Abstract
Hox genes specify the different morphologies of segments along the anteroposterior axis of animals. How they control complex segment morphologies is not well understood. We have studied how the Hox gene Ultrabithorax (Ubx) controls specific differences between the bristle patterns of the second and third thoracic segments (T2 and T3) of Drosophila melanogaster. We find that Ubx blocks the development of two particular bristles on T3 at different points in sensory organ development. For the apical bristle, a precursor is singled out and undergoes a first division in both the second and third legs, but in the third leg further differentiation of the second-order precursors is blocked. For the posterior sternopleural bristle, development on T3 ceases after proneural cluster initiation. Analysis of the temporal requirement for Ubx shows that in both cases Ubx function is required shortly before bristle development is blocked. We suggest that interactions between Ubx and the bristle patterning hierarchy have evolved independently on many occasions, affecting different molecular steps. The effects of Ubx on bristle development are highly dependent on the context of other patterning information. Suppression of bristle development or changes in bristle morphology in response to endogenous and ectopic Ubx expression are limited to bristles at specific locations.
Keywords: Drosophila, Hox, Ubx, sensory organs, bristle, context
Hox genes play a major role in generating the diversity of segment morphology along the anteroposterior axis in arthropods. Hox gene mutations can result in complete transformations of one segment into the likeness of another, affecting a multitude of characters which differ between segments. For instance, loss of Ultrabithorax (Ubx) function in the imaginal tissues of the fly Drosophila melanogaster causes a transformation of the third thoracic segment (T3) to resemble the second (T2): the haltere is transformed to wing, while the shape, size and bristle pattern of the T3 leg and body wall all resemble those of T2 (Lewis 1963). The complexity of this transformation requires us to explain how, in normal development, one transcription factor—the Ubx protein—can instruct all the unique characteristics of the third thoracic segment, affecting many distinct developmental processes.
In the insect thorax and abdomen, the developmental instructions for establishing segments are initially independent of the instructions that specify the differences between segments (Akam 1987). The expression of segment polarity and dorsoventral patterning genes in Drosophila does not require Hox gene function, and is similar in each trunk segment (Chasan and Anderson 1993; Martinez Arias 1993). It constitutes a common segmental ground plan that, in the absence of all Hox gene function, generates a default arthropod segment, with cuticular plates, jointed appendages, etc. (Lewis 1978; Stuart et al. 1991).
In only a few cases do we know how the Hox genes modify this default developmental program. One such case is the control of ventral appendage development in the abdomen. In this case, early expression of either the Ubx or the abd-A Hox gene in cells of the presumptive appendage primordium represses distalless expression, and so blocks formation of the appendage which is a part of the default program (Vachon et al. 1992). The modification of appendage development under homeotic gene control has also been studied in some detail. Hox genes act at multiple points in the genetic network that controls the differential developmental trajectories that result in leg versus antennal development (Wagner-Bernholz et al. 1991; Casares and Mann 1998), and haltere versus wing development (Weatherbee et al. 1998; Roch and Akam 2000). They collaborate with genes that function in all appendages to instruct appendage-specific development (Gorfinkiel et al. 1997).
In the present study we examined how the Hox gene Ubx modulates the bristle pattern in the legs and lateral body wall of D. melanogaster. Bristles are chemosensory and mechanosensory organs of the peripheral nervous system. They are abundant on all fly segments but differentially distributed between segments. Among the mechanosensory bristles there is a clear morphological distinction between large bristles (macrochaetes) and small bristles (microchaetes). Ubx is expressed in both the second and third leg imaginal discs. In both discs, expression levels are modulated throughout development but levels are consistently much higher in the third leg disc than in the second (Brower 1987).
The legs retain the same basic structure in each thoracic segment, with an identical number of leg segments and joints. This allows precisely corresponding parts of different legs to be identified without difficulty. Many characteristics of the bristle pattern are also directly comparable between the legs—notably the morphologically distinct macrochaetes that occur at specific positions with respect to these other landmarks. Thus they are assumed to be homologous elements of the pattern on different legs, and are given generic names (“tibial apical bristle” etc.). Individual macrochaetes may be present on all legs in one species, but only on one or two leg pairs in another species (Wheeler 1981).
The three leg discs develop in parallel as imaginal discs within the body of the larva. These show coordinate changes in morphology and gene expression patterns (Auerbach 1936; Cohen 1993; Goto and Hayashi 1997). Patterning of the imaginal discs ultimately provides instructions for the initiation of bristle development through the formation of proneural clusters—groups of cells characterized by the expression of two genes of the achaete–scute complex (AS-C): achaete (ac) and scute (sc). The patterning information that defines the position of proneural clusters comprises a diverse array of positive and negative regulators which act through site-specific enhancer elements distributed along the AS-C (Gomez-Skarmeta et al. 1995). All the cells in a proneural cluster are competent to give rise to sensory organs (Heitzler and Simpson 1991). Lateral inhibition among these cells prevents most cells from acquiring a definitive sensory organ fate. The singling out of an individual precursor from the proneural cluster is gated by a general enhancer within the AS-C complex which is distinct from the specific enhancers that promote proneural cluster formation (Culi and Modolell 1998). Once singled out, the sensory organ precursors undergo a series of stereotyped divisions. In the case of mechanosensory organs, the first division generates two second-order precursors, one of which will give rise to the external cells of the bristle, the shaft, and the socket, whereas the other will give rise to a neuron, a glia cell, and a neuron sheath cell (Gho et al. 1999).
The parallel development of the three pairs of legs allows precise definition of the points at which Ubx controls the development of leg bristle patterns. As steps of sensory organ development are discrete and stereotyped we can ask, for each bristle affected, where in the program Ubx modifies the default pathway. In this way we can assess whether there are general rules for how Ubx interacts with bristle development. Knowledge of the mechanisms underlying such interaction will allow us to envisage how morphology may evolve between segments of a species—in the case of this study for example, how bristle patterns may diversify between segments.
We have concentrated on three large mechanosensory bristles that differ between the second and third thoracic segment—the posterior macrochaete on the sternopleurum, and the apical and preapical bristles on the tibia. In D. melanogaster, the sternopleural bristles and the apical bristle on the tibia are found only on T2. The preapical bristle on the tibia is found on both second and third legs, but it has a different morphology on these two legs. In flies mutant for adult function of Ubx (e.g., bx3/Ubx1), both second and third thoracic segments display the wild-type pattern of the T2 segment for these bristle traits. For each of these bristles, we examined when the differences between the T2 and T3 segments are laid down, and when Ubx functions in relation to the program of sensory organ development.
Results
The timing of leg bristle development
In wild-type flies, apical and sternopleural bristles appear only on the second thoracic segment, but in Ubx mutant flies, they also develop on the third thoracic segment. Thus, directly or indirectly, Ubx normally blocks the development of these bristles on the third leg. To determine when the block of development occurs for the apical and the posterior sternopleural macrochaete, we monitored early stages of sensory organ development. We started by looking for segregated sensory organ precursors.
The precursors for different types of sensory organs can be distinguished by the combination of markers they express. A reporter construct in the neuralized gene, neu-lacZ, is expressed in all sensory organ precursors. The homeodomain protein Cut is expressed only in the precursors for external sensory organs, and thus allows us to distinguish between external and internal sensory organs (e.g., the chordotonal organs). Among the external sensory organs, chemosensory bristle precursors can be distinguished from mechanosensory precursors by their expression of the paired box protein Pox–neuro. In the folded disc epithelium, the individual mechanosensory precursors can be identified by their location in relation to markers of joint territory and other landmarks in the discs. When the legs elongate, the identity of the sensory organ precursors can be assessed by comparing the position of labeled cells with that of sensory organs in the adult leg segments. In the leg disc, the first bristle precursors develop during the third larval instar. These early segregating precursors include precursors for the tarsal chemosensory bristles, and for a few of the largest mechanosensory bristles in the proximal leg, including the precursor for the posterior sternopleural macrochaete. Precursors for the apical and preapical bristles appear at about the time of puparium formation, and can be detected reliably in the white prepupa (Nottebohm et al. 1994). At this time chemosensory precursors also appear in the tibia. Precursors for the smaller mechanosensory bristles are not formed until 8–12 h after puparium formation (Nottebohm et al. 1994).
