Abstract
Depending on the research question or the public health application, the appropriate resolution of the data varies temporally, spatially, and, for satellite data, spectrally and radiometrically. Regardless of the scale used to address a research or public health question, the temptation is always there to extrapolate from fine-resolution data or to interpolate from coarse resolution studies. In both cases, the relevance of data and analyses conducted on one spatial level to other levels cannot be taken for granted. Spatial heterogeneity on the micro-scale may not be detected using coarse spatial resolution, and conversely, general patterns on the macro-scale may not be detected using fine spatial resolution. Two studies are described where the transmission dynamics and risk of infection was assessed on the micro-scale starting with household level studies in one community, and the study area was extended gradually to consider several communities and sources for vectors or intermediate hosts. In a study of Chagas disease in northwest Argentina, the reinfestation process of communities by the main domestic vector was analyzed using spatial statistics; sources within and outside communities as well as the distance of reinfestation were identified. In a study of urinary schistosomiasis in coastal Kenya, age dependent and directional focal clustering of infections was detected around some aquatic habitats, and a hydrological model was developed to detect least cost dispersal routes that allow snails to reinfest dried-up habitats. Some general aspects of focal statistics are discussed. Several general questions need to be considered in geospatial health studies, including the following: (i) what are the best criteria for selecting the spatial (and temporal) unit of intervention and analysis? (ii) how do the key measures of risk and transmission dynamics vary with scale? (iii) how do we integrate processes occurring at diverse spatial and temporal scales? All of these questions can only be addressed through solid biological, epidemiological and socio-economic understanding of the system in time and space.
Keywords: remote sensing, spatial statistics, scale, clustering, Chagas disease, schistosomiasis
Introduction
Health, as defined in the UN Alma Ata declaration refers to “a state of complete physical, mental and social wellbeing, and not merely the absence of disease or infirmity (International Conference on Primary Health Care, Alma-Ata, USSR, 6-12 September 1978). Since the 1980’s several geospatial tools have become readily available for eco-epidemiological research and applications, including geographic information systems (GIS), global positioning systems (GPS), satellite imagery, geostatistics and other spatial statistics. Although these tools have been developed largely for other reasons and for other applications than health and its improvement, eco-health researchers and some health professionals have embraced these technologies and applied them successfully to study, explain and predict spatio-temporal patterns of diseases, risk factors for disease and, more recently, to study other conditions that impact human welfare, including social and economic factors and their interactions with community health. A recent workshop (30-31 Jan. 2006) held by the US National Research Council was devoted to the contributions of remote sensing for decisions about human welfare (report in preparation).
Depending on the research question or the public health application, the appropriate resolution of the data varies temporally, spatially, and, for satellite data, spectrally and radiometrically (Holmes, 1997; Kitron, 1998, Kitron, 2000; Beck et al., 2000, Beck et al., 2002; Hay, 2000; Hay et al., 2000; Hess et al., 2002). On the spatial scale, research and applications range from mapping the distribution of Anopheles gambiae mosquitoes and malaria risk on the national or continental level in Africa (MARA - http://www.mara.org.za, Kleinschmidt et al., 2000) to household level studies of Aedes aegypti and dengue in San Juan, Puerto Rico and Iquitos, Peru (Morrison et al., 1998; Getis et al., 2003). Regardless of the scale used to address a research or public health question, the temptation is always there to extrapolate from fine-resolution data and conclusions or to interpolate from coarse resolution studies. In both cases, the relevance of data and analyses conducted on one spatial level to other levels cannot be taken for granted. Spatial heterogeneity on the micro-scale may not be detected at a coarse spatial resolution, and conversely, general patterns on the macro-scale may not be detected at a fine spatial resolution (Turner et al., 1989; Levin, 1992; Wiens, 1989; Qi and Wu, 1996).
In several of our studies, we have concentrated on studying the transmission dynamics and risk of infection on the micro-scale starting with household level studies in one community and enlarging our study area gradually to consider several communities and sources for vectors or intermediate hosts. Below, we review some of our findings in two such studies: Chagas disease in northwest Argentina and urinary schistosomiasis in coastal Kenya.
