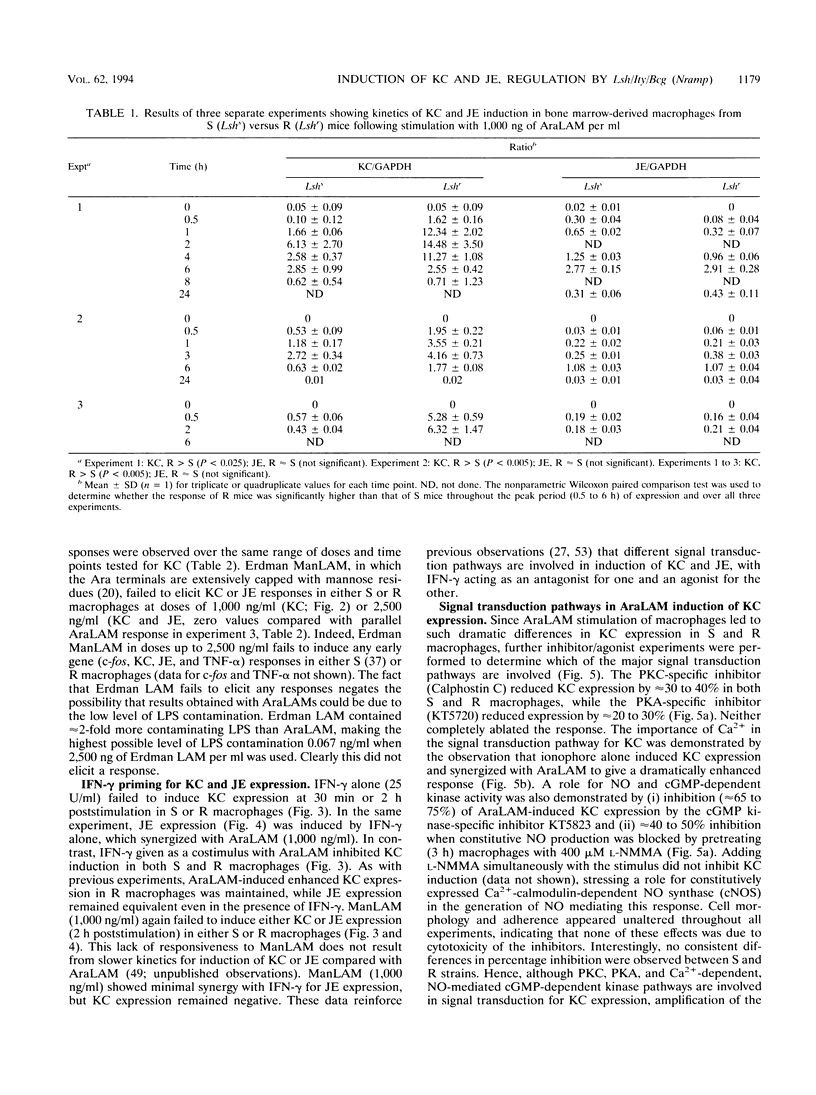
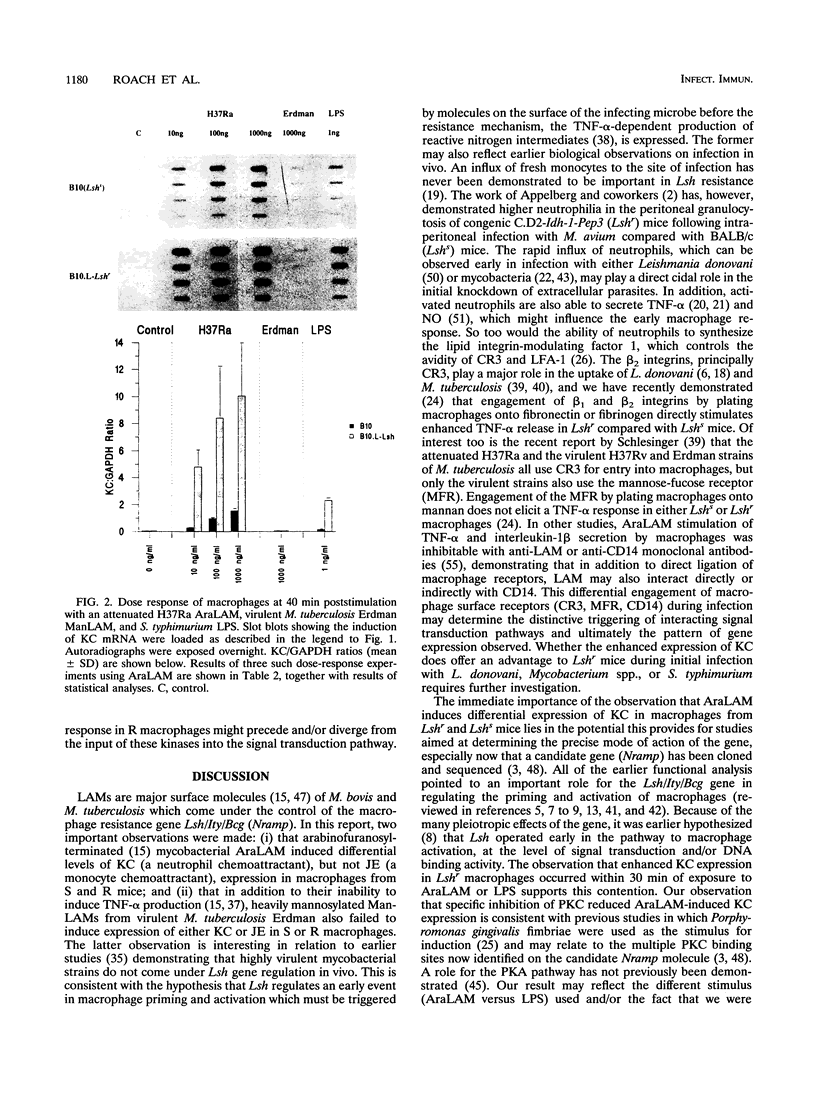
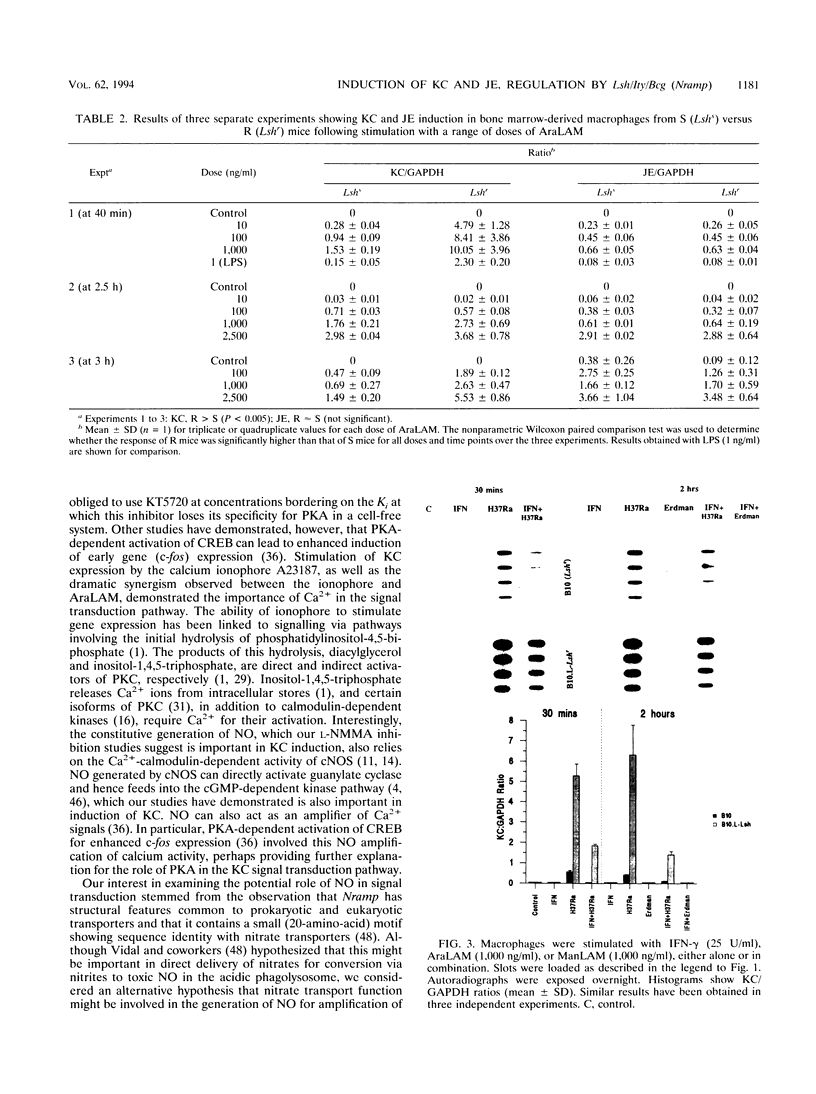
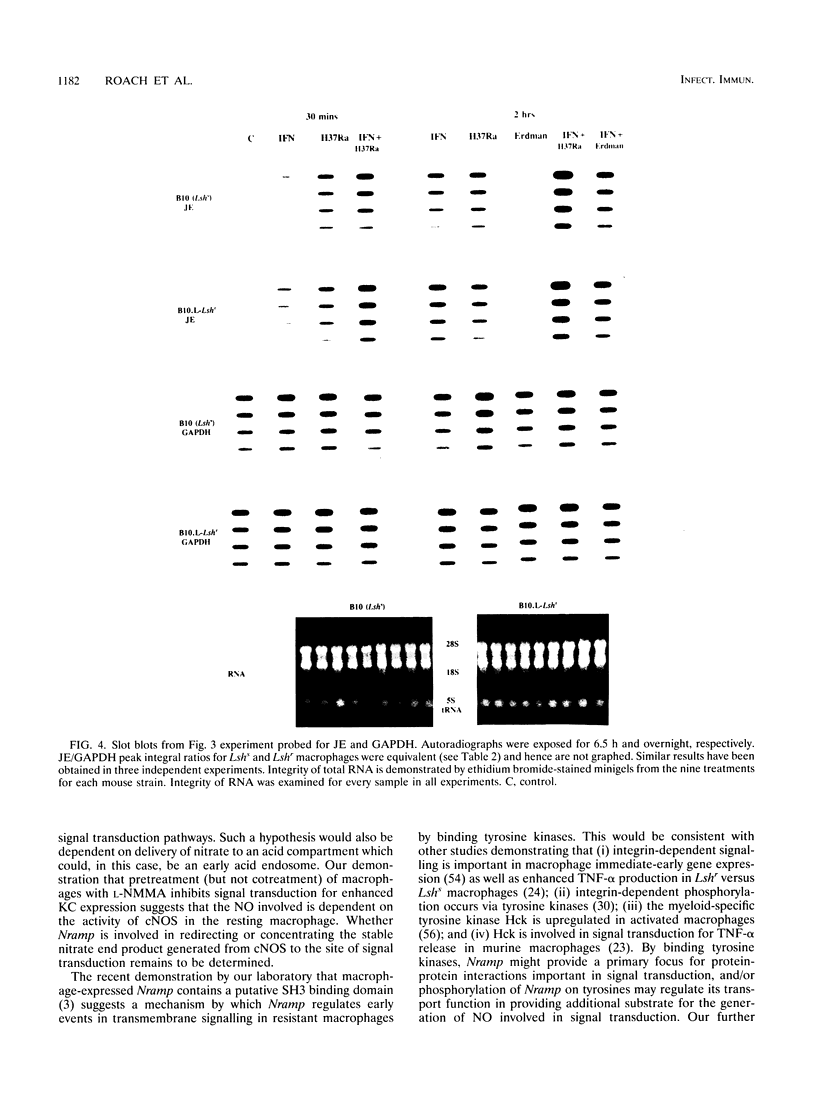
Abstract
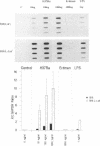
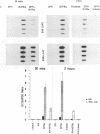
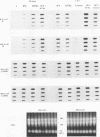
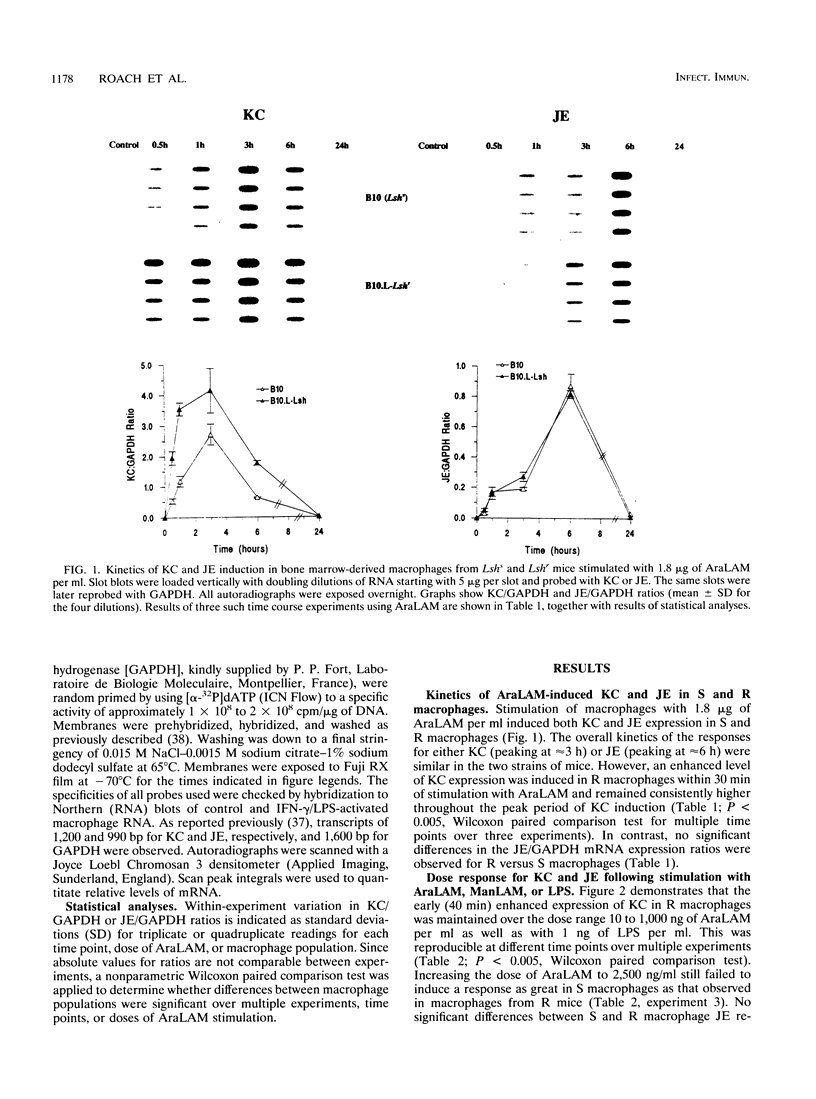
The murine chromosome 1 gene Lsh/Ity/Bcg (candidate Nramp) regulates macrophage activation for antimicrobial activity against Salmonella typhimurium, Leishmania donovani, and Mycobacterium spp. To determine early events in the activation pathway, the ability of mycobacterial lipoarabinomannan (LAM) to induce early gene (KC and JE) expression in macrophages from susceptible (S) C57BL/10ScSn (Lshs) and congenic resistant (R) B10.L-Lshr mice was investigated. Stimulation with 1.8 microgram of arabinofuranosyl-terminated LAM (AraLAM) per ml resulted in similar kinetics for KC or JE expression in S and R macrophages. However, whereas JE/glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA ratios remained equivalent, R macrophages consistently showed enhanced KC/GAPDH ratios within 30 to 40 min of stimulation compared with S macrophages. Significant differences in KC/GAPDH ratios were observed throughout the peak period (0.5 to 6 h) of the KC response and with doses of AraLAM ranging from 0.01 to 2.5 micrograms/ml. Heavily mannosylated LAM from virulent Mycobacterium tuberculosis Erdman, in doses of up to 2.5 micrograms/ml, failed to stimulate KC or JE in S or R macrophages. Gamma interferon alone (25 U/ml) stimulated equivalent JE expression in S and R macrophages and synergized with AraLAM to enhance JE in both. In contrast, AraLAM-induced KC expression was inhibited in the presence of gamma interferon. Agonist/inhibitor studies were undertaken to determine the signal transduction pathways mediating KC expression. The protein kinase C (PKC) inhibitor Calphostin C (200 nM) inhibited AraLAM-induced KC by 34% +/- 4% in S macrophages and 43% +/- 5% in R macrophages; the cyclic AMP-dependent PKA inhibitor KT5720 (2 microM) inhibited AraLAM-induced