---|---|
| Neurons in the arcuate nucleus | ||||
| NPY | 100 ± 5.48 (5) | 98 ± 3.22 (5) | 178 ± 7.44 (5)c | 155 ± 0.57 (3)cd |
| AgRP | 100 ± 4.70 (4) | 95 ± 3.65 (5) | 167 ± 7.28 (5)c | 136 ± 8.38 (3)be |
| POMC | 100 ± 3.91 (5) | 99 ± 4.84 (4) | 33 ± 3.00 (5)c | 39 ± 0.45 (3)c |
| CART | 100 ± 2.21 (5) | 103 ± 2.67 (5) | 23 ± 1.26 (5)c | 26 ± 3.27 (4)c |
| GnRHI neurons in the forebrain | ||||
| Med. Sept. n. | 100 ± 6.63 (5) | 123 ± 2.10 (5)a | 104 ± 4.33 (5) | 122 ± 8.81 (4)ad |
| Preopt. a. | 100 ± 6.42 (5) | 120 ± 4.33 (5)a | 103 ± 4.62 (5) | 120 ± 3.85 (4)ad |
| Neurons in the cortex | ||||
| NPY | 100 ± 4.20 (5) | 98 ± 5.29 (5) | 98 ± 6.04 (5) | 97 ± 7.72 (4) |
| Neurons in the paraventricular nucleus | ||||
| CART | 100 ± 3.36 (4) | 102 ± 4.24 (5) | 96 ± 2.38 (5) | 101 ± 3.10 (3) |
Data represent mean labeling intensity of neurons given as percentage of wild type ± SEM (number of animals); aP < 0.05, bP < 0.01 and cP < 0.001 versus wild-type; dP < 0.05 and eP < 0.01 versus ob/ob. Med. Sept. n. = medial septal nucleus; Preopt. a. = preoptic area.
Figure 9.
Effect of Y4 receptor deletion on GnRH mRNA levels in neurons of the medial septal nucleus. High-power bright-field photomicrographs of dipped sections obtained from wild-type, Y4−/−,ob/ob and Y4−/−,ob/ob double knockout mice after in situ hybridization for GnRH. Bar, 10 μm.
Discussion
This study shows that the infertility of leptin-deficient ob/ob mice involves signaling through Y4 receptors, since crossing our Y4 receptor knockout onto the ob/ob strain conferred the ability to produce live births in breeding pairs consisting of male or female Y4−/−,ob/ob double knockout mice. This was accompanied by significant improvements in gonadotropic function in male mice, partial restoration of estrous cycling in females, and increased GnRH expression in the forebrain, in keeping with mediation by the brain centers that regulate reproduction. Since PP, the only known endogenous high-affinity Y4 receptor agonist, does not cross the blood–brain barrier and is not found in the brain, we propose that the high hypothalamic NPY levels present in ob/ob mice are sufficient to inhibit reproductive functions by agonism of central Y4 receptors. In support of this hypothesis, crossing the ob/ob strain onto NPY-deficient mice has also been shown to rescue fertility in male and female NPY−/−,ob/ob double knockout mice (Erickson et al. 1996). Furthermore, fasting of ob/ob animals, which reduces their body weight without affecting the high NPY levels, does not restore fertility (Mounzih et al. 1997).
Although Y4 receptor deletion in ob/ob mice can restore fertility and lead to improved mammary gland development, pups born of Y4−/−,ob/ob females die from dehydration and/or starvation within 1–2 d after birth. The primary cause appears to be failed lactogenesis, but could also be due to the defective maternal behavior of these females as seen by the scattering of pups in the cage rather than collection within the nest. A similar situation is found in leptin-treated female ob/ob mice, which can conceive and deliver live pups, but also fail to lactate (Chehab et al. 1996). Furthermore, mice with knockout of genes involved in prolactin release or the prolactin signaling pathway show a similar lactational and behavioral defect (Liu et al. 1997; Ormandy et al. 1997; Wynick et al. 1998). This suggests that although Y4 receptor deletion can partially restore gonadotropic function in ob/ob females, pituitary prolactin release may only be restored to a level sufficient to maintain the corpus luteum but insufficient to allow lactogenesis and correct maternal behavior. Two Y4−/−,ob/ob mothers had second litters and both again failed to lactate. This raises the possibility that in addition to leptin's hypothalamic action to reduce NPY signaling, binding to its receptor in the mammary gland may also be required for full development and normal onset of lactogenesis.