Using sensory organ markers, we found that sensory organ development is blocked at different points for the apical and for the posterior sternopleural bristles. In white prepupae, no precursor cell appears in the third leg at the position corresponding to that of the sternopleural precursor in the second leg (Fig. 1F,G). However, apical bristle precursors are present on both the second and third legs (not shown, see below). We conclude that Ubx blocks some event leading to the specification of the posterior sternopleural bristle precursor, but that Ubx does not inhibit formation of the apical bristle precursor on T3.
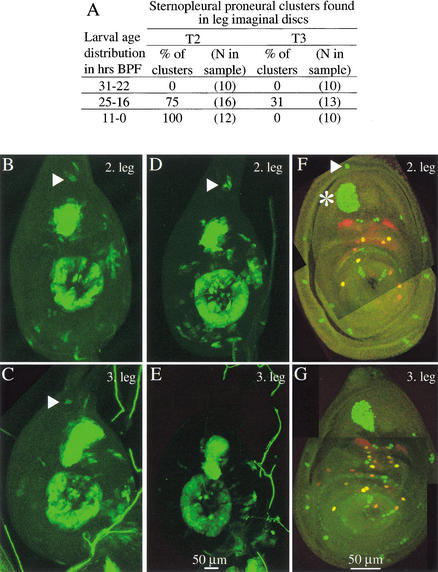
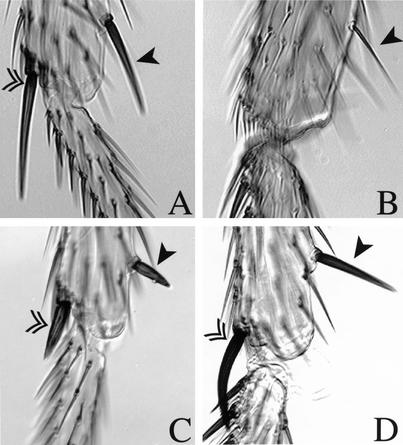
Figure 1.
Development of the posterior sternopleural bristle in the third leg ceases after proneural cluster initiation—no precursor segregation takes place. (A) Summary table. Sternopleural proneural clusters were identified as cells staining for GFP expressed with the sca-Gal4 line. (B–E) Leg imaginal discs of two individuals from the time interval 25–16 h BPF during which the cluster (triangle) emerges in T2 and T3 and also disappears in T3. One individual shows a few GFP positive cells in the dorsal outer ring in the second (B) and the third leg (C). The other individual shows several GFP positive cells in the dorsal outer ring only in the second leg (D), not in the third leg (E). (F,G) Sensory organ precursors at the white prepupa stage. A sternopleural bristle precursor is found in the second leg (triangle) but not the third leg. Discs of the neuralized-lacZ line were stained with anti-β-galactosidase (green) and with anti-Pox-neuro (red). In the second leg disc (F), the sternopleural bristle precursor (triangle) is located in the outer ring of the disc dorsal to the large femoral chordotonal organ (asterisk).
Sternopleural bristle development on T3 is blocked during proneural cluster maturation
No segregated precursor appears for the sternopleural bristle on T3. To find out whether any earlier stage of sensory organ development is reached, we observed the course of proneural cluster development during the third larval instar. We found that a sternopleural proneural cluster forms transiently in the third leg disc, but then regresses (Fig. 1A–E). We have used a scabrous-Gal4 line driving GFP expression to observe the formation of this cluster. scabrous (sca) is a downstream target of achaete and scute (Singson et al. 1994). We recorded sternopleural proneural cluster development in three different time intervals (Fig. 1A). On the second leg, this proneural cluster first emerges between 25 and 16 h before puparium formation (BPF), probably around 20 h BPF. On the third leg, the cluster appears in the same time window as on the second leg (Fig. 1B–E). However, the percentage of clusters observed on the third leg is lower than on the second leg (31% vs. 75%), probably because the cluster exists for only a short time. Indeed, in the time interval from 11–0 h BPF, it is no longer found on the third leg.
In conclusion, sensory organ development for the sternopleural bristle is initiated on T3 but halted at the stage of proneural cluster development, between 25 and 16 h BPF.
Ubx is required at the time of proneural cluster initiation to block development of sternopleural macrochaetes on T3
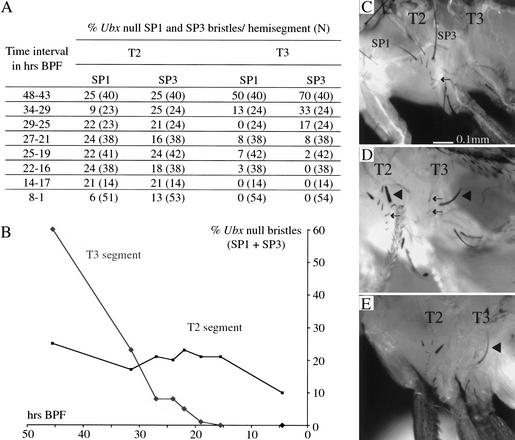
To determine when the Ubx gene is required to suppress development of the sternopleural bristles in the third leg, we induced Ubx null clones (Ubx1) at different times during development. In a first series of experiments, we heatshocked larvae to induce expression of FLP recombinase, thereby controlling the timing of clone induction. These animals were collected as newly formed pupae, and scored for sternopleural bristle clones (marked by bristle morphology) in the adult (Fig. 2). On T2 formation of the sternopleural bristle is not affected by the presence of the Ubx gene, which is expressed only at low levels. Thus, scoring bristle clones on T2 provides a control for the success of clone induction over the entire time course. We find that Ubx null clones induced by heatshock up to 22–16 h BPF can give rise to sternopleural macrochaetes on T3.
Figure 2.
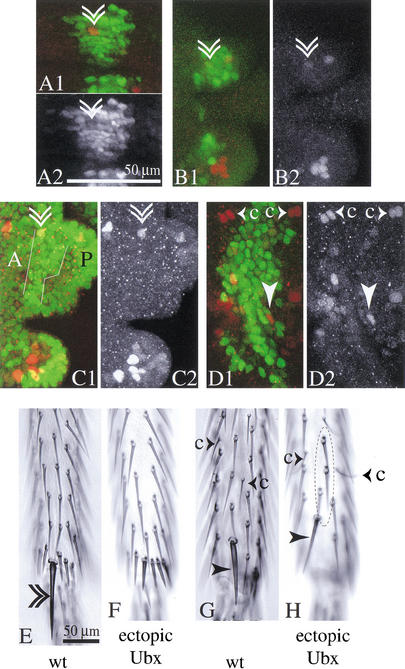
Formation of anterior and posterior sternopleural macrochaetes (SP1 and SP3) in T3 following timed induction of Ubx null clones and Ubx null clone induction targeted to the sternopleural proneural cluster. (A) Summary table. (B) Graph showing the frequency of T2 and T3 anterior and posterior sternopleural bristles as a function of the time of Ubx null clone induction. (C) Lateral thorax of a wild-type fly. Sternopleural bristles are found exclusively on T2 (SP1 anterior and SP3 posterior sternopleural macrochaete; the arrow points to the row of microchaetes). (D) Fly with Sb+-marked Ubx null clones induced with a heatshock 28 h BPF. Ubx null clones induced at this time give rise to sternopleural macrochaetes (triangle) and microchaetes (arrows) on T3; corresponding bristles on T2 are marked in the same way. (E) Fly with Sb+-marked Ubx null clones induced with the scabrous-Gal4 line. sca-Gal4 is transiently expressed in the T3 sternopleural proneural cluster when the cluster is first formed on T2 and T3. Ubx null clones generated at this time give rise to sternopleural macrochaetes on T3 (triangle in E).