KC by 33% +/- 4% (S) and 25% +/- 5% (R). A role for Ca2+ was indicated because ionophore alone stimulated KC expression and synergized with AraLAM to give a dramatically enhanced response. Induction of KC was also inhibited by (i) blocking constitutive nitric oxide (NO) production by preincubation of macrophages with NG-monomethyl-L-arginine (400 microM) (48% +/- 8% [S] and 40% +/- 11% [R]) and (ii) incubation of macrophages with the cyclic GMP-dependent kinase inhibitor KT5823 (4 microM) (65% +/- 4% [S] and 72% +/- 6% [R]). The manner in which these PKC-, PKA-, and Ca(2+)-dependent, NO-mediated cyclic GMP-dependent kinase signal transduction pathways may relate to function of the candidate Lsh/Ity/Bcg gene Nramp is discussed.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman J. Ins and outs of cell signalling. Nature. 1988 Jan 14;331(6152):119–120. doi: 10.1038/331119a0. [DOI] [PubMed] [Google Scholar]
- Appelberg R., Pedrosa J. M., Silva M. T. Host and bacterial factors control the Mycobacterium avium-induced chronic peritoneal granulocytosis in mice. Clin Exp Immunol. 1991 Feb;83(2):231–236. doi: 10.1111/j.1365-2249.1991.tb05620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billiar T. R., Curran R. D., Harbrecht B. G., Stadler J., Williams D. L., Ochoa J. B., Di Silvio M., Simmons R. L., Murray S. A. Association between synthesis and release of cGMP and nitric oxide biosynthesis by hepatocytes. Am J Physiol. 1992 Apr;262(4 Pt 1):C1077–C1082. doi: 10.1152/ajpcell.1992.262.4.C1077. [DOI] [PubMed] [Google Scholar]
- Blackwell J. M., Ezekowitz R. A., Roberts M. B., Channon J. Y., Sim R. B., Gordon S. Macrophage complement and lectin-like receptors bind Leishmania in the absence of serum. J Exp Med. 1985 Jul 1;162(1):324–331. doi: 10.1084/jem.162.1.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell J. M., Roach T. I., Atkinson S. E., Ajioka J. W., Barton C. H., Shaw M. A. Genetic regulation of macrophage priming/activation: the Lsh gene story. Immunol Lett. 1991 Oct;30(2):241–248. doi: 10.1016/0165-2478(91)90032-6. [DOI] [PubMed] [Google Scholar]