In addition to lack of leptin, the genetic background of C57BL/6 ob/ob mice has also been considered to contribute to infertility. Male animals deficient in the leptin gene on a mixed C57BL/6J-BALB/cJ genetic background showed improved fertility, with 41% being able to produce offspring, although none of the ob/ob females on this genetic background were able to do so (Ewart-Toland et al. 1999). It has been suggested that inheritance of so-called modifier genes is responsible for the improvement in male fertility in these animals. Interestingly, one of the loci found to be associated with high testosterone levels in the present study (D14MIT113) is very close to the location of the Y4 receptor gene (D14MIT14), making the Y4 receptor a strong candidate for such a modifier gene. In our studies, the improved fertility of Y4−/−,ob/ob mice was not due to breeding onto the mixed C57BL/6-129/SvJ background, because our ob/ob control mice on the same genetic background were effectively sterile by comparison.
Analysis of our male and female Y4−/− mice also showed a several-fold increase in plasma concentrations of PP compared to wild-type values. PP is released from pancreatic F cells in response to stimuli such as food intake and hypoglycemia, mainly by vagal muscarinic activation (Schwartz 1983; Havel et al. 1993). It is thought to act on Y4 receptors in the brain stem accessible to plasma-borne factors to enhance digestive events such as gastric secretion, motility, and emptying by subsequent activation of vagal cholinergic pathways (McTigue et al. 1997). It is therefore possible that the loss of Y4 receptor function contributed to the reduced body weight gain and reduced adiposity in Y4−/− mice by an inefficiency of nutrient uptake during digestion. It is also possible that the reduced body weight of Y4−/− mice was mediated by the high plasma PP concentrations acting on other peripheral Y receptors besides Y4. In support of this, PP transgenic mice, which have a 20-fold increase in plasma PP levels, have a metabolic phenotype similar to that of our Y4−/− mice. This includes reduced food intake, body weight, and fat mass, as well as reduced glucose-induced insulin secretion and gastric emptying, with effects more evident in male compared to female transgenic mice (Ueno et al. 1999). It appears unlikely that changes in hypothalamic neuropeptide expression are involved in the manifestation of the reduced body weight in Y4−/− mice, because the expression levels of the orexigenic and anorexic peptides (NPY, AgRP, POMC, and CART) were no different in Y4−/− mice compared to controls. In ob/ob mice, Y4 deficiency increased plasma PP levels from the low levels characteristic of congenitally obese rodents (Jia and Taylor 1984) to levels not significantly different from those of wild-type mice. However, this increase was probably of insufficient magnitude to reduce the body weight and adiposity of Y4−/−,ob/ob mice relative to ob/ob mice.
In conclusion, Y4 receptors are specifically involved in the down-regulation of reproductive function and impaired fertility observed in leptin-deficient ob/ob mice. This is likely to occur by NPY-mediated activation of Y4 receptors in the brain regions that control the gonadotropic axis. In contrast, other aspects of obesity in ob/ob mice, such as defects in lactation, as well as hyperphagia, hyperinsulinemia, hyperglycemia, and increased fat mass, are most likely not mediated by Y4 receptor agonism. In normal physiology, the Y4 receptor may contribute to reducing reproductive capacity when hypothalamic NPY expression is increased, such as in food restriction, heavy exercise, lactation, and type 1 diabetes mellitus, representing a possible new target for the treatment of infertility.