Chagas disease in northwest Argentina
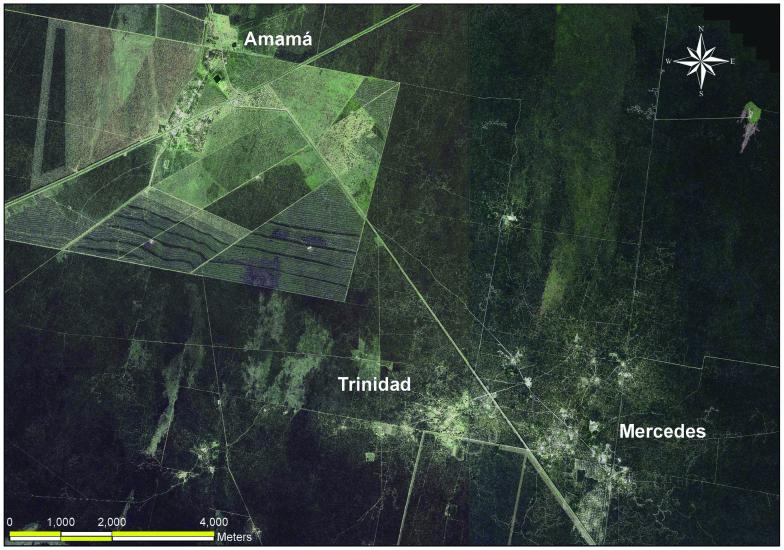
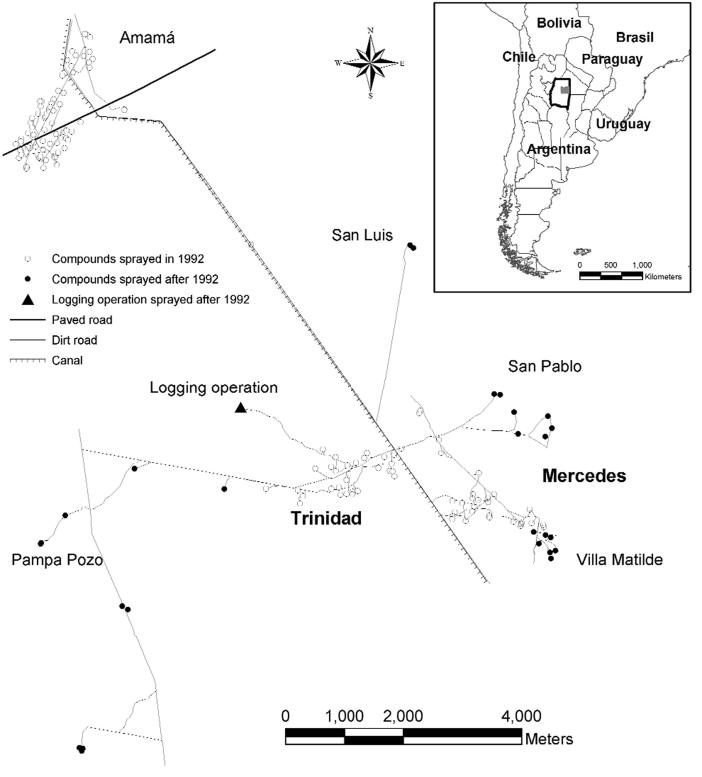
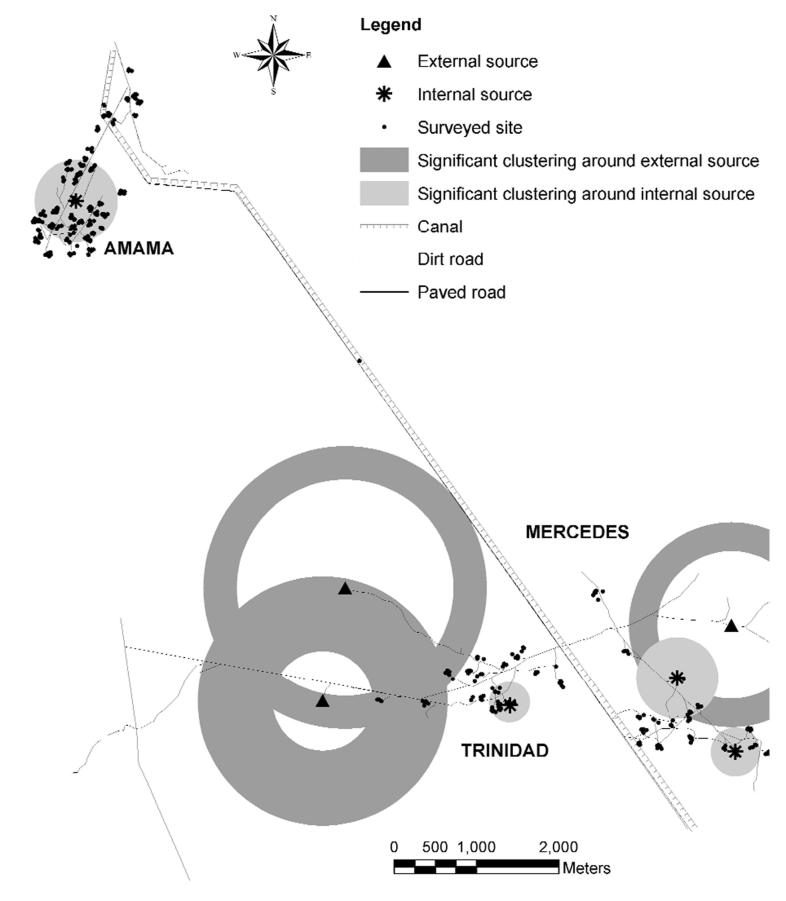
In our study of the eco-epidemiology of Chagas disease in northwest Argentina, we initially studied the pattern of reinfestation by Triatoma infestans (the main domestic vector) in an isolated rural community (Amamá) following a community-wide residual spraying with insecticides in domestic and peridomestic structures. Domiciles of this very impoverished rural area are made of adobe walls, thatched roofs and dirt floors. The peridomestic area comprises the yard and several structures (storerooms, kitchens, chicken coops) typically with thatched roofs and mud walls, and animal corrals fenced with wood sticks, posts, or thorny branches. Moving upscale, we then studied how the reinfestation by T. infestans in two neighboring communities (Trinidad and Mercedes) could be affected by other neighboring communities that were not simultaneously sprayed with residual insecticides. A georeferenced multispectral Ikonos (Space Imaging, Atlanta, GA) satellite image of the three villages (notice the increased deforestation around Amamá and the main road in comparison to neighboring more remote communities) sharpened to 1 m spatial resolution (Fig. 1), and GPS readings were used to identify and map the location of each structure present in 2000-2002. Sketch maps made in the field during 1993-1999 were used with the satellite image to geo-reference each surveyed structure that was not located with the GPS. The sketch maps, though not as accurate as GPS readings or the IKONOS image, were the only historical register of structures during the 1990’s. The 10-year retrospective entomological data were then associated with the geographic locations (x and y coordinates in UTM projection) of each identified structure (Fig. 2). The local spatial statistic Gi(d) (Getis and Ord, 1996) was used as a focal statistic in order to identify and measure spatial clustering of T. infestans abundance around known and suspected epicenters or sources of reinfestation (Fig. 3). Scale clearly affected the reinfestation process of rural communities. In Amamá, reinfestation occurred from one internal source, a pigcorral, where bugs were not eliminated during the community-wide spraying effort and from which infestation spread throughout the community (Cecere et al., 2004). Subsequent infested sites were focally clustered around this source up to a distance of 450 m. When moving upscale, the reinfestation in two more remote communities could be associated with internal sources (a granary and a storeroom) and with external sources (a logging operation and a neighboring community). These unsprayed external sources were heavily infested by T. infestans and were located up to a distance of 1,500 m around the sprayed communities (Cecere et al., 2006). Flight dispersal of T. infestans bugs from the identified sources appeared to be the most likely mechanism explaining the reinfestation process of these