The latest time when the generation of Ubx null clones results in the development of sternopleural macrochaetes on T3 coincides with the time when we see transient formation of a proneural cluster on T3. To test whether the cells of this cluster can in fact give rise to bristles, we induced Ubx null clones by using the sca-Gal4 driver to activate recombinase. Such clones should only be generated after the first division of a cell within the sternopleural proneural cluster. Sternopleural macrochaetes develop from these clones on T3 (Fig. 2E). Therefore, the neural potential of the proneural cluster cells on T3 is intact, despite expressing Ubx up to the point of clone induction. This shows that Ubx function is required after formation of the sternopleural proneural cluster to block macrochaete development on T3.
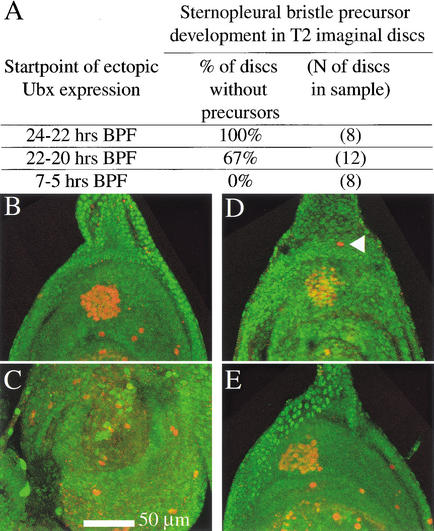
These results suggested that the formation of precursors for sternopleural macrochaetes on T2 would be blocked by ectopic expression of Ubx protein during formation of the proneural cluster. To test this, we expressed Ubx protein under the control of a heatshock promoter (Fig. 3). We found that sternopleural precursor development on the T2 leg can be suppressed when Ubx is induced in developing second leg discs by heatshocks administered from 23±1h BPF, and also from 21±1 h BPF but at lower efficiency. Heatshocks from 6±1 h BPF do not inhibit sternopleural precursor development on T2. Thus, Ubx can only suppress sternopleural macrochaete development until shortly after proneural cluster formation.
Figure 3.
Ectopic Ubx expression from 24–22 h BPF can suppress sternopleural bristle precursor development on T2, but ectopic expression from shortly before puparium formation cannot. Larvae were subjected to heatshock regimes covering different spans of third larval instar development. Larvae were examined for sensory organ precursors at a stage corresponding to the white prepupa. Precursors were visualized by staining with anti-β-galactosidase in the neuralized-lacZ background (red). Ubx levels were assayed by costaining for Ubx protein (green). (A) Summary table. (B–C) Individual for which the heatshock-Ubx regime started between 24 and 22 h BPF. (B) Second leg disc of this individual. No sternopleural bristle precursor is found in the dorsal leg periphery. (C) Third leg disc of this individual. (D–E) Individual for which the hs-Ubx regime started 7 h BPF. (D) Second leg discs of this animal. The sternopleural bristle precursor is present in the dorsal leg periphery (arrowhead). (E) Third leg of this animal. (C,E) In both treatments, ectopic Ubx levels at the time of collection have not sunk below endogenous third leg levels.
Apical bristle development on T3 is blocked after the first division of the bristle mother cell
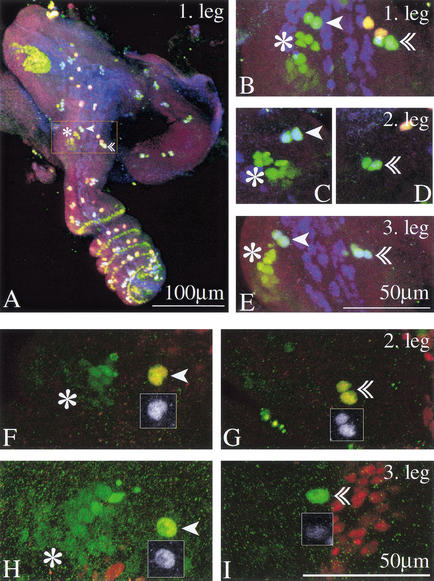
We have seen above that sternopleural macrochaete development on T3 is blocked during proneural cluster development. In contrast, a precursor for the apical bristle is formed on the third leg, while the wild-type condition for this leg is absence of an apical bristle. We monitored the further development of this precursor by analyzing the next stages in bristle development. By 4 h after puparium formation (APF), the precursors for the apical bristle have undergone a first division, giving rise to second-order precursors in all three legs (Fig. 4A–E). On the third leg, the further development of these second-order precursors is then disrupted. Sometimes a three-cell cluster can be detected, but by 5.5 h APF, expression of neural markers is fading (Fig. 4F–I). Staining with anti-Cut antibody is lost first, while detectable neuralized (neu)-lacZ expression persists. To assess whether the cells survive the loss of neural markers, we drove GFP expression in the precursors, using a Gal4 line inserted in the scabrous locus, 109-68 Gal4. This line drives expression in the sensory organ lineage. GFP-expressing cells can be observed for a period of time after they lose neu-lacZ expression (data not shown). We conclude that the loss of neural markers is not accomplished by cell death.
Figure 4.
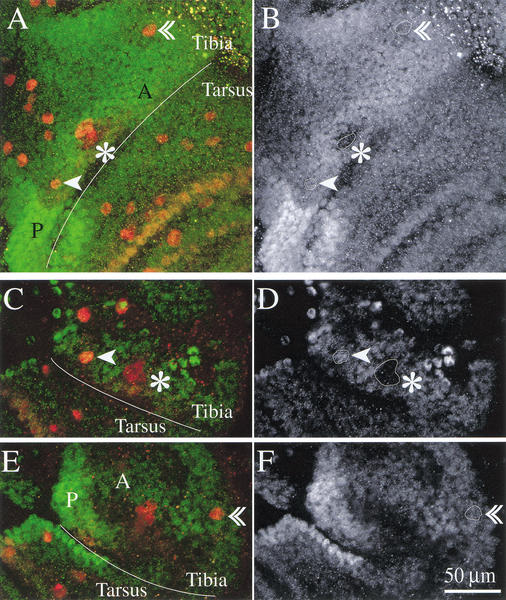
Second-order precursors for both the apical and the preapical bristle form on all three legs. The apical bristle precursors on T3 lose their neural markers but survive. (A–E) Leg imaginal discs of the neu-lacZ line 4–4.5 h APF stained with anti-β-galactosidase (green), with anti-Pox-neuro (red) and with anti-Cut (blue). Mechanosensory bristles appear blue-green. (A) First leg. The inset frames the distal tibia and is magnified in B. (B–E) Close-up of the distal tibia in the three legs. Second-order precursors for the preapical (arrowhead) and the apical bristle (double arrowhead) are found in all three legs. Preapical bristle precursors are in close proximity to the tibial chordotonal organ precursors (asterisk). (F–I) Leg discs from a white prepupa of the neu-lacZ line, 4.5 h APF stained with anti-β-galactosidase (green) and anti-Cut (red). Sensory organs are marked as above. Insets show Cut expression of the precursors. (F) Preapical and (G) apical bristle precursors of the second leg; (H) preapical and (I) apical bristle precursors of the third leg. Cut expression has faded from the apical bristle precursors in the third leg, while anti-β-galactosidase expression still marks the cells (I).
Ubx is required at several stages, prior to and in the sensory organ lineage, to suppress apical bristle development on T3
Apical bristle development is disrupted at the second-order precursor stage. Is it also at this stage that Ubx effects the suppression of further bristle development? To address this question, we generated Ubx null second-order precursors. We targeted the induction of Ubx null clones specifically to the sensory organ lineage, by using the 109-68 Gal4 line. In the development of most sensory organs, activity of the 109-68 Gal4 line is detectable in the first-order precursors but not earlier. We have confirmed this for the apical bristle (see Materials and Methods). Therefore, when this line is used to generate recombination, mitotic clones should first be produced at the division of first-order precursors. We found that apical bristle precursors which are deficient for Ubx from the second-order precursor stage give rise to bristle shaft and socket on the third leg (Fig. 5). We confirmed this late requirement for Ubx by using a timed heatshock to provide Flipase for mitotic recombination. We find that Flipase expression 0–6 h BPF can lead to the development of apical bristles on T3 (T3, 10% presence of apical bristles, n = 29; T2, 17% Sb+ bristles, n = 30). Sensory mother cells are selected from mitotically quiescent clusters of cells arrested in G2 (Usui and Kimura 1992). As the first-order precursor of the apical bristle is generated around the time of puparium formation, a heatshock shortly before puparium formation will provide recombinase in the quiescent proneural cluster cells from which the precursor is selected so that a Ubx null clone will be generated in the second-order precursors. These results show that Ubx is required in the second-order precursors to stop further neural development.