Materials and methods
Targeting vector construction and gene disruption
A 129/SvJ mouse genomic BAC library (GenomSystems) was screened under low stringency for the Y4 receptor gene using a human Y4 cDNA probe (Lundell et al. 1995). Positively hybridizing clones were isolated and mapped. A 12 kb HindIII fragment and a 4.5 kb SacI fragment were subcloned into pBluescript and used to generate the targeting construct. A loxP-flanked Neo cassette was inserted into the HindIII site downstream of the Y4 gene and a third loxP sequence was introduced by cloning two complementary 46 mer oligonucleotides into an XbaI site 5 kb upstream of the Y4 receptor gene initiation codon. A 1.2 kb HindIII/EcoRI fragment 5′ to the Y4 targeting construct was used to screen for positively targeted ES cell clones. Two of these clones were injected into C57BL/6 blastocysts. Chimeric mice were bred either with C57BL/6 mice to generate heterozygous conditional knockout mice (Y4lox/+) or with C57BL/6 transgenic animals containing the Cre-recombinase gene under an oocyte-specific promoter to obtain heterozygous germ line Y4 receptor knockout mice (Y4+/−). Breeding the respective heterozygous mice generated all five possible genotypes (Y4lox/lox; Y4lox/+; Y4+/+; Y4+/−; Y4−/−). The genotype of mice was determined by Southern blot analysis as described in the Results section, and by PCR using oligonucleotide A (5′-ATCCTTCCTGCCTCTATG-3′), oligonucleotide B (5′-GGA TAATACC AGCATGGC-3′), and oligonucleotide C (5′-GCA TCTGTACTGAGTGGC-3′), with 35 cycles of 94°C for 45 sec, 60°C for 1 min, and 72°C for 20 sec.
Measurement of food intake and body weight
Groups of 16–21 animals per genotype originating from 3–4 different breeding pairs were group-housed and fed standard chow. Body weight was monitored at the same time each week from 4 wk of age onwards. Food intake was measured over 7 d in individually-housed mice at 8 and 12 wk of age.
Tissue collection and analysis
At 16–18 wk of age, Y4+/+ and Y4−/− mice derived from crosses of Y4+/−parents, as well as six of the nine possible genotypes obtained from crosses of double heterozygous animals (Y4+/+,OB/OB; Y4−/−,OB/OB; Y4+/+,OB/ob; Y4−/−,OB/ob; Y4+/+,ob/ob; and Y4−/−,ob/ob) were killed by cervical dislocation between 10.00–15.00 h for collection of trunk blood, and brains and plasma were immediately frozen and stored at −80°C. Food was removed from cages 1–3 h before death, which occurred within 90 sec of initial handling and removal from cages to avoid time-related increases in stress hormones (corticosterone) (Sakakura et al. 1976). White adipose tissue depots (right inguinal, right epididymal or periovarian, right retroperitoneal, and mesenteric), pancreas, stomach, small intestine, liver, kidney, heart, ovary, testis, and seminal vesicle were removed and weighed. For the morphology investigation, the third and fourth inguinal mammary glands were dissected out, whole-mounted on glass slides, and fixed in Bouin's solution. Plasma PP was radioimmunoassayed using guinea pig anti-rat PP serum and second Antibody Precipitating System from Linco Research and 125I-PP (Auspep). Plasma leptin and insulin levels were measured by radioimmunoassay kits from Linco Research, corticosteronemia and plasma testosterone concentrations were measured with kits from ICN Biomedicals, and glycemia was determined with a glucose oxidase assay kit (Trace Scientific, Melbourne, Australia).