- Blackwell J. M., Roach T. I., Kiderlen A., Kaye P. M. Role of Lsh in regulating macrophage priming/activation. Res Immunol. 1989 Oct;140(8):798–805. doi: 10.1016/0923-2494(89)90036-9. [DOI] [PubMed] [Google Scholar]
- Blackwell J. M. The macrophage resistance gene Lsh/Ity/Bcg. Res Immunol. 1989 Oct;140(8):767–769. [PubMed] [Google Scholar]
- Blackwell J. M., Toole S., King M., Dawda P., Roach T. I., Cooper A. Analysis of Lsh gene expression in congenic B10.L-Lshr mice. Curr Top Microbiol Immunol. 1988;137:301–309. doi: 10.1007/978-3-642-50059-6_45. [DOI] [PubMed] [Google Scholar]
- Booker G. W., Gout I., Downing A. K., Driscoll P. C., Boyd J., Waterfield M. D., Campbell I. D. Solution structure and ligand-binding site of the SH3 domain of the p85 alpha subunit of phosphatidylinositol 3-kinase. Cell. 1993 May 21;73(4):813–822. doi: 10.1016/0092-8674(93)90259-s. [DOI] [PubMed] [Google Scholar]
- Bredt D. S., Snyder S. H. Isolation of nitric oxide synthetase, a calmodulin-requiring enzyme. Proc Natl Acad Sci U S A. 1990 Jan;87(2):682–685. doi: 10.1073/pnas.87.2.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruns R. F., Miller F. D., Merriman R. L., Howbert J. J., Heath W. F., Kobayashi E., Takahashi I., Tamaoki T., Nakano H. Inhibition of protein kinase C by calphostin C is light-dependent. Biochem Biophys Res Commun. 1991 Apr 15;176(1):288–293. doi: 10.1016/0006-291x(91)90922-t. [DOI] [PubMed] [Google Scholar]
- Buschman E., Taniyama T., Nakamura R., Skamene E. Functional expression of the Bcg gene in macrophages. Res Immunol. 1989 Oct;140(8):793–797. doi: 10.1016/0923-2494(89)90035-7. [DOI] [PubMed] [Google Scholar]
- Busse R., Mülsch A. Calcium-dependent nitric oxide synthesis in endothelial cytosol is mediated by calmodulin. FEBS Lett. 1990 Jun 4;265(1-2):133–136. doi: 10.1016/0014-5793(90)80902-u. [DOI] [PubMed] [Google Scholar]
- Chatterjee D., Roberts A. D., Lowell K., Brennan P. J., Orme I. M. Structural basis of capacity of lipoarabinomannan to induce secretion of tumor necrosis factor. Infect Immun. 1992 Mar;60(3):1249–1253. doi: 10.1128/iai.60.3.1249-1253.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung W. Y. Calmodulin plays a pivotal role in cellular regulation. Science. 1980 Jan 4;207(4426):19–27. doi: 10.1126/science.6243188. [DOI] [PubMed] [Google Scholar]