Figure 5.
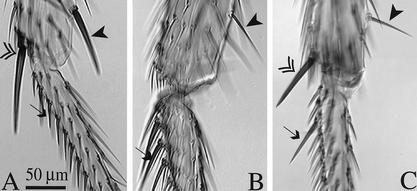
Removal of Ubx from the second-order precursors results in shaft and socket development of the apical bristle on T3 and converts preapical bristle morphology on T3 towards T2. (A–D) Double arrowhead marks the apical bristle, arrowhead marks the preapical bristle. (A) Wild-type second leg and (B) wild-type third leg. (C) Second leg of experimental animal expressing the Sb mutation in apical and preapical bristle. (D) Third leg in which mitotic recombination driven in the sensory organ lineage resulted in Sb+ macrochaetes.
However, Ubx expression in the precursor lineage alone is not sufficient to instruct loss of neural identity. We used the 109-68 Gal4 line to induce ectopic expression of Ubx (protein isoform Ubx Ia) from the time of formation of the first-order precursor onwards. In this ectopic expression regime, no loss of the apical bristle on T2 occurred, despite levels of Ubx protein in the apical bristle precursors comparable with those seen in T3 (data not shown). Even much higher levels of Ubx expression (from a line expressing isoform IVa) do not block apical bristle development at the normal time (data not shown).
Yet, prolonged expression of Ubx in T2 can suppress the development of the apical bristle in a manner that mimics normal development on T3. We used a decapentaplegic (dpp)-Gal4 line to drive ectopic Ubx expression with the same UAS-Ubx line expressing isoform Ia. This dpp-Gal4 line drives Ubx expression in the domain which will give rise to the apical bristle precursor during much of imaginal disc development. In this experiment, the apical bristle is lost from T2 (45 times in 50 cases). In comparison, the preapical bristle is rarely suppressed (10 times in 50 cases) (Fig. 6E–H). Examination of second leg imaginal discs from these flies shows that in the majority of cases, precursors for the apical bristle are found initially within the Ubx-expressing domain (Fig. 6A). At the time when apical bristle precursors normally cease to express neural markers in the third leg, 5–6 h APF, the second leg precursors also show reduced levels of neural markers (anti-β-galactosidase staining in the neu-lacZ line) (Fig. 6B,C). Thus, this ectopic expression regime recreates in T2 the course of apical bristle development normally seen in T3.
Figure 6.
In a regime of prolonged ectopic Ubx expression, neural markers disappear from the apical bristle precursors on T2. The adult flies show selective loss of the apical bristle. (A–D) Confocal images of the apical bristle precursors in prepupal legs with ectopic Ubx expression (Ubx isoform Ia) driven by dpp-Gal4 in the neu-lacZ background. Precursors are marked by anti-β-galactosidase staining (red), and Ubx expression is shown (green). Double arrowhead marks the apical bristle precursors, arrowhead marks the preapical bristle precursors. (A) Second leg about 3 h APF. Second-order precursors for the apical bristle are present within the domain of ectopic Ubx expression. (A2) shows the Ubx channel. (B) Second leg 5–6 h APF. Very low levels of anti-β-galactosidase staining remain in the apical bristle precursors (see red channel in B2). Anti-β-galactosidase staining is also reduced in the apical bristle precursors of the third leg of this individual (C). The stripe of ectopic Ubx expression in the third leg (white outlines in C1) compares to Ubx levels in the posterior third leg (marked P), exceeding levels in the anterior third leg (marked A). (D) In the dorsal second leg, the stripe of ectopic Ubx expression occupies most of the width between the chemosensory precursors (small arrowheads accompanied by the letter c). (E) Ventral aspect of the distal tibia of an adult wild-type fly and (F) of a fly from the above regime of ectopic Ubx expression—the apical bristle is missing from the ventral side of the tibia. From the dorsal domain of ectopic Ubx expression (D) the preapical bristle (arrowhead in H) and mechanosensory microchaetes on the dorsal leg (circle in H) arise normally. (G) Dorsal aspect of the distal tibia of a wild-type fly.
Differences in Ubx levels are not the basis for the differential response of apical and preapical bristle precursors
The experiments above show that a precursor cell for the apical bristle is specified in the developing third leg, but the daughters of this cell lose neural markers at about 5.5 h APF. The precursors for another mechanosensory bristle, the preapical bristle, develop in the same compartment of the same leg segment at a similar time, but do not cease expressing neural markers in response to Ubx: A preapical bristle appears on all three legs of the adult. How does Ubx repress the development of the apical bristle, but not the preapical bristle?
Ubx is widely expressed in the third leg, but it is not expressed at the same level in all cells. Thus one possible reason for the differential development of the apical and preapical bristle precursors is that they are exposed to different levels of Ubx protein, at the time when bristle development is blocked. The need for high levels of Ubx to block apical bristle development is supported by the observation that this function is partially haploinsufficient; flies heterozygous for a Ubx null mutation occasionally develop an apical bristle on T3 (1.4%, n = 138 of T3 legs with apical bristle for Ubx1).
To test whether the different developmental course of the apical and preapical bristles can be explained by differences in Ubx expression, we examined Ubx levels through antibody staining. We have shown above that Ubx function is required in the precursors to suppress apical bristle development. Therefore we examined Ubx expression in the first- and second-order precursors of the apical and preapical bristles 2.5 h APF (Fig. 7A,B) and 4.5 h APF (Fig. 7C–F). At both stages, Ubx levels are modulated in different regions of the disc. It is particularly noteworthy that sensory organ precursors can show levels of Ubx protein very different from those of the surrounding disc epithelium (see the precursors of the tibial chordotonal organ in Fig. 7B,D). However, at both time points, levels of Ubx protein in the apical bristle precursors are similar to those in the preapical bristle precursors and in the surrounding disc epithelium. Therefore, their differential developmental response cannot be attributed to differences in the levels of Ubx protein at these stages.
Figure 7.
Ubx protein levels are modulated in the third leg epithelium, but the levels in apical and preapical bristle precursors are similar. Confocal images of distal tibia and basitarsus of third leg of neu-lacZ flies 2.5 h APF (A,B) and 4.5 h APF (C–F). In green and right-hand panels anti-Ubx staining; in red anti-β-galactosidase staining. (A,B) Third leg 2.5 h APF. In the area reproduced here, Ubx protein levels are modulated: Expression is higher in posterior (P) than anterior leg (A); levels are higher in anterior distal tibia than anterior basitarsus; levels are reduced in anterior distal tibia in a dorsal strip containing sensory organ precursors and in the invaginating cells of the tibial chordotonal organ (see outline marked by asterisk in B). The first-order precursors of apical (arrowhead) and preapical bristle (double arrowhead) show similar levels of Ubx expression (see outlines marked with arrowheads in B). (C,D) Dorsal and (E,F) ventral aspects of distal tibia and basitarsus of a third leg at 4.5 h APF. By this time, the second-order precursors of apical and preapical bristle have formed. Ubx levels in these precursors are still comparable; Ubx levels in the tibial-chordotonal organ remain reduced (see outlines marked with arrowheads respectively asterisk in D and F).
It remains possible that earlier differences in levels of Ubx expression contribute to the different response of these cells. However, the result of ectopically expressing Ubx in the second leg throughout much of disc development argues against this possibility. When Ubx is ectopically expressed using the dpp-Gal4 driver, development of the apical bristle is efficiently suppressed, but development of the preapical bristle is rarely blocked (see above). Very high levels of Ubx protein do seem to suppress the expression of neural markers to some extent in both the apical and preapical bristle precursors, but this does not lead to complete dedifferentiation in the case of the preapical bristle precursors. Thus, the response of the apical and preapical precursors to Ubx appears to be intrinsically different.