In situ hybridization
Coronal brain sections (20 μm) were cut on a cryostat and thaw-mounted on superfrost slides. The sections were kept desiccated at −80°C until their use in the respective experiments. Matching sections from the same coronal brain level of knockout and control mice (4–5 male mice per group) were assayed together, generally following the method of Young (1989) with slight variations (Tsunashima et al. 1997). In short, DNA oligonucleotides complementary to mouse NPY (5′-GAGGGTCAGTC CACACAGCCCCATTCGCTTGTTACCTAGCAT-3′); mouse POMC (5′-TGGCTGCTCTCCAGGCACCAGCTCCACACAT CTATGGAGG-3′); mouse GnRH (5′-CAAACACACAGTCAG CAGTAGAATGCCGGCCATCAGTTTGAGGATC-3′); mouse CART (5′-TCCTTCTCGTGGGACGCATCATCCACGGCAG AGTAGATGTCCAGG-3′); and mouse AGRP (5′-AGCTTGCG GCAGTAGCAAAAGGCATTGAAGAAGCGGCAGTAGCAC3′) mRNAs were labeled with [35S]thio-dATP (1300 Ci/mmol, Amersham) by reaction with terminal deoxynucleotidyltransferase (Roche) and precipitated with ethanol/sodium chloride. Frozen sections were rapidly immersed in 2% paraformaldehyde in 150 mM NaCl and 10 mM phosphate buffer (pH 7.2; phosphate buffered saline, PBS) for 10 min in an ice bath, rinsed in PBS, immersed in 0.25% acetic anhydride in 0.1 M triethylamine hydrochloride (pH 8.2 in saline) for 10 min, dehydrated by ethanol series, and delipidated with chloroform. Air-dried sections were hybridized at 42°C for 18 h with 30 fmole (0.6 × 106 cpm) of labeled oligonucleotide probe in 50 μL hybridization buffer. The hybridization buffer consisted of 50% formamide, 5× SSC (1× SSC is 150 mM NaCl, 15 mM sodium citrate at pH 7.2), 500 μg/mL salmon sperm DNA, 250 μg/mL yeast tRNA, 1× Denhardt's solution (0.02% Ficoll, 0.02 % polyvinylpyrrolidone, and 0.02% bovine serum albumin), 10% dextran sulfate, and 20 mM dithiothreitol. Slides were washed four times in 50% formamide in 2× SSC (42°C, 15 min), cooled to room temperature for 30 min, rinsed in 1× SSC, and dipped briefly in water. Sections were then dipped in 70% ethanol, dried, and exposed to Kodak Biomax MR films for 1–5 d. Subsequently the slides were dipped in Kodak NTB-2 photosensitive emulsion (diluted 1:1 with distilled water), air dried, and exposed for 6 to 14 d. Films and dipped slides were developed with Kodak D19 developer. Sections were counterstained superficially with hematoxylin, dehydrated, and coverslipped with Aquamount (BDH). The corresponding radiolabeled sense DNAs were used to exclude nonspecific hybridization of the probe. Sections hybridized with a 50 times excess of unlabeled probe were included as further controls in some experiments.
Autoradiographs were scanned, and relative optical densities (ROD) were measured over the arcuate nucleus, the medial septal nucleus, or the paraventricular nucleus. Background measured over white matter was deducted. For evaluation of mRNA levels in scattered neurons, images from dipped sections were digitized using a ProgRes 3008 camera (Zeiss) mounted on a Zeiss Axiophot microscope. Silver grain density over single neurons was evaluated using NIH-Image 1.61 software (written by Wayne Rasband and available from anonymous FTP at zippy.nimh.nih.gov).
Histology of mammary gland, vaginal smear, ovary, testis, and seminal vesicle
Mammary development was investigated using carmine-stained whole mounts followed by paraffin embedding and staining of 5-μm sections using hematoxylin/eosin. Lobuloalveoli in hematoxylin/eosin-stained sections were quantified using digital photography and automated area measurement by a macro running the NIH Image analysis software (http://rsb.info.nih.gov/nih-image/). The success of lactation and suckling was assessed by examination of the stomach contents of pups; milk where present was visible through the skin. Estrous cyclicity was studied using DiffQuick staining of vaginal swabs spread on glass slides. Ovaries were fixed in 4% paraformaldehyde and embedded in paraffin before examination of 5-μm hematoxylin/eosin-stained sections. For examination of morphology, seminal vesicle was separated from the coagulating gland and cut from the urethra at their junction. The epididymis/vas deferens was removed from the testis. Testes were fixed in Bouin's fixative, transferred to 10% glucose solution, paraffin embedded, sectioned at 5 μm, and stained with hematoxylin-eosin.