- Chiu R., Boyle W. J., Meek J., Smeal T., Hunter T., Karin M. The c-Fos protein interacts with c-Jun/AP-1 to stimulate transcription of AP-1 responsive genes. Cell. 1988 Aug 12;54(4):541–552. doi: 10.1016/0092-8674(88)90076-1. [DOI] [PubMed] [Google Scholar]
- Cooper A., Rosen H., Blackwell J. M. Monoclonal antibodies that recognize distinct epitopes of the macrophage type three complement receptor differ in their ability to inhibit binding of Leishmania promastigotes harvested at different phases of their growth cycle. Immunology. 1988 Dec;65(4):511–514. [PMC free article] [PubMed] [Google Scholar]
- Davies E. V., Singleton A. M., Blackwell J. M. Differences in Lsh gene control over systemic Leishmania major and Leishmania donovani or Leishmania mexicana mexicana infections are caused by differential targeting to infiltrating and resident liver macrophage populations. Infect Immun. 1988 May;56(5):1128–1134. doi: 10.1128/iai.56.5.1128-1134.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djeu J. Y., Serbousek D., Blanchard D. K. Release of tumor necrosis factor by human polymorphonuclear leukocytes. Blood. 1990 Oct 1;76(7):1405–1409. [PubMed] [Google Scholar]
- Dubravec D. B., Spriggs D. R., Mannick J. A., Rodrick M. L. Circulating human peripheral blood granulocytes synthesize and secrete tumor necrosis factor alpha. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6758–6761. doi: 10.1073/pnas.87.17.6758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards D., Kirkpatrick C. H. The immunology of mycobacterial diseases. Am Rev Respir Dis. 1986 Nov;134(5):1062–1071. doi: 10.1164/arrd.1986.134.5.1062. [DOI] [PubMed] [Google Scholar]