Other bristles not affected by the prolonged ectopic expression of Ubx in this regime include the ventral and dorsal mechanosensory microchaetes on the distal tibia. The dorsal microchaetes lie in a space between two rows of chemosensory bristles, the precursors for which demarcate the border of the domain of ectopic Ubx expression driven by dpp-Gal4 in the leg disc (Fig. 6D). All of 24 legs surveyed showed mechanosensory bristles in this domain (e.g., Fig. 6H). Thus, continuous ectopic Ubx expression does not halt the development of these bristles. In conclusion, the specificity of the response of the apical bristle precursors to Ubx expression is not explained by a particular profile of Ubx expression.
Ubx induces different morphological changes in the apical and preapical bristles
Ectopic Ubx expression in the sensory organ lineage (Ubx isoform Ia driven by the 109-68 Gal4 line at 18°C) transforms the stout shaft characteristic of the normal preapical bristle on T2 to a much finer shaft characteristic of the preapical bristle on T3 (Fig. 8). The same regime of ectopic Ubx expression leaves the apical bristle largely unchanged (Fig. 8). Conversely, removal of Ubx protein from the preapical precursors on T3 causes them to form a stout shaft resembling the preapical bristle on T2 (Fig. 5).
Figure 8.
Ectopic Ubx expression in the sensory organ lineage has different effects on bristle morphology depending on bristle identity or bristle location. (A) Wild-type second leg and (B) wild-type third leg. (C) Second leg in which Ubx has been expressed in the sensory organ lineage with the 109-68 Gal4 driver at 18°C. In the ectopic Ubx expression regime (C), the stout preapical bristle on T2 is transformed toward the morphology of the thin preapical bristle normally found on T3 (arrowhead), whereas short bristles on the ventral basitarsus of T2 can be transformed toward the long stout morphology of those on the T3 leg (arrows). The morphology of the T2 apical bristle remains largely unchanged in this ectopic Ubx expression regime (double arrowhead).
Other bristles show specific responses to Ubx expression, but in the opposite direction to that of the preapical bristle: the short and relatively thin bristles on the T2 ventral basitarsus can be transformed by ectopic Ubx expression in the sensory organ lineage to long thick bristles, similar to those found on the T3 leg at this position (Fig. 8).
Discussion
Hox genes lie at the core of morphological diversification along the anteroposterior body axis. One Hox gene can control a multitude of traits that differ between segments, involving many different cell types and developmental pathways. To dissect aspects of this complexity, we have studied the segment-specific development of a class of organs that are repeated at many places within each segment—the mechanosensory bristles. Some representatives of this class are similar between segments, whereas others are restricted to certain segments, or show morphological variation between segments. We concentrated on individual large mechanosensory bristles which occur at a characteristic position in each segment. On the third thoracic segment, Ubx suppresses the development of some of these bristles and modifies the morphology of others.
Mechanisms of interference of Ubx with bristle development
For two particular bristles whose development is suppressed on T3, we find that Ubx acts directly on steps in sensory organ development, and not at some earlier stage of pattern formation.
In the two cases studied, it acts at different points in the hierarchy of sensory organ development. Development of the posterior sternopleural bristle is intercepted in midthird larval instar, shortly after initiation of a sternopleural proneural cluster. Ubx function is required at and restricted to this phase. Development of the tibial apical bristle is intercepted at 4.5–5 h APF, at the second-order precursor stage. However, the requirement for Ubx is not limited to this phase of sensory organ development. To suppress the apical bristle, Ubx function is required both in the second-order precursor cells, and prior to mother cell specification, possibly in the proneural cluster. Ubx accomplishes apical bristle suppression on T3 by additive mechanisms which are temporally separated.
It was previously suggested that the placement of segment-specific sensory organs would likely be executed by the interaction of Hox genes with the discrete regulatory elements of the AS-C complex that integrate prepattern factors to locate individual proneural clusters (Gerhart and Kirschner 1997). Our results suggest that no exclusive preference has been given to this mechanism. For both apical and sternopleural bristles, a proneural cluster is initially specified but later developmental steps are blocked.
We suggest that interactions between homeotic genes and sensory organ development have evolved independently on many occasions, affecting different molecular steps. We have not found evidence for a potential constraint in respect to mechanisms of bristle suppression. Evolution appears to have fixed a specific interaction between Ubx and the developmental pathway of individual bristles, not affecting other morphological features.
The specificity of Ubx function in bristle development
Ubx blocks the development of some bristles on the third thoracic segment (sternopleural bristles, edge bristle, apical bristle). It alters the morphology of others (preapical bristle, ventral basitarsal row). Yet other bristles are indistinguishable on the second and third thoracic segments, which suggests that they are unaffected by Ubx. It is not likely that the proneural genes or later acting genes controlling bristle development provide this specificity, for all of these mechanosensory bristles express the same subset of known neural markers.
The differences in Ubx action might in principle result from the differential expression of Ubx in different bristle lineages within the T3 segment. Spatiotemporal regulation of this type allows Ubx to define the specific segment morphologies of both third thoracic and first abdominal segments during larval development (Castelli-Gair et al. 1994), and instructs the distribution of trichomes during pupal leg development (Stern 1998).
However, our data suggest that the regulation of Ubx levels within the third leg disc does not play a role in eliciting the differential response of sternopleural, apical, and preapical bristles. Ubx levels are similar in the first- and second-order precursors of the apical and preapical bristles (Fig. 7). Moreover, the ectopic expression of Ubx does not eliminate their specific responses even though it imposes essentially the same profile of Ubx expression on a number of domains from which different bristles will arise. We have seen this in the regime of ectopic Ubx expression directed by the dpp-Gal4 driver, which results in the suppression of the apical but not the preapical bristle (Fig. 6). Similarly ubiquitous ectopic expression of Ubx in T2 during the last 24 h of the third larval instar consistently suppresses the sternopleural bristle precursor, but none of the seven other bristle precursors segregating at this time in the periphery of the leg disc (data not shown).
Other factors that are differentially distributed in the disc will provide bristles with individual identities, and so restrict Ubx action. Leg patterning is already well advanced at the late larval and early pupal stages when Ubx intercepts bristle development. Numerous molecular markers, many of them transcription factors, demonstrate the subdivision of the proximodistal axis into a number of domains (e.g., Abu-Shaar and Mann 1998). Prospective joint territories are marked by complex gene expression patterns (e.g., Rauskolb and Irvine 1999). Decapentaplegic (Dpp) and Wingless (Wg) signaling pathways are active in dorsal and ventral leg territories, respectively (Brook and Cohen 1996). Any one of these factors may act combinatorially with Ubx to control the targets that modify bristle development.
Held and Heup (1996) showed that wg null clones induced on the ventral side of the leg eliminate the apical bristle and result in the development of a second preapical bristle. Conversely, dpp null clones on the dorsal side of the leg eliminate the preapical bristle and cause the formation of a second apical bristle. Clones that constitutively activate the Wg pathway (shaggy null clones) can result in ectopic apical bristle formation on the dorsal side of the leg next to the wild-type preapical bristle (Wilder and Perrimon 1995). This demonstrates an intimate link between dorsal or ventral cell identity and sensory organ patterning and identity. However, we cannot conclude from these results that wg or dpp are directly involved in modulating Ubx effects on bristle development.
The idea that individual macrochaetes have distinct molecular identities is supported by the observation that many have unique morphologies. In some cases, this is further documented by the branching pattern of their associated neurons in the leg neuromere. For example, whereas most mechanosensory bristles show either anterior or posterior branching in the neuromere, the apical bristle has a C-shaped branching pattern extending into both the anterior and posterior neuromere (Murphey et al. 1989). These distinct molecular identities are constituted by or derived from the fine-patterning of the leg that is generated during larval and pupal stages.
We propose that the specific effects of Ubx on sensory organ development depend on these additional factors. We can then comprehend how a single homeotic gene can specify the complex differences in sensory organ distribution and sensory organ morphology that characterize the legs and other body surfaces. The generic sensory organ program is unresponsive to the homeotic protein on its own; only in the context of other patterning information is an instruction conveyed. This allows for specific responses to the Hox gene to be elicited locally, affecting one or a small group of sensory organs, and not all organs of the same class throughout the segment.