Statistical analyses
Results for body weight were compared among groups by repeated measures ANOVA followed by Fisher's post-hoc tests. Differences in food intake were assessed by Student's t-tests. Differences between Y4−/− and Y4+/+ mice in tissue and organ weights and plasma hormone and metabolite concentrations were assessed by 2-way ANOVA (effect of Y4 deletion and gender, with subsequent Fisher's post-hoc tests). Comparison of these parameters among male Y4+/+,OB/OB; Y4−/−,OB/OB; Y4+/+,OB/ob; Y4−/−,OB/ob; Y4+/+,ob/ob; and Y4−/−,ob/ob mice was made by 2-way ANOVA (effect of Y4 deletion and ob mutation). When the Y4 or Y4*ob interaction effect was significant, Fisher's post-hoc tests were used to locate significant differences between groups. Alterations in neuronal neuropeptide mRNA expression were assessed be ANOVA with Fisher's post-hoc tests. StatView version 4.5 (Abacus Concepts) was used for all statistical analyses, and P < 0.05 was accepted as being statistically significant.
Acknowledgments
We thank Simon Junankar for his expertise in mammary gland preparation and histology, Dr. Julie Ferguson for invaluable veterinary advice, and the staff of the Garvan Institute Biological Testing Facility. We thank Prof. R. Sutherland for critical review of the manuscript. This research was supported by a Garvan Project Grant donated by Mr. Ray Williams, the National Health and Medical Research Council of Australia Centre Block Grant, a Peter Doherty Post-Doctoral Fellowship (987122) to A.S., and a Fellowship from the Auslandsabteilung of the University of Innsbruck to C.S.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL h.herzog@garvan.org.au; FAX 61-2-9295-828.
Article and publication are at http://www.genesdev.org/cgi/doi/10.1101/gad.979102.
References
- Aubert ML, Pierroz DD, Gruaz NM, d'Alleves V, Vuagnat BA, Pralong FP, Blum WF, Sizonenko PC. Metabolic control of sexual function and growth: Role of neuropeptide Y and leptin. Mol Cell Endocrinol. 1998;140:107–113. doi: 10.1016/s0303-7207(98)00058-6. [DOI] [PubMed] [Google Scholar]
- Baldock PA, Sainsbury A, Couzens M, Enriquez RF, Ghomas GP, Gardiner EM, Herzog H. Hypothalamic Y2 receptors regulate bone formation. J Clin Invest. 2002;109:915–921. doi: 10.1172/JCI14588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bard JA, Walker MW, Branchek TA, Weinshank RL. Cloning and functional expression of a human Y4 subtype receptor for pancreatic polypeptide, neuropeptide Y, and peptide YY. J Biol Chem. 1995;270:26762–26765. doi: 10.1074/jbc.270.45.26762. [DOI] [PubMed] [Google Scholar]
- Blomqvist AG, Herzog H. Y-receptor subtypes—How many more? Trends Neurosci. 1997;20:294–298. doi: 10.1016/s0166-2236(96)01057-0. [DOI] [PubMed] [Google Scholar]
- Caprio M, Fabbrini E, Isidori AM, Aversa A, Fabbri A. Leptin in reproduction. Trends Endocrinol Metab. 2001;12:65–72. doi: 10.1016/s1043-2760(00)00352-0. [DOI] [PubMed] [Google Scholar]