rural communities. By joining the results of our research with long-term detailed field data we were able to recommend to the Argentinean vector control program that in order to prevent the subsequent propagation of T. infestans in communities under surveillance, an effective control at the community level would entail residual spraying with insecticides of the colonized sites and all sites within a radius of 450 m, as well as of all communities within 1,500 m of the target community. This recommendation replaced the prevailing concept of spraying 200 m around colonized sites, a strategy that was based on anecdotal and circumstantial evidence rather than scientifically based knowledge.
Fig. 1.
Ikonos Image of study area in the Department of Moreno, Province of Santiago del Estero, Argentina. The area around Amamá is nearly completely deforested.
Fig. 2.
Chagas disease study area in the Department of Moreno, Province of Santiago del Estero, Argentina.
Fig. 3.
Focal clustering around internal, and external sources in the communities of Amamá, Trinidad and Mercedes, Department of Moreno, Province of Santiago del Estero, Argentina.
Clustering detection methods are classified as global, local and focused (Besag and Newell, 1991; Lawson, 2001). Global and local spatial statistics are exploratory tools that allow for the finding and identification of clusters without a pre-determined hypothesis about cluster location, whereas focal spatial statistics allow for testing the hypothesis of whether a disease, its vectors or its hosts cluster around a suspected location. Focal statistics are one approach to deal with fine resolution health data, since clustering of vector-borne diseases can occur at very short distances, as observed for dengue fever cases and Ae. aegypti mosquitoes (Morrison et al., 1998; Getis et al., 2003), for LaCrosse encephalitis (Kitron et al., 1997) and for malaria (Chadee and Kitron, 1999). In our studies we applied the Getis local spatial statistic Gi(d) as focused statistic and detected significant clustering around suspected sources for T. infestans dispersal. These analyses were performed on the number of insects per site for each evaluation date. However, focal clustering can also be tested for individual-level data such as disease occurrence or infested sites, by means of the local K-function (Getis, 1984; Getis and Franklin, 1987; Kitron et al., 1992; Morrison et al., 1998).
This approach provides three measures of clustering:
the nearest neighbor distance (i.e. the distance from the tested location where cases begin to appear);
the maximum clustering distance (the distance where clustering is maximized); and
the significant clustering distance (i.e. the distance at which clustering is statistically significant) (Getis and Franklin, 1987).
Statistical significance can be ascertained either by Monte Carlo simulations or by accepting the value of the expression ±1.42 √ Area/(number of points within the tested distance - 1) as an approximation of the 5% significance level (Getis and Franklin, 1987).
