Abstract
The aim of the study was to examine the mechanisms by which ACh, acting via m2 receptors, regulates GRK2-mediated VPAC2 receptor desensitization in gastric smooth muscle cells. VIP induced VPAC2 receptor phosphorylation and internalization in freshly dispersed smooth muscle cells. Co-stimulation with acetylcholine (ACh), in the presence of m3 receptor antagonist, 4-DAMP, augmented VPAC2 receptor phosphorylation and internalization. The m2 receptor antagonist methoctramine or the c-Src inhibitor PP2 blocked the effect of ACh, suggesting that the augmentation was mediated by c-Src, derived from m2 receptor activation. ACh induced activation of c-Src and phosphorylation of GRK2 and the effects of ACh were blocked by methoctramine, PP2, or by uncoupling of m2 receptors from Gi3 with pertussis toxin. In conclusion, we identified a novel mechanism of cross-regulation of GRK2-mediated phosphorylation and internalization of Gs-coupled VPAC2 receptors by Gi-coupled m2 receptors via tyrosine phosphorylation of GRK2 and stimulation of GRK2 activity.
Keywords: Smooth muscle, receptor desensitization, vasoactive intestinal peptide, GRK2 phosphorylation
Smooth muscle of the gastrointestinal tract exhibits tone on which rhythmic contractions are superimposed. Both, the frequency and amplitude of rhythmic contractions and the muscle tone, are modulated by excitatory and inhibitory neural inputs from the myenteric plexus of the enteric nervous system. Excitatory transmitters consist of acetylcholine (ACh) and tachykinins, and inhibitory transmitters consist of vasoactive intestinal peptide (VIP), pituitary adenylyl cyclase-activating peptide (PACAP), and nitric oxide (NO) [1, 2]. About 30% of the myenteric neurons contain VIP, PACAP, and the NO synthase, the enzyme responsible for generation of neural NO. About 40% of the neurons contain ACh and tachykinins. There is no overlap between these groups of nerve fibers and the fibers project into muscle layers. Excitatory neurons mainly release acetylcholine, whereas inhibitory neurons co-release VIP/PACAP and NO [1, 2].
On the smooth muscle of the gastrointestinal tract, ACh interacts with two muscarinic receptors to activate distinct signaling pathways[3–5]. The more abundant (~80%) m2 receptors are coupled to inhibition of adenylyl cyclase and activation of PI 3-kinase/c-Src pathways via α and β γ subunits of pertussis toxin (PTx)-sensitive G protein Gi3 [3]. m3 receptors are coupled to activation phospholipase C (PLC-β1) and RhoA via Gαq and Gα13, respectively, to mediate initial Ca2+-dependent and sustained Ca2+-independent MLC20 phosphorylation and muscle contraction [4].
VIP and PACAP interact with three types of receptors that belong to the family of class II G protein-coupled receptors: VPAC1 and VPAC2 receptors, which possess equal affinity for VIP and PACAP, and PAC1 receptors, which possess high affinity for PACAP only [6–9]. Smooth muscle cells of the gut express predominantly VPAC2 receptors, but not VPAC1 or PAC1 receptors [7]. VPAC2 receptors are coupled to stimulation of adenylyl cyclase, cAMP formation, cAMP-dependent protein kinase (PKA) activation, and muscle relaxation [10]. VPAC2 receptor regulation appears to be distinct from VPAC1 and PAC1 receptors and from other members of the class II G protein-coupled receptors such as secretin [11–14]. We have recently shown that VPAC2 receptor phosphorylation, internalization, and desensitization are exclusively mediated by G protein-coupled receptor kinase 2 (GRK2) [15]. VPAC2 receptor phosphorylation internalization and desensitization, however, are regulated by PKA in a feedback mechanism. PKA phosphorylates GRK2 at serine 685 and enhances its ability to mediate VPAC2 receptor phosphorylation, internalization, and desensitization [15, 16].
Most studies on receptor regulation have used GRK2 as a prototype kinase that phosphorylates many G protein-coupled receptors [16–18]. The activity and localization of GRK2 are regulated by phosphorylation by kinases such as PKA, protein kinase C (PKC), c-Src, and extracellular regulated kinases (ERK1/2) and binding to signaling molecules such as phosphatidylinositol 4,5-bisphosphate, calmodulin, caveolin, and Gβ γ subunits [14, 16, 18–27]. The interaction of Gβ γ with GRK2 assists the kinase to target to the membrane and stimulates its catalytic activity toward the receptor. We postulate that concomitant activation of muscarinic m2 receptors modulates VPAC2 receptor phosphorylation and desensitization, and we demonstrate that activation of c-Src by Gi3-coupled m2 receptors induces phosphorylation of GRK2 resulting in augmentation of GRK2-mediated VPAC2 receptor phosphorylation and internalization.
MATERIALS AND METHODS
Dispersion and culture of gastric smooth muscle cells
Smooth muscle cells were isolated from the circular muscle layer of rabbit stomach by sequential enzymatic digestion, filtration, and centrifugation as described previously [4, 5, 10]. Briefly, smooth muscle strips were incubated at 31°C for 20 min in HEPES medium containing type II collagenase (0.1%) and soybean trypsin inhibitor (0.1%). Muscle cells were harvested by filtration through 500 μM Nitex and centrifuged twice at 350 g for 10 min.