- Chehab FF, Lim ME, Lu R. Correction of the sterility defect in homozygous obese female mice by treatment with the human recombinant leptin. Nat Genet. 1996;12:318–320. doi: 10.1038/ng0396-318. [DOI] [PubMed] [Google Scholar]
- Clark JT, Kalra PS, Kalra SP. Neuropeptide Y stimulates feeding but inhibits sexual behavior in rats. Endocrinol. 1985;117:2435–2442. doi: 10.1210/endo-117-6-2435. [DOI] [PubMed] [Google Scholar]
- Erickson JC, Hollopeter G, Palmiter RD. Attenuation of the obesity syndrome of ob/ob mice by the loss of neuropeptide Y. Science. 1996;274:1704–1707. doi: 10.1126/science.274.5293.1704. [DOI] [PubMed] [Google Scholar]
- Ewart-Toland A, Mounzih K, Qiu J, Chehab FF. Effect of the genetic background on the reproduction of leptin-deficient obese mice. Endocrinol. 1999;140:732–738. doi: 10.1210/endo.140.2.6470. [DOI] [PubMed] [Google Scholar]
- Gehlert DR. Role of hypothalamic neuropeptide Y in feeding and obesity. Neuropeptides. 1999;33:329–338. doi: 10.1054/npep.1999.0057. [DOI] [PubMed] [Google Scholar]
- Havel PJ, Akpan JO, Curry DL, Stern JS, Gingerich RL, Ahren B. Autonomic control of pancreatic polypeptide and glucagon secretion during neuroglucopenia and hypoglycemia in mice. Am J Physiol. 1993;265:R246–R254. doi: 10.1152/ajpregu.1993.265.1.R246. [DOI] [PubMed] [Google Scholar]
- Hokfelt T, Broberger C, Zhang X, Diez M, Kopp J, Xu Z, Landry M, Bao L, Schalling M, Koistinaho J, et al. Neuropeptide Y: Some viewpoints on a multifaceted peptide in the normal and diseased nervous system. Brain Res Brain Res Rev. 1998;26:154–166. doi: 10.1016/s0165-0173(97)00052-0. [DOI] [PubMed] [Google Scholar]
- Jain MR, Pu S, Kalra PS, Kalra SP. Evidence that stimulation of two modalities of pituitary luteinizing hormone release in ovarian steroid-primed ovariectomized rats may involve neuropeptide Y Y1 and Y4 receptors. Endocrinol. 1999;140:5171–5177. doi: 10.1210/endo.140.11.7107. [DOI] [PubMed] [Google Scholar]
- Jia BQ, Taylor IL. Failure of pancreatic polypeptide release in congenitally obese mice. Gastroenterology. 1984;87:338–343. [PubMed] [Google Scholar]
- Kalra SP, Dube MG, Pu SY, Xu B, Horvath TL, Kalra PS. Interacting appetite-regulating pathways in the hypothalamic regulation of body weight. Endocrine Rev. 1999;20:68–100. doi: 10.1210/edrv.20.1.0357. [DOI] [PubMed] [Google Scholar]
- Krysiak R, Obuchowicz E, Herman ZS. Interactions between the neuropeptide Y system and the hypothalamic-pituitary-adrenal axis. Eur J Endocrinol. 1999;140:130–136. doi: 10.1530/eje.0.1400130. [DOI] [PubMed] [Google Scholar]
- Larsen PJ, Kristensen P. Central Y4 receptor distribution. Radioactive ribonucleotide probe in situ hybridization with in vitro receptor autoradiography. Methods Mol Biol. 2000;153:185–198. doi: 10.1385/1-59259-042-X:185. [DOI] [PubMed] [Google Scholar]
- Liu X, Robinson GW, Wagner KU, Garrett L, Wynshaw-Boris A, Hennighausen L. Stat5a is mandatory for adult mammary gland development and lactogenesis. Genes & Dev. 1997;11:179–186. doi: 10.1101/gad.11.2.179. [DOI] [PubMed] [Google Scholar]