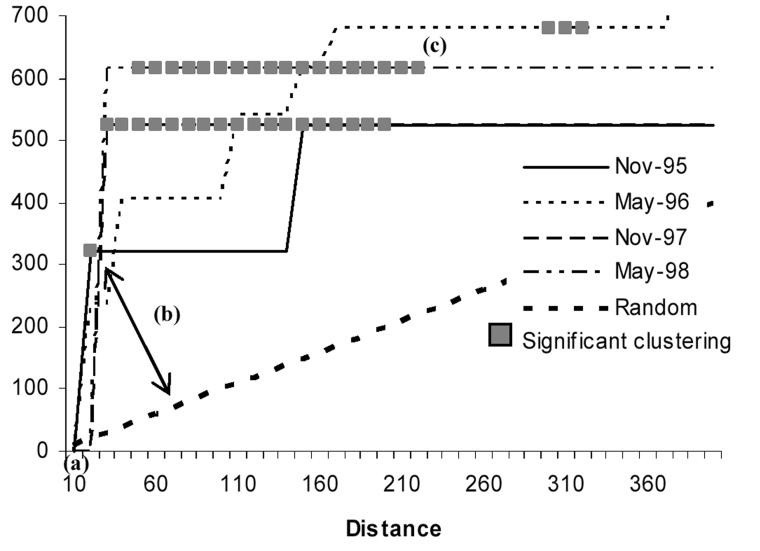
By using data from Vazquez-Prokopec et al. (2005), we analyzed the appearance of new infested sites around a source of reinfestation by Triatoma guasayana, a sylvatic vector of Chagas disease, in the community of Amamá during the 5 years following community-wide spraying with insecticides. In order to disentangle the clustering of infested sites around the suspected source from the background clustering of all sites (domestic and peridomestic), local K-function was applied separately for infested sites and for all sites. Clustering of T. guasayana infestation around the suspected source occurred when the local K-function value of infested sites was statistically significant at the 5% level, and was higher than the local K-function value (Li(d)) for all sites. The nearest neighbor distance was very short(4 m), indicating that new infestations occurred very close to the source (Fig. 4). The maximum clustering distance ranged from 20 m to 300 m, and significant clustering distance varied over time, ranging from 20 m in November 1995 to 200 m or more starting in May 1996, indicating an overall expansion of infestation over time.
Fig. 4.
Focal K-function (Li(d)) performed on the occurrence of Triatoma guasayana infested sites around a suspected source of reinfestation of the rural community of Amamá following a community-wide spraying with residual insecticides. Letters indicate: (a) the nearest neighbor distance; (b) the maximum clustering distance; and (c) the significant clustering distance.
Other methods for detecting clusters of disease cases around suspected sources of disease (i.e. children leukemia around a nuclear power station) such as the Diggle’s method (Diggle, 1990; Diggle and Rowlinson, 1994; Gatrell et al., 1996; Morris and Wakefield, 2000) compare the spatial pattern of case locations with the spatial pattern of control subjects. However, for assessing the distribution of vectors or intermediate hosts of disease selecting “control” locations is not always feasible. The application of the Gi(d) and Li(d) as focal statistics is a more suitable approach for addressing the question of whether the vectors or disease cluster around a suspected source. In general, the analysis of spatial data has to be carried out at an appropriate resolution, i.e., the spatial resolution at which the phenomena being studied occurs. Whereas disease data on a coarse spatial scale (counts of disease in administrative areas) are appropriate for mapping disease distribution and for performing regional or national level analyses, the analysis of disease data on a fine spatial resolution (location of individual cases) allows for the assessment of specific spatial associations, such as the relative contribution of specific risk factors, or the relation between a cluster of cases and a putative source of disease (Besag and Newell, 1991; Gatrell et al., 1996; Lawson, 2001).
While disease data are more commonly available as aggregated summaries within arbitrary areas (i.e. census tracts) rather than as exact case locations, interpolation from a coarse to a fine scale cannot consider local clustering of vectors or disease (Robinson, 2000; Cromley and McLafferty, 2002). In our research on Chagas disease we were able to consider a range of scales to analyze different reinfestation scenarios, but the spatial resolution of our data remained the same (at the household and structure levels), enabling us to determine the processes and mechanisms underlying the reinfestation process in different rural communities. Indeed, fine scale resolution data, when available, can provide detailed information on the processes responsible for disease clustering, allowing disease control agencies to target the sources of disease and to improve human health more efficiently. However, data and decision often take place on a much coarser resolution, and more general mechanisms may not be inferred from such fine resolution data. We are currently testing some of our findings on Ministry of Health data for a much larger administrative unit (the Department of Moreno in the Province of Santiago del Estero) (Vazquez-Prokopec et al., unpublished data).
Schistosomiasis in coastal Kenya
In our study of the distribution and impact of Schistosoma haematobium in Msambweni, coastal Kenya, we were interested first in the question of where, when and why human infections are clustered.