[125I]VIP binding to smooth muscle cells
Binding of [125I]VIP to freshly dispersed muscle cells was done as described previously [28]. Cell aliquots (2 × 106 cell/ml) were incubated for 60 min at 4° C with 50 pM [125I]VIP, followed by separation of bound and free radioligands by rapid filtration through 5 μm polycarbonate Nucleophore filters. Non-specific binding was calculated as the amount of radioactivity in the presence of 10 μM of VIP. [125I]VIP binding was measured in the control cells and in cells pretreated for 30 min with 1 μM VIP to induce VPAC2 receptor internalization. VIP was added alone or in the presence of ACh (0.1 μM) plus m3 receptor antagonist diphenylacetoxy-N-methylpiperidine (4-DAMP, 0.1 μM) to co-activate m2 receptors.
Phosphorylation of VPAC2 receptor and GRK2
Phosphorylation of VPAC2 receptor, or GRK2 was measured in cells labeled with 32P followed by immunoprecipitation with antibody to VPAC2 receptor or GRK2. Samples (3 × 106 ml) were incubated with various concentrations of VIP (1 pM to 1 μM) for 5 min and homogenized in lysis buffer (10 mM Tris (pH 7.5), 50 mM NaCl, 1% Triton X-100, and 60 mM octyl glucoside). For cross-regulation, samples were incubated with sub-maximal concentrations of VIP (10 nM) in the presence or absence of 0.1 μM ACh (plus the m3 receptor antagonist 4-DAMP). The cell lysates were incubated with antibody to VPAC2 receptor or GRK2 for 2 h at 4°C and with 40 μl of protein A-Sepharose for another 1 h. The immunoprecipitates were extracted with Laemmli sample buffer, boiled for 5 min, and separated by SDS-PAGE. After transfer to nitrocellulose membranes, [32P]GRK2 or [32P]VPAC2 receptor was visualized by autoradiography, and the amount of radioactivity in the protein band was measured. The results were expressed as counts per minute [29].
GRK2 phosphorylation was also measured in non-labeled cells using phospho-tyrosine antibody in GRK2 immunoprecipitates. GRK2 immunoprecipitates were separated by SDS-PAGE, transferred to PVDF membrane, and probed with an antibody to the phospho-tyrosine residue. After incubation with a secondary antibody, the proteins were visualized using enhanced chemiluminescence.
Activation of c-Src
Activation of c-Src was measured by Western blot analysis. using a phospho-Src (Tyr527) antibody. Dispersed muscle cells were treated with ACh plus 4-DAMP in the presence or absence of a selective c-Src inhibitor, PP2 (1 μM), and solubilized on ice for 2 h in 20 mM Tris/HCl medium containing 1 mM DTT, 100 mM NaCl, 0.5% SDS, 1 mM PMSF, 10 μg/ml leupeptin, and 100 μg/ml aprotinin. The proteins were resolved by SDS-PAGE and transferred electrophoretically to PVDF membrane. The membranes were incubated for 12 h with phospho-Src (Tyr527) antibody and then for 1 h with horseradish peroxidase-conjugated secondary antibody. The bands were identified by enhanced chemiluminiscence.
Materials
[125I]VIP and [32P]orthophosphate were obtained from NEN Life Sciences Products (Boston, MA); antibodies to VPAC2 receptors, GRK2, c-Src, phospho-c-Src (Tyr527), and phospho-tyrosine were obtained from Santa Cruz Biotechnologies (Santa Cruz, CA); Western blotting and chromatography materials were obtained from Bio-Rad Laboratories (Hercules, CA); collagenase and soybean trypsin inhibitor were obtained from Worthington Biochemical (Freehold, NJ); all other reagents were from Sigma.
Statistical analysis
All values are expressed as means ± SE; n represents the number of animal studies. Regression analysis was performed using GraphPad Prism® 4. Statistical analysis was performed by unpaired t-test and P< 0.05 was considered statistically significant.
RESULTS
Concentration-dependent phosphorylation and internalization of VPAC2 receptors by VIP
Treatment of freshly dispersed smooth muscle cells with VIP induced phosphorylation of VPAC2 receptor in a concentration-dependent fashion with an EC50 of 4±3 nM and maximal phosphorylation was obtained with 1 μM VIP (Fig. 1). VPAC2 receptor internalization was assessed by the decrease in 125I-VIP binding to the surface receptors after treatment with VIP. Pretreatment of cells with different concentration VIP (1 pM to 1 μM) caused a decrease in [125I]VIP binding to surface receptors, suggesting agonist-induced internalization of VPAC2 receptors. The effect of VIP on receptor nternalization was concentration-dependent with an EC50 of 8±5 nM and maximal internalization was obtained with 1 μM VIP (Fig. 1). The concentration-response curve for VPAC2 receptor phosphorylation was similar to that for VPAC2 receptor internalization, suggesting that the extent of receptor phosphorylation was related to the extent of receptor internalization.
Figure 1. Normalized concentration-response curves for VPAC2 receptor phosphorylation and internalization in freshly dispersed gastric smooth muscle cells.