- Lundell I, Blomqvist AG, Berglund MM, Schober DA, Johnson D, Statnick MA, Gadski RA, Gehlert DR, Larhammar D. Cloning of a human receptor of the NPY receptor family with high affinity for pancreatic polypeptide and peptide YY. J Biol Chem. 1995;270:29123–29128. doi: 10.1074/jbc.270.49.29123. [DOI] [PubMed] [Google Scholar]
- McTigue DM, Hermann GE, Rogers RC. Effect of pancreatic polypeptide on rat dorsal vagal complex neurons. J Physiol. 1997;499:475–483. doi: 10.1113/jphysiol.1997.sp021942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mounzih K, Lu R, Chehab FF. Leptin treatment rescues the sterility of genetically obese ob/ob males. Endocrinol. 1997;138:1190–1193. doi: 10.1210/endo.138.3.5024. [DOI] [PubMed] [Google Scholar]
- Naveilhan P, Neveu I, Arenas E, Ernfors P. Complementary and overlapping expression of Y1, Y2 and Y5 receptors in the developing and adult mouse nervous system. Neuroscience. 1998;87:289–302. doi: 10.1016/s0306-4522(98)00141-9. [DOI] [PubMed] [Google Scholar]
- Ormandy CJ, Camus A, Barra J, Damotte D, Lucas B, Buteau H, Edery M, Brousse N, Babinet C, Binart N, et al. Null mutation of the prolactin receptor gene produces multiple reproductive defects in the mouse. Genes & Dev. 1997;11:167–178. doi: 10.1101/gad.11.2.167. [DOI] [PubMed] [Google Scholar]
- Parker RM, Herzog H. Regional distribution of Y-receptor subtype mRNAs in rat brain. Eur J Neurosci. 1999;11:1431–1448. doi: 10.1046/j.1460-9568.1999.00553.x. [DOI] [PubMed] [Google Scholar]
- Pierroz DD, Catzeflis C, Aebi AC, Rivier JE, Aubert ML. Chronic administration of neuropeptide Y into the lateral ventricle inhibits both the pituitary-testicular axis and growth hormone and insulin-like growth factor I secretion in intact adult male rats. Endocrinol. 1996;137:3–12. doi: 10.1210/endo.137.1.8536627. [DOI] [PubMed] [Google Scholar]
- Raposinho PD, Broqua P, Hayward A, Akinsanya K, Galyean R, Schteingart C, Junien J, Aubert ML. Stimulation of the gonadotropic axis by the neuropeptide Y receptor Y1 antagonist/Y4 agonist 1229U91 in the male rat. Neuroendocrinol. 2000;71:2–7. doi: 10.1159/000054514. [DOI] [PubMed] [Google Scholar]
- Reznikov AG, McCann SM. Effects of neuropeptide Y on gonadotropin and prolactin release in normal, castrated or flutamide-treated male rats. Neuroendocrinol. 1993;57:1148–1154. doi: 10.1159/000126481. [DOI] [PubMed] [Google Scholar]
- Sabatino FD, Collins P, McDonald JK. Investigation of the effects of progesterone on neuropeptide Y-stimulated luteinizing hormone-releasing hormone secretion from the median eminence of ovariectomized and estrogen-treated rats. Neuroendocrinol. 1990;52:600–607. doi: 10.1159/000125651. [DOI] [PubMed] [Google Scholar]
- Sakakura M, Saito Y, Takebe K, Yamashita I, Ishii K. Time course of hypothalamic CRF activity after the administration of two different stresses. Endocrinol Jpn. 1976;23:413–416. doi: 10.1507/endocrj1954.23.413. [DOI] [PubMed] [Google Scholar]
- Schober DA, Van Abbema AM, Smiley DL, Bruns RF, Gehlert DR. The neuropeptide Y Y1 antagonist, 1229U91, a potent agonist for the human pancreatic polypeptide-preferring (NPY Y4) receptor. Peptides. 1998;19:537–542. doi: 10.1016/s0196-9781(97)00455-5. [DOI] [PubMed] [Google Scholar]