Infection with S. haematobium is highly prevalent (90% of school-aged children are infected in some villages) in Mswambweni Division (Kwale District, Coast Province, Kenya), although <1% of the intermediate host snails (Bulinus nasutus) in the area are ever found shedding S. haematobium cercariae. To understand why prevalence of infection is so high, despite low rates of shedding by intermediate host, we studied the infection pattern of humans and the hydrology of the area. We initially studied the spatial structure of infection patterns by considering infection levels of school-aged children in a single village (Milalani), covering an area of ∼2.5 km2 and located near one pond (Nimbodze Pond, which had the highest numbers of intermediate host snails found shedding in 2001, Fig. 5) (Clennon et al., 2004). We then considered a larger extent and examined how infection patterns varied throughout Msambweni in a ten village area (∼25 km2; Clennon et al., in press). We also mapped a variety of water sources that the residents use (ponds, spring fed rivers and a stream, in addition to manmade open wells and boreholes). Household and water use site locations were then entered into a GIS, and linked to demographic, parasitological, malacological, and environmental data.
Fig. 5.
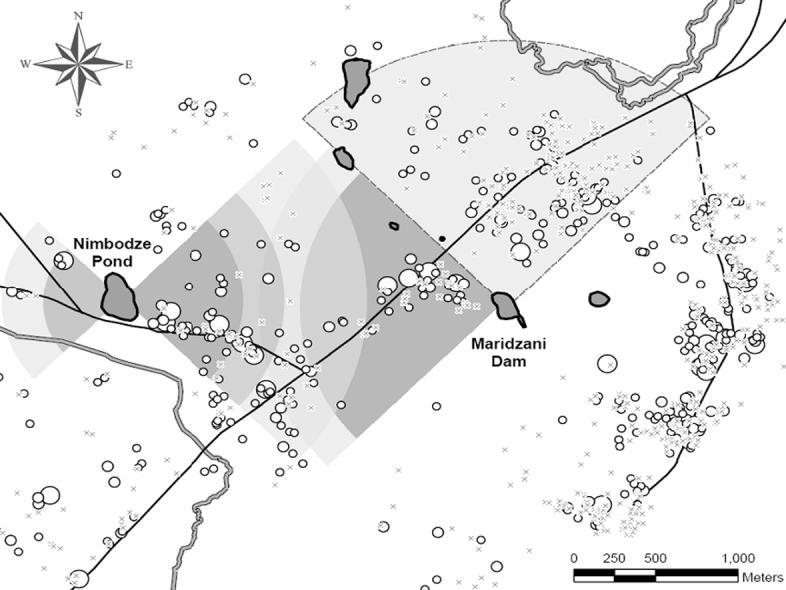
Positive and negative clustering of human infection with Schistosoma haematobium in Msambweni, Kenya. Negative clustering occurs among children 6-9 years-old in the NW near the river where no cercaria-shedding snails are found (light grey with dashed border); positive clustering occurs to the east of Nimbodze pond and to the west of Maridzani pond in the non-bordered gray areas near these ponds (clustering decreasing with reduced intensity of color). Size of circles is proportional to infection density.
Using global (weighted K-function), local (Gi*(d)) and focal spatial analyses (Gi(d)), Clennon et al. (2004) examined the spatial structure of S. haematobium infection patterns and identified significant clustering of elevated infection levels among children (ages: <5, 6-9, 10-13, 14-17, and 18-21 years-old) in Milalani Village. Because no global clustering was identified using a weighted K-function analysis (Getis, 1984), local Gi(d) and Gi*(d) spatial statistics were applied. While Ord and Getis (1995) introduced the use of the local Gi*(d) as a focal statistic among locations of a similar type (all counties), Clennon et al. (2004) demonstrated that by using Gi(d) and incorporating a “source” location within the household location matrix, clustering of household infection density could be associated with a known human water contact site where high numbers of shedding intermediate host snails can be found. Significant local clustering of elevated infection levels was detected only on the eastside of Nimbodze Pond, and was most prominent for 6-13 year-olds. Clustering of infection in children <5 years-old was highly significant very close to the pond, whereas clustering for 10-13 year-olds did not occur until 550 m away from the pond. The relationship between age, household distance to Nimbodze Pond, and clustering of high infection suggests that children <6 year-old who live close to Nimbodze Pond have increased exposure to cercariae infested waters, whereas children living farther away are not exposed until later in life.
Moving upscale to consider the entire Msambweni area with eight water sources, we assessed the spatial patterns of household S. haematobium infection density among school-aged children, and compared recent data with a historical cohort (Muchiri et al., 1996) as well as the distribution patterns of cercariae-shedding snails (Kariuki et al., 2004). Global, local and focal spatial analyses were again applied, but now a directional application of the Gi(d) focal statistic was also used (Fig. 5). The application of focal spatial statistics allowed us to assess transmission levels around a river with no intermediate host snails and ponds with various levels of cercarial contamination. Directional focal statistics (e.g. Score Test, Diggle’s) compare decay models of infection prevalence from a single source and do not allow one to assess how spatial dependency changes across different distances. Also, they do not allow the assessment of interactions from multiple foci.