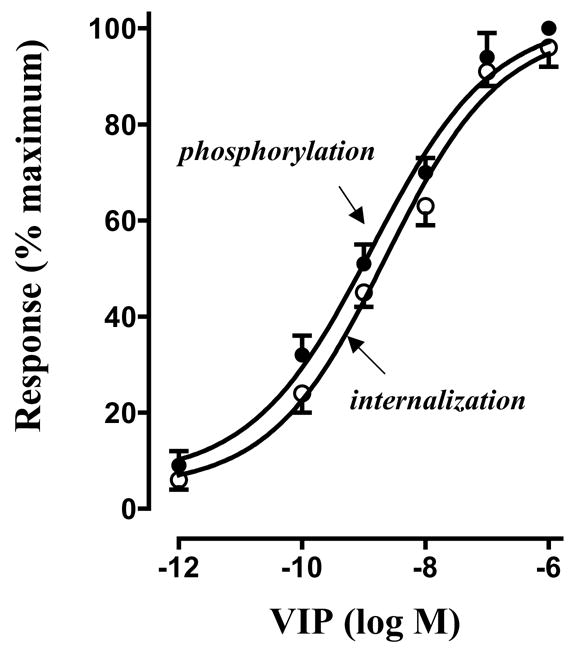
VPAC2 receptor phosphorylation in response to various concentrations of VIP (1 pM to 1 μM) was measured in cells labeled with 32P. Immunoprecipitates derived from 500 μg of protein using VPAC2 receptor antibody were separated on SDS-PAGE. Bands corresponding to VPAC2 receptor (p-VPAC2 R) were identified by autoradiography. Radioactivity in the bands was expressed as counts per minute (cpm). VPAC2 receptor internalization was assessed by the decrease in 125I-VIP binding to the surface receptors after pretreatment with VIP (1 pM to 1 μM). Non-specific binding was determined as binding of [125I]VIP in the presence of 10 μM unlabeled VIP. Specific [125I]VIP binding was expressed as counts per minute. Response to 1 μM VIP calculated from the regression curve was defined as 100% for each response. Values are expressed as means ± SE of four experiments.
Augmentation of VPAC2 receptor phosphorylation by m2 receptor via c-Src
Treatment of dispersed smooth muscle cells with VIP (10 nM) induced significant phosphorylation of VPAC2 receptors. Co-treatment of muscle cells with ACh in the presence of m3 antagonist 4-DAMP (i.e., by activation of m2 receptors) significantly (P<0.01) enhanced the effect of VIP on VPAC2 receptor phosphorylation (Fig. 2). The stimulatory effect of ACh was blocked by the m2 receptor antagonist, methoctramine (0.1 μM), or by the c-Src inhibitor, PP2 (1 μM) (Fig. 2). ACh alone in the absence of VIP had no effect on VPAC2 receptor phosphorylation.
Figure 2. Stimulation VPAC2 receptor phosphorylation by m2 receptor activation via c-Src.
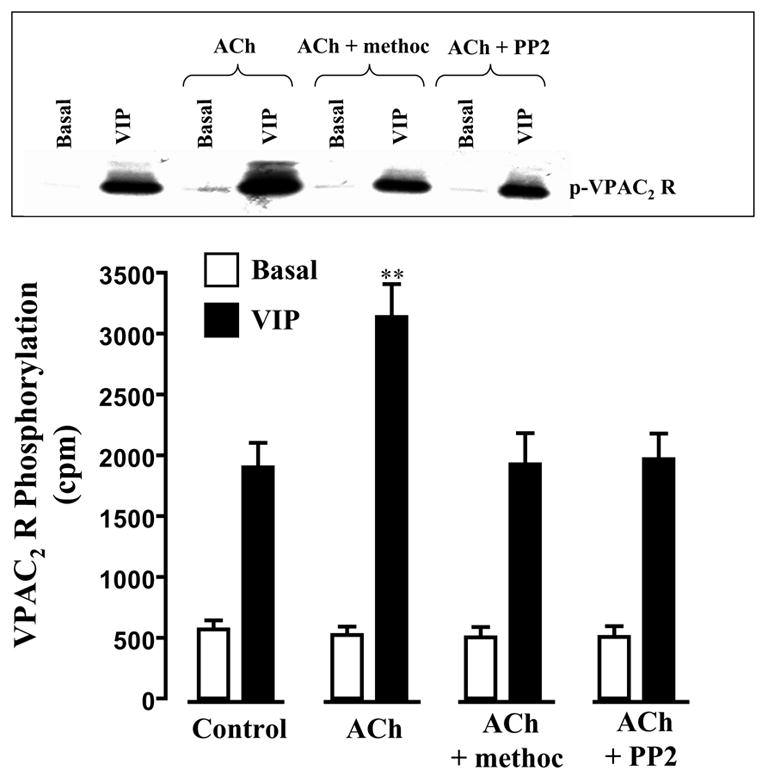
Gastric smooth muscle cells labeled with 32P were incubated with VIP (10 nM) or VIP plus ACh (0.1 μM plus m3 receptor antagonist 4-DAMP) for 5 min in the absence or presence of m2 receptor antagonist methoctramine (0.1 μM) or c-Src inhibitor PP2 (1 μM). VPAC2 receptor phosphorylation was measured as described in the Methods. Immunoblot analysis showed equal amounts of loaded protein (not shown). Values are means ± SE of four experiments. ** P<0.01 significant increase in VPAC2 receptor phosphorylation in the presence of ACh compared with phosphorylation by VIP alone
Augmentation of VPAC2 receptor internalization by m2 receptor via c-Src
Pretreatment of cells with 1 nM VIP caused a significant decrease (43±4 % decrease) in the binding of [125I]VIP to the surface receptors (Fig. 3). Co-treatment of cells with ACh in the presence of m3 antagonist 4-DAMP caused a further decrease (79±6 % decrease, P<0.01) in [125I]VIP binding. The effect of ACh was blocked by methoctramine (01. μM) or PP2 (1 μM) (Fig. 3). ACh alone, in the absence of VIP, had no effect on [125I]VIP binding. These results suggest that co-activation of m2 receptors augments VPAC2 receptor internalization via c-Src.