- English B. K., Ihle J. N., Myracle A., Yi T. Hck tyrosine kinase activity modulates tumor necrosis factor production by murine macrophages. J Exp Med. 1993 Sep 1;178(3):1017–1022. doi: 10.1084/jem.178.3.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanazawa S., Murakami Y., Takeshita A., Kitami H., Ohta K., Amano S., Kitano S. Porphyromonas gingivalis fimbriae induce expression of the neutrophil chemotactic factor KC gene of mouse peritoneal macrophages: role of protein kinase C. Infect Immun. 1992 Apr;60(4):1544–1549. doi: 10.1128/iai.60.4.1544-1549.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermanowski-Vosatka A., Van Strijp J. A., Swiggard W. J., Wright S. D. Integrin modulating factor-1: a lipid that alters the function of leukocyte integrins. Cell. 1992 Jan 24;68(2):341–352. doi: 10.1016/0092-8674(92)90475-r. [DOI] [PubMed] [Google Scholar]
- Introna M., Bast R. C., Jr, Tannenbaum C. S., Hamilton T. A., Adams D. O. The effect of LPS on expression of the early "competence" genes JE and KC in murine peritoneal macrophages. J Immunol. 1987 Jun 1;138(11):3891–3896. [PubMed] [Google Scholar]
- Introna M., Hamilton T. A., Kaufman R. E., Adams D. O., Bast R. C., Jr Treatment of murine peritoneal macrophages with bacterial lipopolysaccharide alters expression of c-fos and c-myc oncogenes. J Immunol. 1986 Oct 15;137(8):2711–2715. [PubMed] [Google Scholar]
- Kishimoto A., Takai Y., Mori T., Kikkawa U., Nishizuka Y. Activation of calcium and phospholipid-dependent protein kinase by diacylglycerol, its possible relation to phosphatidylinositol turnover. J Biol Chem. 1980 Mar 25;255(6):2273–2276. [PubMed] [Google Scholar]
- Lipfert L., Haimovich B., Schaller M. D., Cobb B. S., Parsons J. T., Brugge J. S. Integrin-dependent phosphorylation and activation of the protein tyrosine kinase pp125FAK in platelets. J Cell Biol. 1992 Nov;119(4):905–912. doi: 10.1083/jcb.119.4.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumdar S., Rossi M. W., Fujiki T., Phillips W. A., Disa S., Queen C. F., Johnston R. B., Jr, Rosen O. M., Corkey B. E., Korchak H. M. Protein kinase C isotypes and signaling in neutrophils. Differential substrate specificities of a translocatable calcium- and phospholipid-dependent beta-protein kinase C and a phospholipid-dependent protein kinase which is inhibited by long chain fatty acyl coenzyme A. J Biol Chem. 1991 May 15;266(14):9285–9294. [PubMed] [Google Scholar]