Combined results from global and local spatial analyses indicate that the process driving the spatial pattern of infection in the area is intrinsically non-stationary. When directionality was considered around known S. haematobium transmission foci, differences in clustering by direction were detected. The majority of clustering was found to be flanked by two transmission foci (Nimbodze Pond and Maridzani Dam, Fig. 5). A change in the local and focal clustering patterns between 1984 and 2000 was found, suggesting a shift in the principal transmission source in Msambweni, and possible changes in intermediate host snail populations or water contacts.
Our findings suggest that infection levels of human urinary schistosomiasis are clustered as a function of the spatial distribution of infested water sources, as well as non-infested ones, such as the river (Fig. 5). By comparing recent and historical data, a change in the primary source of S. haematobium transmission was recognized, which could be related to the El ño flooding during 1997-98 and/or the conversion of sugar cane fields to rice fields during the 1990s.
Once we explained where, when and (partially) why infections are clustered based on snail distribution and on human demography and behavior, and in light of the ongoing drought in our study area, we needed to address a new set of questions:
what happens when there is no transmission?
where do these aquatic snails hide?
how do they come back?
To help answer some of these questions, area-wide aquatic snail shell collection surveys were conducted and a terrain-based hydrological model were integrated with LandSat moisture data to developed a least cost of dispersal model for intermediate host snails from source to sink habitats. Then, a tassel-cap transformed Ikonos imagery and a LandSat moisture stress image were used to predict possible locations of aestivating snails. Based on these studies, we concluded that specific aquatic sites were responsible for most of the transmission, that aquatic sites are inter-connected, at least during heavy rains (or through irrigation), and that snail dispersal routes can be determined using remote sensing and field data. Spatially explicit infection patterns and dispersal models allow for improved snail surveillance and can contribute to targeted local control of urinary schistosomiasis.
Conclusion and future research
Regardless of the scale considered in a spatial analysis of disease and risk factors, it is important to remember that, as is the case with most statistical analyses, these can only describe a pattern and changes in a pattern; only if the biology of the system is well-understood, some underlying processes can be inferred (Hay et al., 2000; Kitron, 2000; Malone, 2005; Raso et al., 2006). In the examples described above, the spatial statistical analysis was used to describe changes in dispersion pattern, from which we could infer on the process of dispersal. Indeed, both of these studies are based on long-term (10-20 years) extensive field and experimental work and on strong collaboration with the National Ministries of Health (Division of Vector-borne Diseases in Kenya and the National Vector Control Program in Argentina). While in the examples described above, we have emphasized the advantages of considering fine resolution data, other studies have successfully applied spatial tools to regional, national or even continental level data (Snow et al., 1999, Snow et al., 2005; Carbajo et al., 2001; Dumonteil and Gourbiere, 2004; Guerra et al., 2006). General questions that need to be considered in geospatial health studies include the following:
what are the best criteria for selecting the spatial (and temporal) unit of intervention and analysis?
how do the key measures of risk and transmission dynamics vary with scale?
how do we integrate processes occurring at diverse spatial and temporal scales?
are we uncovering new relevant information or covering up the lack of data with massive environmental correlates?
how do we decide which environmental or climate changes to follow (the most important as far as actual and potential health impacts)?
how do we move beyond considering disease (or its absence) to considering overall human welfare and the factors contributing to it?
All of these questions can only be addressed through solid biological, epidemiological and socioeconomic understanding of the system in time and space. When using remotely sensed data one always has to find a compromise between spatial and temporal resolution. As far as whether to go upscale (extrapolate) or downscale (interpolate), we quote Levins (1968), who stated, “the detailed analysis of a model for purposes other than that which it was constructed may be as meaningless as studying a map under a microscope.”
Acknowledgments
The writing of this review and the research described in the examples was supported by awards from the NIH/NSF Ecology of Infectious Disease program (TW05836 from the Fogarty International Center and the National Institute of Environmental Health Sciences, and TW/ES01543 from the Fogarty International Center).