Figure 3. Stimulation of VPAC2 receptor internalization by m2 receptor activation via c-Src.
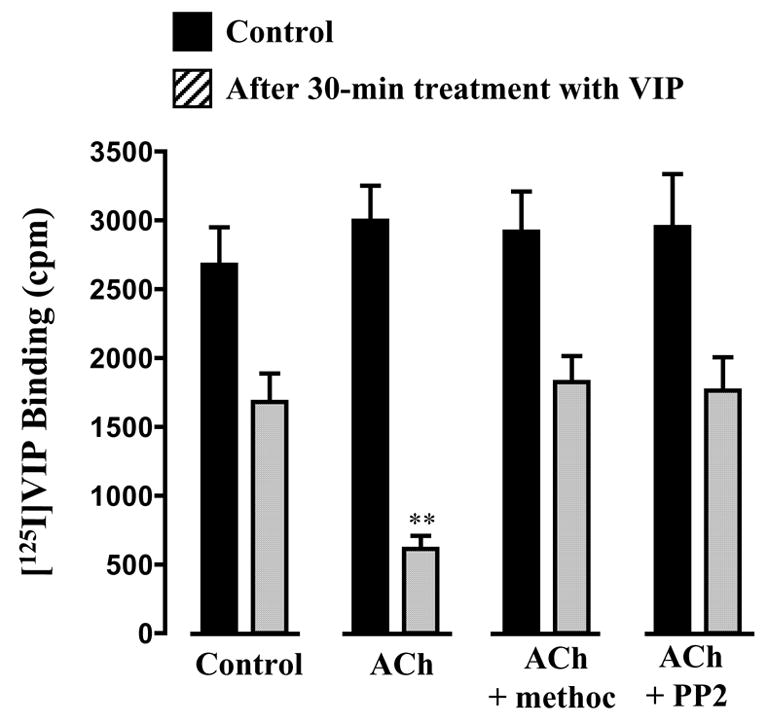
Freshly dispersed gastric muscle cells were incubated for 30 min with VIP (10 nM) or VIP plus ACh (0.1 μM plus m3 receptor antagonist 4-DAMP) in the absence or presence of methoctramine (0.1 μM) or PP2 (1 μM). After washing, muscle cells were incubated for 60 min at 4°C with 50 pM [125I]VIP. VPAC2 receptor internalization was assessed as described in the methods. Values are expressed as means ± SE of four experiments. ** P<0.001 significant increase in VPAC2 receptor internalization in the presence of ACh compared with internalization with VIP alone.
Phosphorylation of GRK2 by m2 receptor via c-Src
Previous studies have shown that agonist-induced VPAC2 receptor phosphorylation and internalization were blocked in cells expressing dominant negative GRK2(K220R), suggesting that phosphorylation and internalization were mediated exclusively by GRK2 [15]. To elucidate the possible role of c-Src-mediated phosphorylation of GRK2 in the augmentation of VPAC2 receptor internalization, we measured c-Src activation and c-Src-mediated GRK2 phosphorylation in response to m2 receptor activation.
ACh induced activation of c-Src, measured as phosphorylation of tyrosine 527 using phospho-specific (Phospho-Src (Tyr527)) antibody, in smooth muscle cells. The effect of ACh on c-Src activity was blocked by methoctramine, PTx or PP2, suggesting that activation of c-Src was mediated by the m2 receptor via a PTx-sensitive G protein, Gi3 (Fig. 4). ACh also induced phosphorylation of GRK2 measured in GRK2 immunoprecipitates in metabolically labeled cells and in non-labeled cells using a phospho-tyrosine antibody. Phosphorylation of GRK2 was blocked by methoctramine, PTx, or PP2 (Fig. 5). The results imply that activation of c-Src by m2 receptors leads to phosphorylation of GRK2.
Figure 4. Activation of c-Src by m2 receptors.
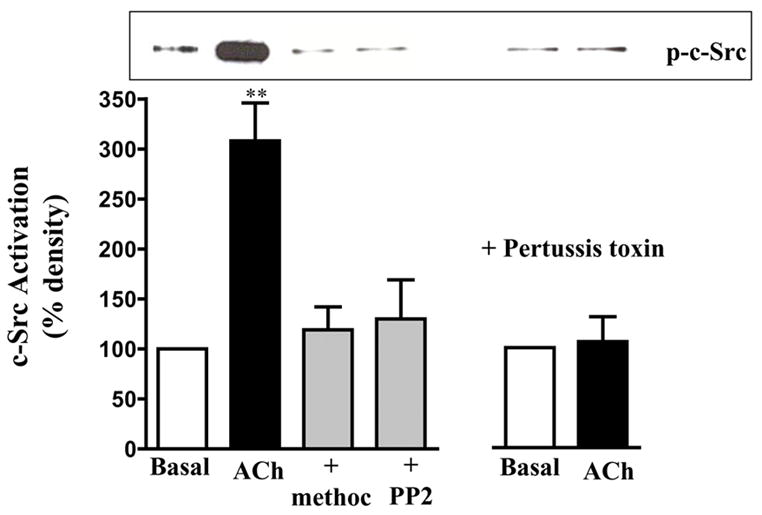
Gastric muscle cells were treated with ACh (0.1μM) in the presence or absence of methoctramine (0.1 μM) or PP2 (1 μM) for 5 min and activation of c-Src (p-c-Src) was measured using phospho-Src (Tyr527) antibody. Pertussis toxin (400 ng/ml) was added for 60 min and then stimulated with ACh. Immunoblot analysis showed equal amounts of loaded protein (not shown). Values are expressed as means ± SE of four experiments. ** P<0.001 significant increase in c-Src activation by ACh.