- Mayer B. J., Baltimore D. Signalling through SH2 and SH3 domains. Trends Cell Biol. 1993 Jan;3(1):8–13. doi: 10.1016/0962-8924(93)90194-6. [DOI] [PubMed] [Google Scholar]
- Miller M. D., Krangel M. S. Biology and biochemistry of the chemokines: a family of chemotactic and inflammatory cytokines. Crit Rev Immunol. 1992;12(1-2):17–46. [PubMed] [Google Scholar]
- Oppenheim J. J., Zachariae C. O., Mukaida N., Matsushima K. Properties of the novel proinflammatory supergene "intercrine" cytokine family. Annu Rev Immunol. 1991;9:617–648. doi: 10.1146/annurev.iy.09.040191.003153. [DOI] [PubMed] [Google Scholar]
- Peunova N., Enikolopov G. Amplification of calcium-induced gene transcription by nitric oxide in neuronal cells. Nature. 1993 Jul 29;364(6436):450–453. doi: 10.1038/364450a0. [DOI] [PubMed] [Google Scholar]
- Roach T. I., Barton C. H., Chatterjee D., Blackwell J. M. Macrophage activation: lipoarabinomannan from avirulent and virulent strains of Mycobacterium tuberculosis differentially induces the early genes c-fos, KC, JE, and tumor necrosis factor-alpha. J Immunol. 1993 Mar 1;150(5):1886–1896. [PubMed] [Google Scholar]
- Roach T. I., Kiderlen A. F., Blackwell J. M. Role of inorganic nitrogen oxides and tumor necrosis factor alpha in killing Leishmania donovani amastigotes in gamma interferon-lipopolysaccharide-activated macrophages from Lshs and Lshr congenic mouse strains. Infect Immun. 1991 Nov;59(11):3935–3944. doi: 10.1128/iai.59.11.3935-3944.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger L. S., Bellinger-Kawahara C. G., Payne N. R., Horwitz M. A. Phagocytosis of Mycobacterium tuberculosis is mediated by human monocyte complement receptors and complement component C3. J Immunol. 1990 Apr 1;144(7):2771–2780. [PubMed] [Google Scholar]
- Schlesinger L. S. Macrophage phagocytosis of virulent but not attenuated strains of Mycobacterium tuberculosis is mediated by mannose receptors in addition to complement receptors. J Immunol. 1993 Apr 1;150(7):2920–2930. [PubMed] [Google Scholar]