References
- Beck L, Bobo M, Kitron U. Remote sensing, GIS and landscape ecology: means for studying disease and global change. In: Martens P, McMichael T, editors. Environmental Change, Climate and Health: Issues and Research Methods. Cambridge University Press; 2002. pp. 226–252. [Google Scholar]
- Beck LR, Lobitz BM, Wood BL. Remote sensing and human health: New sensors and new opportunities. Emerg Infect Dis. 2000;6:217–227. doi: 10.3201/eid0603.000301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besag J, Newell J. The detection of clusters in rare diseases. J Roy Stat Soc, Ser A. 1991;154:143–155. [Google Scholar]
- Carbajo AE, Schweigmann N, Curto SI, Garín A, Bejarán R. Dengue transmission risk maps of Argentina. Trop Med Int Health. 2001;6:170–183. doi: 10.1046/j.1365-3156.2001.00693.x. [DOI] [PubMed] [Google Scholar]
- Cecere MC, Vazquez-Prokopec GM, Gürtler RE, Kitron U. Spatio-temporal analysis of reinfestation by Triatoma infestans (hemiptera: reduviidae) following insecticide spraying in a rural community in northwestern argentina. Am J Trop Med Hyg. 2004;71:803–810. [PMC free article] [PubMed] [Google Scholar]
- Cecere MC, Vazquez-Prokopec GM, Gürtler RE, Kitron U. Reinfestation sources for Chagas disease vector, Triatoma infestans, Argentina. Emerg Infect Dis. 2006;12:1096–1102. doi: 10.3201/eid1207.051445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadee DD, Kitron U. Malaria surveillance in Trinidad: imported cases and risk of outbreaks. Am J Trop Med Hyg. 1999;61:513–517. doi: 10.4269/ajtmh.1999.61.513. [DOI] [PubMed] [Google Scholar]
- Clennon JA, King CH, Muchiri EM, Kariuki HC, Ouma JH, Mungai P, Kitron U. Spatial patterns of urinary schistosomiasis infection in a highly endemic area of coastal Kenya. Am J Trop Med Hyg. 2004;70:443–448. [PubMed] [Google Scholar]
- Clennon JA, Mungai P, Muchiri EM, King CH, Kitron U. Spatial and temporal variations in local transmission of Schistosoma haematobium in Msambweni, Kenya. Am J Trop Med Hyg. in press. [PubMed] [Google Scholar]
- Cromley EK, McLafferty SL. GIS and Public Health. The Guilford Press; 2002. [Google Scholar]
- Diggle PJ. A point process modeling approach to raised incidence of a rare phenomenon in the vicinity of a prespecified point. J Roy Stat Soc. 1990;153:349–362. [Google Scholar]
- Diggle PJ, Rowlinson BS. A conditional approach to point process modeling of elevated risk. J Roy Stat Soc. 1994;157:433–440. [Google Scholar]
- Dumonteil E, Gourbiere S. Predicting Triatoma dimidiata abundance and infection rate: A risk map for natural transmission of Chagas disease in the Yucatan Peninsula of Mexico. Am J Trop Med Hyg. 2004;70:514–519. [PubMed] [Google Scholar]
- Gatrell AC, Bailey TC, Diggle PJ, Rowlingson BS. Spatial point pattern analysis and its application in geographical epidemiology. Trans Inst Brit Geog. 1996;21:256–274. [Google Scholar]
- Getis A. Interaction modeling using second-order analysis. Env Plan A. 1984;16:173–183. [Google Scholar]
- Getis A, Franklin J. Second-order neighborhood analysis of mapped point patterns. Ecology. 1987;68:473–477. [Google Scholar]
- Getis A, Morrison AC, Gray K, Scott TW. Characteristics of the spatial pattern of the dengue vector, Aedes aegypti, in Iquitos, Peru. Am J Trop Med Hyg. 2003;6:494–505. [PubMed] [Google Scholar]
- Getis A, Ord JK. Local Spatial Statistics: An Overview. In: Longley P, Batty M, editors. Spatial analysis: modeling in a GIS environment. Geoinformation International; Cambridge: 1996. pp. 261–277. [Google Scholar]
- Guerra CA, Snow RW, Hay SI. A global assessment of closed forests, deforestation and malaria risk. Ann Trop Med Parasit. 2006;100:189–204. doi: 10.1179/136485906X91512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay SI. An overview of remote sensing and geodesy for epidemiology and public health application. Adv Parasit. 2000;47:1–35. doi: 10.1016/s0065-308x(00)47005-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay SI, Randolph SE, Rogers DJ. Adv Parasit. Vol. 47. London Academic Press; 2000. Remote sensing and geographical information systems in epidemiology. [Google Scholar]
- Hess GR, Randolph SE, Arneberg P, Chemini C, Furlanello C. Spatial aspects of disease dynamics. In: Hudson PJ, Rizzoli A, Grenfell BT, Heesterbeek H, Dobson AP, editors. The Ecology of Wildlife Diseases. Oxford University Press; 2002. pp. 102–118. [Google Scholar]
- Holmes EE. Basic epidemiological concepts in a spatial context. In: Tilman D, Kareiva P, editors. Spatial Ecology: the role of space in population dynamics and interspecific interactions. Princeton Univ. Press; 1997. [Google Scholar]