Figure 5. Phosphorylation of GRK2 by c-Src.
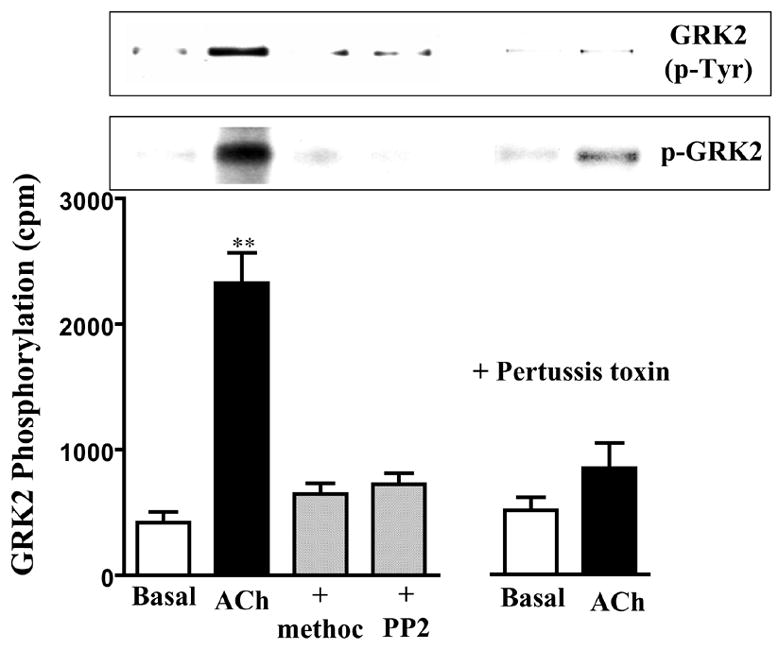
Gastric smooth muscle cells labeled with 32P were incubated with ACh (0.1μM) in the presence of absence of methoctramine (0.1 μM) or PP2 (1 μM) for 5 min. Pertussis toxin (400 ng/ml) was added for 60 min and then stimulated with ACh. Immunoprecipitates derived from 500 μg of protein using GKR2 antibody were separated on SDS-PAGE. Bands corresponding to GRK2 (p-GRK2) were identified by autoradiography. Radioactivity in the bands was expressed as counts per minute (cpm). Immunoblot analysis showed equal amounts of loaded protein (not shown). GRK2 phosphorylation was also measured in non-labeled cells using phospho-tyrosine antibody in GRK2 immunoprecipitates (GRK2(p-Tyr)). Values are means ± SE of four experiments. ** P<0.01 significant increase in GRK2 phosphorylation by ACh.
DISCUSSION
Although the peristalsis of the gastrointestinal tract involves sequential contractions and relaxations mediated by excitatory (e.g., ACh) and inhibitory transmitters (e.g., VIP), signaling by the neurotransmitters often overlaps or develops concurrently. The present study characterized the cross-regulation of VIP-induced VPAC2 receptor internalization by concurrent activation of predominant m2 receptors by ACh in smooth muscles and provided evidence that co-activation of m2 receptors augmented VPAC2 receptor phosphorylation and internalization. The mechanism involves Gi3-dependent activation of c-Src by m2 receptors leading to tyrosine phosphorylation of GRK2 and stimulation of GRK2-dependent VPAC2 receptor phosphorylation and internalization. The evidence for the mechanism is based on a combination of experimental strategies.
VIP-induced phosphorylation and internalization of VPAC2 receptors were augmented upon concurrent activation of m2 receptors by ACh.
The stimulatory effect of ACh was blocked by the m2 receptor antagonist, methoctramine, PTx, or by the c-Src inhibitor, PP2.
The studies were also complimented by direct measurements of c-Src activity and GRK2 phosphorylation by ACh.
Activation of c-Src and phosphorylation GRK2 by ACh was mediated by the m2 receptors, since methoctramine or PTx blocked both events. Previous studies have shown that methoctramine-sensitive receptors were selectively uncoupled from Gi3 by PTx [4, 5]. GRK2 phosphorylation, in addition, was blocked by PP2 suggesting that co-activation of Gi3-coupled m2 receptors augments the activity GRK2 via c-Src. Recent studies have shown that activation of c-Src by m2 receptors was mediated via Gβγ-dependent activation of PI 3-kinase. c-Src activity was blocked by the PI 3 kinase inhibitor, LY294002, or in cells expressing Gβγ-scavenging peptide [30]. ACh, in the absence of VIP, had no direct effect on VPAC2 receptor phosphorylation, providing evidence that any increase in VPAC2 receptor phosphorylation and internalization by m2 receptors resulted from phosphorylation of GRK2 and stimulation of its activity.