- Schwartz TW. Pancreatic polypeptide: A unique model for vagal control of endocrine systems. J Autonom Nerv Syst. 1983;9:99–111. doi: 10.1016/0165-1838(83)90134-0. [DOI] [PubMed] [Google Scholar]
- Schwartz MW, Baskin DG, Bukowski TR, Kuijper JL, Foster D, Lasser G, Prunkard DE, Porte DJ, Woods SC, Seeley RJ, et al. Specificity of leptin action on elevated blood glucose levels and hypothalamic neuropeptide Y gene expression in ob/ob mice. Diabetes. 1996;45:531–535. doi: 10.2337/diab.45.4.531. [DOI] [PubMed] [Google Scholar]
- Schwenk F, Baron U, Rajewsky K. A cre-transgenic mouse strain for the ubiquitous deletion of loxP-flanked gene segments including deletion in germ cells. Nucleic Acids Res. 1995;23:5080–5081. doi: 10.1093/nar/23.24.5080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens TW, Basinski M, Bristow PK, Bue–Valleskey JM, Burgett SG, Craft L, Hale J, Hoffmann J, Hsiung HM, Kriauciunas A, et al. The role of neuropeptide Y in the antiobesity action of the obese gene product. Nature. 1995;377:530–532. doi: 10.1038/377530a0. [DOI] [PubMed] [Google Scholar]
- Tsunashima K, Schwarzer C, Kirchmair E, Sieghart W, Sperk G. GABA(A) receptor subunits in the rat hippocampus III: Altered messenger RNA expression in kainic acid-induced epilepsy. Neuroscience. 1997;80:1019–1032. doi: 10.1016/s0306-4522(97)00144-9. [DOI] [PubMed] [Google Scholar]
- Ueno N, Inui A, Iwamoto M, Kaga T, Asakawa A, Okita M, Fujimiya M, Nakajima Y, Ohmoto Y, Ohnaka M, et al. Decreased food intake and body weight in pancreatic polypeptide-overexpressing mice. Gastroenterology. 1999;117:1427–1432. doi: 10.1016/s0016-5085(99)70293-3. [DOI] [PubMed] [Google Scholar]
- Urban JH, Das I, Levine JE. Steroid modulation of neuropeptide Y-induced luteinizing hormone releasing hormone release from median eminence fragments from male rats. Neuroendocrinol. 1996;63:112–119. doi: 10.1159/000126947. [DOI] [PubMed] [Google Scholar]
- Widdowson PS, Wilding JP. Hypothalamic neuropeptide Y and its neuroendocrine regulation by leptin. Front Horm Res. 2000;26:71–86. doi: 10.1159/000061016. [DOI] [PubMed] [Google Scholar]
- Wynick D, Small CJ, Bacon A, Holmes FE, Norman M, Ormandy CJ, Kilic E, Kerr NC, Ghatei M, Talamantes F, et al. Galanin regulates prolactin release and lactotroph proliferation. Proc Natl Acad Sci. 1998;95:12671–12676. doi: 10.1073/pnas.95.21.12671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu B, Sahu A, Crowley WR, Leranth C, Horvath T, Kalra SP. Role of neuropeptide-Y in episodic luteinizing hormone release in ovariectomized rats: An excitatory component and opioid involvement. Endocrinol. 1993;133:747–754. doi: 10.1210/endo.133.2.8344213. [DOI] [PubMed] [Google Scholar]
- Young WS., 3rd In situ hybridization. Histochemical detection of neuropeptide mRNA using DNA and RNA probes. Methods Enzymol. 1989;168:702–710. doi: 10.1016/0076-6879(89)68051-2. [DOI] [PubMed] [Google Scholar]