- Schurr E., Malo D., Radzioch D., Buschman E., Morgan K., Gros P., Skamene E. Genetic control of innate resistance to mycobacterial infections. Immunol Today. 1991 Mar;12(3):A42–A45. doi: 10.1016/S0167-5699(05)80012-X. [DOI] [PubMed] [Google Scholar]
- Schurr E., Radzioch D., Malo D., Gros P., Skamene E. Molecular genetics of inherited susceptibility to intracellular parasites. Behring Inst Mitt. 1991 Feb;(88):1–12. [PubMed] [Google Scholar]
- Silva M. T., Silva M. N., Appelberg R. Neutrophil-macrophage cooperation in the host defence against mycobacterial infections. Microb Pathog. 1989 May;6(5):369–380. doi: 10.1016/0882-4010(89)90079-x. [DOI] [PubMed] [Google Scholar]
- Stoeckle M. Y., Barker K. A. Two burgeoning families of platelet factor 4-related proteins: mediators of the inflammatory response. New Biol. 1990 Apr;2(4):313–323. [PubMed] [Google Scholar]
- Tannenbaum C. S., Hamilton T. A. Lipopolysaccharide-induced gene expression in murine peritoneal macrophages is selectively suppressed by agents that elevate intracellular cAMP. J Immunol. 1989 Feb 15;142(4):1274–1280. [PubMed] [Google Scholar]
- Tsou K., Snyder G. L., Greengard P. Nitric oxide/cGMP pathway stimulates phosphorylation of DARPP-32, a dopamine- and cAMP-regulated phosphoprotein, in the substantia nigra. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3462–3465. doi: 10.1073/pnas.90.8.3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venisse A., Berjeaud J. M., Chaurand P., Gilleron M., Puzo G. Structural features of lipoarabinomannan from Mycobacterium bovis BCG. Determination of molecular mass by laser desorption mass spectrometry. J Biol Chem. 1993 Jun 15;268(17):12401–12411. [PubMed] [Google Scholar]
- Vidal S. M., Malo D., Vogan K., Skamene E., Gros P. Natural resistance to infection with intracellular parasites: isolation of a candidate for Bcg. Cell. 1993 May 7;73(3):469–485. doi: 10.1016/0092-8674(93)90135-d. [DOI] [PubMed] [Google Scholar]