GRK2 is a ubiquitous serine/threonine kinase that phosphorylates agonist occupied G protein-coupled receptors to initiate their internalization and desensitization [26]. Phosphorylation of the receptor by GRK2 allows interaction of β-arrestin with receptor and β-arrestin-mediated recruitment of clathrin, thus initiating receptor internalization, as a prelude to deposphorylation and recycling of the receptor. GRK2 activity was shown to be regulated by phosphorylation on serine residues by other kinases such as PKA, PKC ERK1/2 and on tyrosine residue by kinases such as c-Src [16, 18, 22, 27]. Direct phosphorylation of GRK2 on serine 29 located in the calmodulin binding of GRK2 by PKC leads to an increase in GRK2 activity by eliminating the inhibition exerted by the binding of calmodulin, whereas phosphorylation of Raf-kinase inhibitor protein (RKIP) by PKC and subsequent switching of RKIP binding from its known target, Raf-1 to GRK2, leads to inhibition of GRK2 activity [24, 31]. Phosphorylation on serine 670 by ERK1/2 inhibits GRK2 activity and sensitivity to Gβγ, whereas expression of dominant negative MEK1, the upstream activator of ERK1/2, increases agonist-induced β-adrenergic receptor phosphorylation suggesting inhibition of GRK2 activity by ERK1/2 [26]. GRK2 activity was also regulated by a feedback phosphorylation via PKA derived from activation of Gs-coupled receptors such as β-adrenergic receptors and VPAC2 receptors[15, 16]. Phosphorylation of GRK2 on serine 685 increases its binding to Gβγ and the ability of GRK2 to phosphorylate VPAC2 receptors. Inhibition of PKA activity or expression of PKA phosphorylation-deficient GRK2 (GRK2-S685A) blocked VIP-induced VPAC2 receptor phosphorylation, internalization, and desensitization[15].
Sarngo et al. [27] have reported that GRK2 is a high affinity substrate for non-receptor tyrosine kinase, c-Src, and that agonist-stimulation of β-adrenergic receptors results in rapid increase in GRK2 phosphorylation on tyrosine residues. The concept that GRK2 is one of the targets of c-Src is consistent with GRK2 being the partner of the multi-protein complex formed upon activation of many G protein-coupled receptors [16]. Within this multi-protein complex, c-Src could potentially phosphorylate GRK2 and thereby increase the catalytic activity of GRK2. Phosphorylation by c-Src was shown to promote an increase in the catalytic activity of GRK2 toward an exogenous substrate in vitro as well as receptor phosphorylation in intact cells [23, 27]. The mechanism(s) by which tyrosine phosphorylation affects the activity of GRK2 is not known. Studies by Panella et al. [25] however, using c-Src phosphorylation-d eficient GRK2 (GRK2-Y/13/86/92F), have shown that neither the activity nor the subcellular localization of GRK2 was affected by phosphorylation via c-Src. In a purified protein system, the catalytic activity of GRK2-Y13/86/92F was equivalent to that of wild-type GRK2. In contrast, these studies provided evidence that phosphorylation by c-Src increased GRK2 degradation. The presence of tyrosine kinase inhibitor or expression of GRK2-Y13/86/92F leads to an increase in steady-state GRK2 expression in different cell lines [25]. Stimulation of c-Src activity and phosphorylation of GRK2 by VPAC2 receptor internalization alone does not seem to occur, since c-Src inhibitor in the absence of m2 receptor activation had no effect on VPAC2 receptor phosphorylation and internalization. c-Src activation and augmentation of VPAC2 receptor phosphorylation and internalization were selective for Gi3-coupled m2 receptors and the effects are blocked by blocking m2 receptors, either with a selective antagonist or by uncoupling the receptors from Gi proteins with PTx. Thus, VPAC2 receptor phosphorylation and internalization are cross-regulated by co-activation of m2 receptor, but not by feedback regulation by VPAC2 receptor activation alone.
In summary, the present studies provide evidence for a cross-talk mechanism by which activation of c-Src via Gi-coupled receptors regulates signaling by Gs-coupled receptors. Our studies also suggest that GRK2 could be a potential target for cross-regulation of receptors when there is concurrent activation of c-Src. The results are of particular interest in the context of many studies characterizing the signaling by VIP and PACAP in muscle tissue pre-contracted with contractile agents that could result in the activation of c-Src. In addition, the increase in VPAC2 receptor internalization by m2 receptors could play a role in the attenuation of VIP response during inflammation with augmented m2 receptor function [32].
Acknowledgments
This work was supported by grant DK28300 from the National Institute of Diabetes, and Digestive and Kidney Diseases.