Materials and methods
Staging of animals
The main reference point for staging animals was the time of puparium formation, defined as the white prepupa. White prepupae were selected as motionless animals with everted spiracles, but retaining a white puparium. This stage lasts about 30 min. For experiments on third instar larvae, the age at the time of experimental intervention was back-calculated from the time at which they formed a puparium. All stages are given for development at 25°C.
Immunocytochemistry
For staining of larval discs, larvae were dissected in phosphate buffered saline (PBS; pH 7.4) and turned inside out; internal organs were then removed. For staining of prepupal discs, the cuticle of the pupal case was opened at the posterior end and removed in rings. All inner organs were removed until only the brain surrounded by the evaginating imaginal discs was left in the anterior pupal case. The discs were then incubated in solutions in this pupal case basket. Dissected specimens were collected in a vial of 4% paraformaldehyde on ice and then fixed for 20 min at RT on a shaker. Washes before incubation with primary and secondary antibodies were in PBS with 0.3% Triton X-100 and 0.2% goat serum (PBST with GS). Incubation with the primary antibodies was generally overnight at 4°C and with the secondary antibodies for 4–6 h at RT. Stained specimens were dissected, recording leg disc identity, and mounted in Vectashield (Vector Laboratories). Sensory organs were monitored by anti-β-gal staining in the neuA101 lacZ line P{lArB} neu[A101] (Huang et al. 1991). Anti-β-gal antibodies were mouse (Promega) 1/100 and rabbit (Cappel) 1/1000. Anti-Cut antibody (rat) (Blochlinger et al. 1990) was used at a dilution of 1/200 to stain external sensory organ precursors, and anti-Pox-neuro antibody (rabbit) (Bopp et al. 1989) was used at a dilution of 1/100 to stain chemosensory organ precursors. Ubx levels were monitored with anti-Ubx antibody FP 3.38 (mouse monoclonal) (White and Wilcox 1984) used at a dilution of 1/25 to 1/100. Secondary antibodies were Cy5-conjugated anti-rat and anti-rabbit (Jackson Laboratories), FITC-conjugated anti-mouse and Texas Red-conjugated anti-rabbit (Vector Laboratories) used at dilutions of 1/100 or 1/200.
Confocal imaging
Confocal z-series were taken with a Bio-Rad 1024 confocal microscope or a Leica SP confocal microscope. The distance between sections in series was 0.5 μm. Projections of sections taken at the Bio-Rad confocal were produced with the software Laser Sharp. We generated single views within the software using the replicate projection mode. Projections of sections taken at the Leica confocal were generated with the projection option in the Leica TCS NT confocal software.
Time series of sternopleural proneural cluster development
GFP expression driven by a sca-Gal4 line was used for monitoring proneural cluster development during the third larval instar. We crossed the w; D1-8a2 arIII UAS-GFP 65/167/TM6B line (gift from Dr. Andrea Brand) and the P{GawB} sca[537.4] line (Hinz et al. 1994). Egg laying and rearing of larvae were on apple juice plates with yeast. Samples from a cohort of larvae moulting to the third instar during a 6 h period were dissected at different time points. Their siblings were allowed to develop to puparium formation, and scored to provide an estimate of the age of the samples BPF. The peak of puparium formation was estimated to be 47 h after the midpoint of the second to third instar moult, with a spread of approximately 12 h. We estimate that at the three times of sampling, the age range of the larvae will have been approximately 31–22, 25–16, and 11–0 h BPF.
Scoring of bristle phenotypes in the adult
Specific bristles were identified on the animals by position, bristle morphology, and the presence or absence of an accompanying bract. All mechanosensory microchaetes on the distal tibia show a dorsally located bract. Chemosensory bristles are recurved and have no bract associated with them. Apical and preapical bristles also do not have a bract associated with them, while each of the spurs on the second leg is accompanied by a bract.
Flies were preserved in 70% ethanol/30% glycerol. For analysis of bristle development following ectopic Ubx expression, legs were examined under a compound microscope. For this purpose, legs were dissected in 70% ethanol/30% glycerol, dried on filter paper, and then mounted in Hoyer's medium (Stern and Sucena 2000). The bristle pattern was scored under bright-field illumination. For quantitative analysis of Ubx null clones, flies were scored under a dissecting scope at 50× magnification.
Manipulation of Hox gene expression
Heatshock-Ubx expression
For timed ectopic Ubx expression, we used the hs-Ubx-Ia line (original stock: w1118; HsUbx Ia/TM3) (Mann and Hogness 1990). We generated flies of the genotype hs-Ubx Ia/neu-LacZ for monitoring sensory organ precursor development. Heatshocks were applied at 37°C for a duration of 45 to 60 min by incubating culture tubes in a waterbath. The heatshock regimes used for the ectopic expression of Ubx were the following: For the startpoint at 23±1 h BPF: 1 h heatshock, 4 h at 25°C, 1 h heatshock, 5 h at 25°C, 1 h heatshock, and final transfer to 25°C. For the startpoint at 21±1 h BPF: 1 h heatshock, 4 h at 25°C, 45 min heatshock, and final transfer to 25°C. For the startpoint 6±1 h BPF, conditions were: one heatshock for 1 h and final transfer to 25°C. Ubiquitous ectopic Ubx expression in the third instar impairs puparium formation. Larvae become immobile but the puparium is not properly formed. Therefore, staging of the animals at the time of the heatshock cannot be done as usual by collecting white prepupae at a certain time after the heatshock. Instead, immobile third instar larvae were collected and dissected. The age of the dissected specimens was assigned according to the degree of imaginal disc eversion observed. Imaginal disc eversion begins in the white prepupa and is a certain indication that this developmental stage has been reached. Specimens were included in the sample only if they showed some but not advanced leg disc eversion. These discs will range in age from 0 to ∼2 h APF.
Expression patterns of the Gal4 lines employed for Ubx null clone induction or ectopic Ubx expression
We employed the Gal4-UAS system for targeted gene expression (Brand and Perrimon 1993).
dpp-Gal4: P {Gal4-dpp.blk1} (Staehling-Hampton et al. 1994). Driven by dpp-Gal4, Ubx is expressed in a robust dorsal stripe and a more patchy ventral stripe in the leg. We used Ubx isoforms Ia and IVa (lines UAS-Ubx Ia1 and UAS-Ubx IVa1; Reed 1996). At 18°C, ectopic Ubx expression covers only the preapical bristle precursors. At 25°C, the stripes of ectopic Ubx cover the sternopleural, preapical, and apical bristle precursors. Continuous animal rearing after embryogenesis at 25°C results in severe leg malformations. For these reasons, we shifted animals from 18°C to 25°C 40–50 h BPF.
sca-Gal4: P{GawB} sca[537.4] (Hinz et al. 1994). sca-Gal4 drives expression in proneural clusters and in the sensory organ lineage (Wang et al. 1997). In the presumptive domain of the sternopleural proneural cluster, no sca-Gal4 activity is detected during third larval instar prior to 22 h BPF (observation from “time series of sternopleural proneural cluster development”). This ensures that clone induction with this line is only initiated on the sternopleurum when the proneural cluster forms.
109-68 Gal4: P {Gal4} sca[109-68] (Guo et al. 1996). For the majority of sensory organs in the leg discs, the 109-68 Gal4 line drives expression in the sensory organ lineage from the first-order precursor onwards. We assayed this by activating GFP expression with this line and costaining for β-galactosidase in the neu-lacZ background. To obtain a summary picture of 109-68 Gal4 activity in the leg disc we used a FRT-LacZ construct (Act5C FRT Draf+ FRT nuc-lacZ; Struhl and Basler 1993) which we activated permanently with this driver via a UAS-Flipase construct. This experiment closely mimics our experimental clone induction regime (see below). We observed the lacZ pattern in second and third leg APF. We found that first-order precursors of the apical bristle exhibit very low levels of lacZ, whereas second-order precursors show moderate levels. This suggests that 109-68 Gal4 expression is initiated in the first-order precursors. In addition, in the majority of cases (11 of 12), no other lacZ-positive cells displaying similar lacZ levels are found adjacent to the apical bristle precursors. Thus, we are confident that clone induction with this driver is targeted to the apical bristle sensory organ lineage.