- Watanabe K., Konishi K., Fujioka M., Kinoshita S., Nakagawa H. The neutrophil chemoattractant produced by the rat kidney epithelioid cell line NRK-52E is a protein related to the KC/gro protein. J Biol Chem. 1989 Nov 25;264(33):19559–19563. [PubMed] [Google Scholar]
- Wilson M. E., Innes D. J., Sousa A. D., Pearson R. D. Early histopathology of experimental infection with Leishmania donovani in hamsters. J Parasitol. 1987 Feb;73(1):55–63. [PubMed] [Google Scholar]
- Wright C. D., Mülsch A., Busse R., Osswald H. Generation of nitric oxide by human neutrophils. Biochem Biophys Res Commun. 1989 Apr 28;160(2):813–819. doi: 10.1016/0006-291x(89)92506-0. [DOI] [PubMed] [Google Scholar]
- Yoshimura T., Yuhki N., Moore S. K., Appella E., Lerman M. I., Leonard E. J. Human monocyte chemoattractant protein-1 (MCP-1). Full-length cDNA cloning, expression in mitogen-stimulated blood mononuclear leukocytes, and sequence similarity to mouse competence gene JE. FEBS Lett. 1989 Feb 27;244(2):487–493. doi: 10.1016/0014-5793(89)80590-3. [DOI] [PubMed] [Google Scholar]
- Yu S. F., Koerner T. J., Adams D. O. Gene regulation in macrophage activation: differential regulation of genes encoding for tumor necrosis factor, interleukin-1, JE, and KC by interferon-gamma and lipopolysaccharide. J Leukoc Biol. 1990 Nov;48(5):412–419. doi: 10.1002/jlb.48.5.412. [DOI] [PubMed] [Google Scholar]
- Yurochko A. D., Liu D. Y., Eierman D., Haskill S. Integrins as a primary signal transduction molecule regulating monocyte immediate-early gene induction. Proc Natl Acad Sci U S A. 1992 Oct 1;89(19):9034–9038. doi: 10.1073/pnas.89.19.9034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Doerfler M., Lee T. C., Guillemin B., Rom W. N. Mechanisms of stimulation of interleukin-1 beta and tumor necrosis factor-alpha by Mycobacterium tuberculosis components. J Clin Invest. 1993 May;91(5):2076–2083. doi: 10.1172/JCI116430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler S. F., Wilson C. B., Perlmutter R. M. Augmented expression of a myeloid-specific protein tyrosine kinase gene (hck) after macrophage activation. J Exp Med. 1988 Nov 1;168(5):1801–1810. doi: 10.1084/jem.168.5.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]