Abbreviations
- GRK
G protein-coupled receptor kinase
- VIP
vasoactive intestinal peptide
- VPAC2
VIP/PACAP receptor 2
- PAGE
polyacrylamide gel electrophoresis
- ACh
acetylcholine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bornstein JC, Costa M, Grider JR. Enteric motor and interneuronal circuits controlling motility. Neurogastroenterol Motil. 2004;16 (Suppl 1):34–8. doi: 10.1111/j.1743-3150.2004.00472.x. [DOI] [PubMed] [Google Scholar]
- 2.Kunze WA, Furness JB. The enteric nervous system and regulation of intestinal motility. Annu Rev Physiol. 1999;61:117–42. doi: 10.1146/annurev.physiol.61.1.117. [DOI] [PubMed] [Google Scholar]
- 3.Zhou H, Das S, Murthy KS. Erk1/2- and p38 MAP kinase-dependent phosphorylation and activation of cPLA2 by m3 and m2 receptors. Am J Physiol Gastrointest Liver Physiol. 2003;284:G472–80. doi: 10.1152/ajpgi.00345.2002. [DOI] [PubMed] [Google Scholar]
- 4.Murthy KS, Zhou H, Grider JR, Brautigan DL, Eto M, Makhlouf GM. Differential signalling by muscarinic receptors in smooth muscle: m2-mediated inactivation of myosin light chain kinase via Gi3, Cdc42/Rac1 and p21-activated kinase 1 pathway, and m3-mediated MLC20 (20 kDa regulatory light chain of myosin II) phosphorylation via Rho-associated kinase/myosin phosphatase targeting subunit 1 and protein kinase C/CPI-17 pathway. Biochem J. 2003;374:145–55. doi: 10.1042/BJ20021274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murthy KS, Makhlouf GM. Differential coupling of muscarinic m2 and m3 receptors to adenylyl cyclases V/VI in smooth muscle. Concurrent M2-mediated inhibition via Gαi3 and m3-mediated stimulation via Gβγ J Biol Chem. 1997;272:21317–24. doi: 10.1074/jbc.272.34.21317. [DOI] [PubMed] [Google Scholar]
- 6.Harmar AJ, Arimura A, Gozes I, Journot L, Laburthe M, Pisegna JR, Rawlings SR, Robberecht P, Said SI, Sreedharan SP, Wank SA, Waschek JA. International Union of Pharmacology. XVIII. Nomenclature of receptors for vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide. Pharmacol Rev. 1998;50:265–70. [PMC free article] [PubMed] [Google Scholar]
- 7.Teng B, Murthy KS, Kuemmerle JF, Grider JR, Makhlouf GM. Selective expression of vasoactive intestinal peptide (VIP)2/pituitary adenylate cyclase-activating polypeptide (PACAP)3 receptors in rabbit and guinea pig gastric and tenia coli smooth muscle cells. Regul Pept. 1998;77:127–34. doi: 10.1016/s0167-0115(98)00112-8. [DOI] [PubMed] [Google Scholar]
- 8.Ulrich CD, 2nd, Holtmann M, Miller LJ. Secretin and vasoactive intestinal peptide receptors: members of a unique family of G protein-coupled receptors. Gastroenterology. 1998;114:382–97. doi: 10.1016/s0016-5085(98)70491-3. [DOI] [PubMed] [Google Scholar]
- 9.Zhou H, Huang J, Murthy KS. Molecular cloning and functional expression of a VIP-specific receptor. Am J Physiol. 2006 doi: 10.1152/ajpgi.00138.2006. In press. [DOI] [PubMed] [Google Scholar]
- 10.Murthy KS, Makhlouf GM. Interaction of cA-kinase and cG-kinase in mediating relaxation of dispersed smooth muscle cells. Am J Physiol. 1995;268:C171–80. doi: 10.1152/ajpcell.1995.268.1.C171. [DOI] [PubMed] [Google Scholar]
- 11.Shetzline MA, Premont RT, Walker JK, Vigna SR, Caron MG. A role for receptor kinases in the regulation of class II G protein-coupled receptors. Phosphorylation and desensitization of the secretin receptor. J Biol Chem. 1998;273:6756–62. doi: 10.1074/jbc.273.12.6756. [DOI] [PubMed] [Google Scholar]
- 12.Shetzline MA, Walker JK, Valenzano KJ, Premont RT. Vasoactive intestinal polypeptide type-1 receptor regulation. Desensitization, phosphorylation, and sequestration. J Biol Chem. 2002;277:25519–26. doi: 10.1074/jbc.M201815200. [DOI] [PubMed] [Google Scholar]
- 13.McDonald TP, Dinnis DM, Morrison CF, Harmar AJ. Desensitization of the human vasoactive intestinal peptide receptor (hVIP2/PACAP R): evidence for agonist-induced receptor phosphorylation and internalization. Ann N Y Acad Sci. 1998;865:64–72. doi: 10.1111/j.1749-6632.1998.tb11164.x. [DOI] [PubMed] [Google Scholar]
- 14.Walker JK, Premont RT, Barak LS, Caron MG, Shetzline MA. Properties of secretin receptor internalization differ from those of the beta(2)-adrenergic receptor. J Biol Chem. 1999;274:31515–23. doi: 10.1074/jbc.274.44.31515. [DOI] [PubMed] [Google Scholar]
- 15.Zhou H, Grider JR, Murthy KS. Agonist-dependent PKA-mediated phosphorylation of GRK2 augments homologous desensitization of VPAC2 receptors in smooth muscle. Gastroenterology. 2002;122:A136. [Google Scholar]