Ubx null clone induction
For clone induction, we made use of the FLP-FRT system (Golic and Lindquist 1989; Golic 1991). Two variants of Flipase-mediated Ubx null clone induction were employed. One relied on heatshock to induce Flipase, the other on Flipase expressed under the control of Gal4 lines expressed in proneural clusters or in the sensory organ lineage. Timed Ubx null clones were induced by heatshock of 1 h at 37°C. No background clone induction was observed in flies raised at 25°C. The allele of Ubx used was Ubx1, a null allele of the Ubx locus (Lindsley and Zimm 1992). As a marker for Ubx null clones in bristles, we used Sb. Mitotic recombination facilitated by the FRT sites generates cells which are deficient for Ubx and wild-type for Sb. Wild-type bristle shafts can easily be scored in an Sb heterozygous background. An exception to this is the preapical bristle on T3. Its morphology is very similar in Sb heterozygotes and wild-type animals. Ubx null clones of this bristle were scored by their phenotypic transformation to T2 preapical bristles.
The original stocks used to construct composite stocks for clone induction were:
scabrous-Gal4: P{GawB} sca[537.4] (Hinz et al. 1994)
109-68 Gal4: P {Gal4} sca[109-68] (Guo et al. 1996)
UAS-Flp: yw; p[UAS-Flp, w+] Pin/CyO (Frise et al. 1996)
Hs-FLP: yw; P{hsFLP22} (Baonza et al. 2000)
FRT82: yw; P[ry+, hs-neo, FRT]82B
FRTSb: w; P[ry+, hs-neo, FRT]82B Sb[63b]/TM6B (Xu and Rubin 1993)
FRT82 Ubx1 e11: w; P[ry+, hs-neo, FRT]82B Ubx1 e11/TM6B
FRT82 Ubx1: yw; P[ry+, hs-neo, FRT]82B Ubx1/TM6B
Flies scored for Ubx null clones were of the genotypes:
yw Hs-FLP; FRTSb/FRTUbx1
yw UAS-FLP; sca-Gal4; FRT Ubx1e11/FRTSb,
yw UAS-FLP; 109-68 Gal4; FRT Ubx1e11/FRTSb
Timed Ubx null clone induction via heatshock was before puparium formation. Ubx null clones were induced at different times during third instar larval development. Specimens were collected after heatshock as newly formed pupae over time intervals of 5–6 h. For the assessment of time of Ubx action in the suppression of sternopleural bristles on T3 we scored animals within each collection interval for the number of Ubx null anterior (SP1) and posterior (SP3) sternopleural bristles marked by Sb+ and calculated the percentage of wild-type bristles per hemisegment. We plotted these percentages in relation to the midpoints of clone induction BPF in the different collection intervals (Fig. 2).
Acknowledgments
We thank Torsten Bossing, Andrea Brand, Gerard Campbell, Jose Casal, Yuh-Nung Jan, Todd Laverty, Peter Lawrence, Hilary Reed, Fernando Roch, Gerald Rubin, and David Stern for fly stocks. We thank Steve Jackson, Markus Noll, and Robert White for antibodies. Thanks to Fernando Roch, James Castelli-Gair, and Laurent Seugnet for critical reading of the manuscript. This work was supported by the Wellcome Trust and the Evangelisches Studienwerk Villigst.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL akam@mole.bio.cam.ac.uk; FAX 44-1223-336679.
Article and publication are at http://www.genesdev.org/cgi/doi/10.1101/gad.219302.
References
- Abu-Shaar M, Mann RS. Generation of multiple antagonistic domains along the proximodistal axis during Drosophila leg development. Development. 1998;125:3821–3830. doi: 10.1242/dev.125.19.3821. [DOI] [PubMed] [Google Scholar]
- Akam M. The molecular basis for metameric pattern in the Drosophila embryo. Development. 1987;101:1–22. [PubMed] [Google Scholar]
- Auerbach C. The development of the legs, wings, and halteres in wild type and some mutant strains of Drosophila melanogaster. Proc R Soc Edinb B. 1936;58:787–815. [Google Scholar]
- Baonza A, Roch F, Martin-Blanco E. DER signaling restricts the boundaries of the wing field during Drosophila development. Proc Natl Acad Sci. 2000;97:7331–7335. doi: 10.1073/pnas.97.13.7331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blochlinger K, Bodmer R, Jan LY, Jan YN. Patterns of expression of Cut, a protein required for external sensory organ development in wild-type and cut mutant Drosophila embryos. Genes & Dev. 1990;4:1322–1331. doi: 10.1101/gad.4.8.1322. [DOI] [PubMed] [Google Scholar]
- Bopp D, Jamet E, Baumgartner S, Burri M, Noll M. Isolation of two tissue-specific Drosophila paired box genes, Pox meso and Pox neuro. EMBO J. 1989;8:3447–3457. doi: 10.1002/j.1460-2075.1989.tb08509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook WJ, Cohen SM. Antagonistic interactions between Wingless and Decapentaplegic responsible for dorsal-ventral pattern in the Drosophila leg. Science. 1996;273:1373–1377. doi: 10.1126/science.273.5280.1373. [DOI] [PubMed] [Google Scholar]
- Brower DL. Ultrabithorax gene expression in Drosophila imaginal discs and larval nervous system. Development. 1987;101:83–92. doi: 10.1242/dev.101.1.83. [DOI] [PubMed] [Google Scholar]
- Casares F, Mann RS. Control of antennal versus leg development in Drosophila. Nature. 1998;392:723–726. doi: 10.1038/33706. [DOI] [PubMed] [Google Scholar]
- Castelli-Gair J, Greig S, Micklem G, Akam M. Dissecting the temporal requirements for homeotic gene function. Development. 1994;120:1983–1995. doi: 10.1242/dev.120.7.1983. [DOI] [PubMed] [Google Scholar]
- Chasan R, Anderson KV. Maternal control of dorsal-ventral polarity and pattern in the embryo. In: Bate M, Martinez Arias A, editors. The development of Drosophila melanogaster. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1993. pp. 387–424. [Google Scholar]
- Cohen SM. Imaginal disc development. In: Bate M, Martinez Arias A, editors. The development of Drosophila melanogaster. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1993. pp. 747–841. [Google Scholar]
- Culi J, Modolell J. Proneural gene self-stimulation in neural precursors: An essential mechanism for sense organ development that is regulated by Notch signaling. Genes & Dev. 1998;12:2036–2047. doi: 10.1101/gad.12.13.2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frise E, Knoblich JA, Younger-Shepherd S, Jan LY, Jan YN. The Drosophila Numb protein inhibits signaling of the Notch receptor during cell–cell interaction in sensory organ lineage. Proc Natl Acad Sci. 1996;93:11925–11932. doi: 10.1073/pnas.93.21.11925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhart J, Kirschner M. Cells, embryos, and evolution. Maldem, MA: Blackwell Science; 1997. [Google Scholar]
- Gho M, Bellaiche Y, Schweisguth F. Revisiting the Drosophila microchaete lineage: A novel intrinsically asymmetric cell division generates a glial cell. Development. 1999;126:3573–3584. doi: 10.1242/dev.126.16.3573. [DOI] [PubMed] [Google Scholar]
- Golic KG. Site-specific recombination between homologous chromosomes in Drosophila. Science. 1991;252:958–961. doi: 10.1126/science.2035025. [DOI] [PubMed] [Google Scholar]
- Golic FG, Lindquist S. The FLP recombinase of yeast catalyses site-specific recombination in the Drosophila genome. Cell. 1989;59:499–509. doi: 10.1016/0092-8674(89)90033-0. [DOI] [PubMed] [Google Scholar]