- Kariuki CH, Clennon JA, Brady MS, Kitron U, Sturrock RF, Hoffman O, Hamburger J, Ouma J, Tosha S, Ndzovu M, Mungai P, Pellegrini C, Muchiri E, King CH. Distribution patterns and cercarial shedding of Bulinus nasutus and other snails in Msambweni Area, Coast Province. Kenya. Am J Trop Med Hyg. 2004;70:449–456. [PubMed] [Google Scholar]
- Kitron U. Landscape ecology and epidemiology of vector-borne diseases: tools for spatial analysis. J Med Entomol. 1998;35:435–445. doi: 10.1093/jmedent/35.4.435. [DOI] [PubMed] [Google Scholar]
- Kitron U. Risk maps: Transmission and burden of vector-borne diseases. Parasitol Today. 2000;16:324–325. doi: 10.1016/s0169-4758(00)01708-7. [DOI] [PubMed] [Google Scholar]
- Kitron U, Jones CJ, Bouseman JK, Nelson JA, Baumgartner DL. Spatial analysis of the distribution of Ixodes dammini (Acari:Ixodidae) on white-tailed deer in Ogle county, Illinois. J Med Entomol. 1992;29:259–266. doi: 10.1093/jmedent/29.2.259. [DOI] [PubMed] [Google Scholar]
- Kitron U, Michael J, Swanson J, Haramis L. Spatial analysis of the distribution of LaCrosse encephalitis in Illinois, using a geographic information system and local and global spatial statistics. Am J Trop Med Hyg. 1997;57:469–475. doi: 10.4269/ajtmh.1997.57.469. [DOI] [PubMed] [Google Scholar]
- Kleinschmidt I, Clarke GPY, Bagayoko M, Craig MH, le Sueur D. A spatial statistical approach to malaria mapping. Int J Epid. 2000;29:355–361. doi: 10.1093/ije/29.2.355. [DOI] [PubMed] [Google Scholar]
- Lawson AB. Wiley Series in Probability and Statistics. John Wiley & Sons; 2001. Statistical Methods in Spatial Epidemiology; p. 298. [Google Scholar]
- Levin S. The problem of pattern and scale in ecology. Ecology. 1992;73:1943–1967. [Google Scholar]
- Levins R. Evolution in changing environments. Princeton University Press; 1968. p. 120. [Google Scholar]
- Malone JB. Biology-based mapping of vector-borne parasites by Geographic Information Systems and Remote Sensing. Parassitologia. 2005;47:27–50. [PubMed] [Google Scholar]
- Mapping malaria risk in Africa MARA/ARMA. http://www.mara.org.za/
- Morris SE, Wakefield JC. Assessment of disease risk in relation to a pre-specified source. In: Elliot P, Wakefield J, Best N, Briggs D, editors. Spatial epidemiology. Methods and applications. Oxford University press; 2000. pp. 153–184. [Google Scholar]
- Morrison AC, Getis A, Santiago M, Rigau-Perez JG, Reiter P. Exploratory space-time analysis of reported dengue cases during an outbreak in Florida, Puerto Rico, 1991-1992. Am J Trop Med Hyg. 1998;58:287–298. doi: 10.4269/ajtmh.1998.58.287. [DOI] [PubMed] [Google Scholar]
- Muchiri EM, Ouma JH, King CH. Dynamics and control of Schistosoma haematobium transmission in Kenya: an overview of the Msambweni Project. Am J Trop Med Hyg. 1996;55:127–134. doi: 10.4269/ajtmh.1996.55.127. [DOI] [PubMed] [Google Scholar]
- Ord JK, Getis A. Local spatial autocorrelation statistics: distributional issues and an application. Geographical Analysis. 1995;27:286–306. [Google Scholar]
- Qi Y, Wu J. Effects of changing spatial resolution on the results of landscape pattern analysis using spatial autocorrelation indices. Landscape Ecol. 1996;11:39–49. [Google Scholar]
- Raso G, Vounatsou P, Singer BH, N’Goran EK, Tanner M, Utzinger J. An integrated approach for risk profiling and spatial prediction of Schistosoma mansoni-hookworm coinfection. PNAS. 2006;103:6934–6939. doi: 10.1073/pnas.0601559103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TP. Spatial statistics and geographical information systems in epidemiology and public health. Adv Parasit. 2000;47:82–120. doi: 10.1016/s0065-308x(00)47007-7. [DOI] [PubMed] [Google Scholar]
- Snow RW, Craig MH, Deichmann U, le Sueur D. A preliminary continental risk map for malaria mortality among African Children. Parasit Today. 1999;15:99–104. doi: 10.1016/s0169-4758(99)01395-2. [DOI] [PubMed] [Google Scholar]
- Snow RW, Guerra CA, Noor AM, Myint HY, Hay SI. The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature. 2005;434:214–217. doi: 10.1038/nature03342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner MG, O’Neill RV, Gardner RH, Milne BT. Effects of changing spatial scale on the analysis of landscape pattern. Landscape Ecol. 1989;3:153–162. [Google Scholar]
- Vazquez-Prokopec GM, Cecere MC, Canale DM, Gürtler RE, Kitron U. Spatiotemporal patterns of reinfestation by Triatoma guasayana (Hemiptera: Reduviidae) in a rural community of north-western Argentina. J Med Entomol. 2005;42:571–581. doi: 10.1093/jmedent/42.4.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiens JA. Spatial scaling in ecology. Function Ecol. 1989;3:385–397. [Google Scholar]