- 16.Cong M, Perry SJ, Lin FT, Fraser ID, Hu LA, Chen W, Pitcher JA, Scott JD, Lefkowitz RJ. Regulation of membrane targeting of the G protein-coupled receptor kinase 2 by protein kinase A and its anchoring protein AKAP79. J Biol Chem. 2001;276:15192–9. doi: 10.1074/jbc.M009130200. [DOI] [PubMed] [Google Scholar]
- 17.Grady EF, Bohm SK, Bunnett NW. Turning off the signal: mechanisms that attenuate signaling by G protein-coupled receptors. Am J Physiol. 1997;273:G586–601. doi: 10.1152/ajpgi.1997.273.3.G586. [DOI] [PubMed] [Google Scholar]
- 18.Aragay AM, Ruiz-Gomez A, Penela P, Sarnago S, Elorza A, Jimenez-Sainz MC, Mayor F., Jr G protein-coupled receptor kinase 2 (GRK2): mechanisms of regulation and physiological functions. FEBS Lett. 1998;430:37–40. doi: 10.1016/s0014-5793(98)00495-5. [DOI] [PubMed] [Google Scholar]
- 19.Carman CV, Lisanti MP, Benovic JL. Regulation of G protein-coupled receptor kinases by caveolin. J Biol Chem. 1999;274:8858–64. doi: 10.1074/jbc.274.13.8858. [DOI] [PubMed] [Google Scholar]
- 20.Corbit KC, Trakul N, Eves EM, Diaz B, Marshall M, Rosner MR. Activation of Raf-1 signaling by protein kinase C through a mechanism involving Raf kinase inhibitory protein. J Biol Chem. 2003;278:13061–8. doi: 10.1074/jbc.M210015200. [DOI] [PubMed] [Google Scholar]
- 21.Elorza A, Sarnago S, Mayor F., Jr Agonist-dependent modulation of G protein-coupled receptor kinase 2 by mitogen-activated protein kinases. Mol Pharmacol. 2000;57:778–83. doi: 10.1124/mol.57.4.778. [DOI] [PubMed] [Google Scholar]
- 22.Elorza A, Penela P, Sarnago S, Mayor F., Jr MAPK-dependent degradation of G protein-coupled receptor kinase 2. J Biol Chem. 2003;278:29164–73. doi: 10.1074/jbc.M304314200. [DOI] [PubMed] [Google Scholar]
- 23.Fan G, Shumay E, Malbon CC, Wang H. c-Src tyrosine kinase binds the beta 2-adrenergic receptor via phospho-Tyr-350, phosphorylates G-protein-linked receptor kinase 2, and mediates agonist-induced receptor desensitization. J Biol Chem. 2001;276:13240–7. doi: 10.1074/jbc.M011578200. [DOI] [PubMed] [Google Scholar]
- 24.Krasel C, Dammeier S, Winstel R, Brockmann J, Mischak H, Lohse MJ. Phosphorylation of GRK2 by protein kinase C abolishes its inhibition by calmodulin. J Biol Chem. 2001;276:1911–5. doi: 10.1074/jbc.M008773200. [DOI] [PubMed] [Google Scholar]
- 25.Penela P, Elorza A, Sarnago S, Mayor F., Jr Beta-arrestin- and c-Src-dependent degradation of G-protein-coupled receptor kinase 2. Embo J. 2001;20:5129–38. doi: 10.1093/emboj/20.18.5129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pitcher JA, Tesmer JJ, Freeman JL, Capel WD, Stone WC, Lefkowitz RJ. Feedback inhibition of G protein-coupled receptor kinase 2 (GRK2) activity by extracellular signal-regulated kinases. J Biol Chem. 1999;274:34531–4. doi: 10.1074/jbc.274.49.34531. [DOI] [PubMed] [Google Scholar]
- 27.Sarnago S, Elorza A, Mayor F., Jr Agonist-dependent phosphorylation of the G protein-coupled receptor kinase 2 (GRK2) by Src tyrosine kinase. J Biol Chem. 1999;274:34411–6. doi: 10.1074/jbc.274.48.34411. [DOI] [PubMed] [Google Scholar]
- 28.Murthy KS, Jin JG, Grider JR, Makhlouf GM. Characterization of PACAP receptors and signaling pathways in rabbit gastric muscle cells. Am J Physiol. 1997;272:G1391–9. doi: 10.1152/ajpgi.1997.272.6.G1391. [DOI] [PubMed] [Google Scholar]
- 29.Murthy KS, Makhlouf GM. Differential regulation of phospholipase A2 (PLA2)-dependent Ca2+ signaling in smooth muscle by cAMP- and cGMP-dependent protein kinases. Inhibitory phosphorylation of PLA2 by cyclic nucleotide-dependent protein kinases. J Biol Chem. 1998;273:34519–26. doi: 10.1074/jbc.273.51.34519. [DOI] [PubMed] [Google Scholar]
- 30.Huang JMS, Murthy KS. Feedback inhibition of Gαi function by Gβ γi subunits: A novel mechanism involving phosphorylation of Gαi by Gβ γ-dependent Src kinase, recruitment to RGS12, and inactivation of GTP-bound Gαi. Gastroenterolgy. 2006;130:A-30. [Google Scholar]
- 31.Lorenz K, Lohse MJ, Quitterer U. Protein kinase C switches the Raf kinase inhibitor from Raf-1 to GRK-2. Nature. 2003;426:574–9. doi: 10.1038/nature02158. [DOI] [PubMed] [Google Scholar]
- 32.Shi X-Z, Sarna SK. G protein-mediated dysfunction of excitation-contraction coupling in ileal inflammation. Am J Physiol gastrointes Liver Physiol. 2004;286:G899–G905. doi: 10.1152/ajpgi.00408.2003. [DOI] [PubMed] [Google Scholar]