- Gomez-Skarmeta JL, Rodriguez I, Martinez C, Culi J, Ferres-Marco D, Beamonte D, Modolell J. Cis-regulation of achaete and scute: Shared enhancer-like elements drive their coexpression in proneural clusters of the imaginal discs. Genes & Dev. 1995;9:1869–1882. doi: 10.1101/gad.9.15.1869. [DOI] [PubMed] [Google Scholar]
- Gorfinkiel N, Morata G, Guerrero I. The homeobox gene Distal-less induces ventral appendage development in Drosophila. Genes & Dev. 1997;11:2259–2271. doi: 10.1101/gad.11.17.2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto S, Hayashi S. Specification of the embryonic limb primordium by graded activity of Decapentaplegic. Development. 1997;124:125–132. doi: 10.1242/dev.124.1.125. [DOI] [PubMed] [Google Scholar]
- Guo M, Jan LY, Jan YN. Control of daughter cell fates during asymmetric division: Interaction of Numb and Notch. Neuron. 1996;17:27–41. doi: 10.1016/s0896-6273(00)80278-0. [DOI] [PubMed] [Google Scholar]
- Heitzler P, Simpson P. The choice of cell fate in the epidermis of Drosophila. Cell. 1991;64:1083–1092. doi: 10.1016/0092-8674(91)90263-x. [DOI] [PubMed] [Google Scholar]
- Held LI, Heup MA. Genetic mosaic analysis of decapentaplegic and wingless gene function in the Drosophila leg. Dev Genes Evol. 1996;206:180–194. doi: 10.1007/s004270050044. [DOI] [PubMed] [Google Scholar]
- Hinz U, Giebel B, Campos-Ortega JA. The basic-helix-loop-helix domain of Drosophila lethal of scute protein is sufficient for proneural function and activates neurogenic genes. Cell. 1994;76:77–87. doi: 10.1016/0092-8674(94)90174-0. [DOI] [PubMed] [Google Scholar]
- Huang F, Dambly-Chaudiere C, Ghysen A. The emergence of sense organs in the wing disc of Drosophila. Development. 1991;111:1087–1095. doi: 10.1242/dev.111.4.1087. [DOI] [PubMed] [Google Scholar]
- Lewis EB. Genes and developmental pathways. Am Zool. 1963;3:33–56. [Google Scholar]
- ————— A gene complex controlling segmentation in Drosophila. Nature. 1978;276:565–570. doi: 10.1038/276565a0. [DOI] [PubMed] [Google Scholar]
- Lindsley DL, Zimm GG. The genome of Drosophila melanogaster. San Diego, CA: Academic Press; 1992. [Google Scholar]
- Mann RS, Hogness DS. Functional dissection of Ultrabithorax proteins in D. melanogaster. Cell. 1990;60:597–610. doi: 10.1016/0092-8674(90)90663-y. [DOI] [PubMed] [Google Scholar]
- Martinez Arias A. Development and patterning of the larval epidermis of Drosophila. In: Bate M, Martinez Arias A, editors. The development of Drosophila melanogaster. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1993. pp. 517–608. [Google Scholar]
- Murphey RK, Possidente DR, Vandervorst P, Ghysen A. Compartments and the topography of leg afferent projections in Drosophila. J Neurosci. 1989;9:3209–3217. doi: 10.1523/JNEUROSCI.09-09-03209.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nottebohm E, Usui A, Therianos S, Kimura K, Dambly-Chaudiere C, Ghysen A. The gene poxn controls different steps of the formation of chemosensory organs in Drosophila. Neuron. 1994;12:25–34. doi: 10.1016/0896-6273(94)90149-x. [DOI] [PubMed] [Google Scholar]
- Rauskolb C, Irvine KD. Notch-mediated segmentation and growth control of the Drosophila leg. Dev Biol. 1999;210:339–350. doi: 10.1006/dbio.1999.9273. [DOI] [PubMed] [Google Scholar]
- Reed H. “The role of alternatively spliced microexons and hexapeptide motif in modulating Ubx specificity in vivo.” Ph.D. thesis. Cambridge, UK: Department of Genetics, University of Cambridge; 1996. [Google Scholar]
- Roch F, Akam M. Ultrabithorax and the control of cell morphology in Drosophila halteres. Development. 2000;127:97–107. doi: 10.1242/dev.127.1.97. [DOI] [PubMed] [Google Scholar]
- Singson A, Leviten MW, Bang AG, Hua XH, Posakony JW. Direct downstream targets of proneural activators in the imaginal disc include genes involved in lateral inhibitory signaling. Genes & Dev. 1994;8:2058–2071. doi: 10.1101/gad.8.17.2058. [DOI] [PubMed] [Google Scholar]
- Staehling-Hampton K, Jackson PD, Clark MJ, Brand AH, Hoffmann FM. Specificity of bone morphogenetic protein-related factors: Cell fate and gene expression changes in Drosophila embryos induced by decapentaplegic but not 60A. Cell Growth Differ. 1994;5:585–593. [PubMed] [Google Scholar]
- Stern D. A role of Ultrabithorax in morphological differences between Drosophila species. Nature. 1998;396:463–466. doi: 10.1038/24863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern D, Sucena E. Preparation of larval and adult cuticles for light microscopy. In: Sullivan W, Ashburner M, Hawley RS, editors. Drosophila protocols. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2000. pp. 601–615. [Google Scholar]
- Struhl G, Basler K. Organizing activity of wingless protein in Drosophila. Cell. 1993;72:527–540. doi: 10.1016/0092-8674(93)90072-x. [DOI] [PubMed] [Google Scholar]
- Stuart JJ, Brown SJ, Beeman RW, Denell RE. A deficiency of the homeotic complex of the beetle Tribolium. Nature. 1991;350:72–74. doi: 10.1038/350072a0. [DOI] [PubMed] [Google Scholar]
- Usui K, Kimura K. Sensory mother cells are selected from among mitotically quiescent cluster of cells in the wing disc of Drosophila. Development. 1992;116:601–610. [Google Scholar]
- Vachon G, Cohen B, Pfeifle C, McGuffin ME, Botas J, Cohen SM. Homeotic genes of the Bithorax complex repress limb development in the abdomen of the Drosophila embryo through the target gene Distal-less. Cell. 1992;71:437–450. doi: 10.1016/0092-8674(92)90513-c. [DOI] [PubMed] [Google Scholar]
- Wagner-Bernholz JT, Wilson C, Gibson G, Schuh R, Gehring WJ. Identification of target genes of the homeotic gene Antennapedia by enhancer detection. Genes & Dev. 1991;5:2467–2480. doi: 10.1101/gad.5.12b.2467. [DOI] [PubMed] [Google Scholar]
- Wang S, Younger-Shepherd S, Jan LY, Jan YN. Only a subset of the binary cell fate decisions mediated by Numb/Notch signaling in Drosophila sensory organ lineage requires Suppressor of Hairless. Development. 1997;124:4435–4446. doi: 10.1242/dev.124.22.4435. [DOI] [PubMed] [Google Scholar]
- Weatherbee SD, Halder S, Kim J, Hudson A, Carroll SB. Ultrabithorax regulates genes at several levels of the wing-patterning hierarchy to shape the development of the Drosophila haltere. Genes & Dev. 1998;12:1474–1482. doi: 10.1101/gad.12.10.1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler MR. The Drosophilidae: A taxonomic overview. In: Ashburner M, Carson HL, Thompson JN, editors. The genetics and biology of Drosophila. London, UK: Academic Press; 1981. pp. 1–97. [Google Scholar]
- White RA, Wilcox M. Protein products of the bithorax complex in Drosophila. Cell. 1984;39:163–171. doi: 10.1016/0092-8674(84)90202-2. [DOI] [PubMed] [Google Scholar]
- Wilder E, Perrimon N. Dual functions of wingless in the Drosophila leg imaginal disc. Development. 1995;121:477–488. doi: 10.1242/dev.121.2.477. [DOI] [PubMed] [Google Scholar]
- Xu T, Rubin GM. Analysis of genetic mosaics in developing and adult Drosophila tissues. Development. 1993;117:1223–1237. doi: 10.1242/dev.117.4.1223. [DOI] [PubMed] [Google Scholar]