Abstract
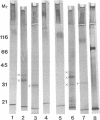
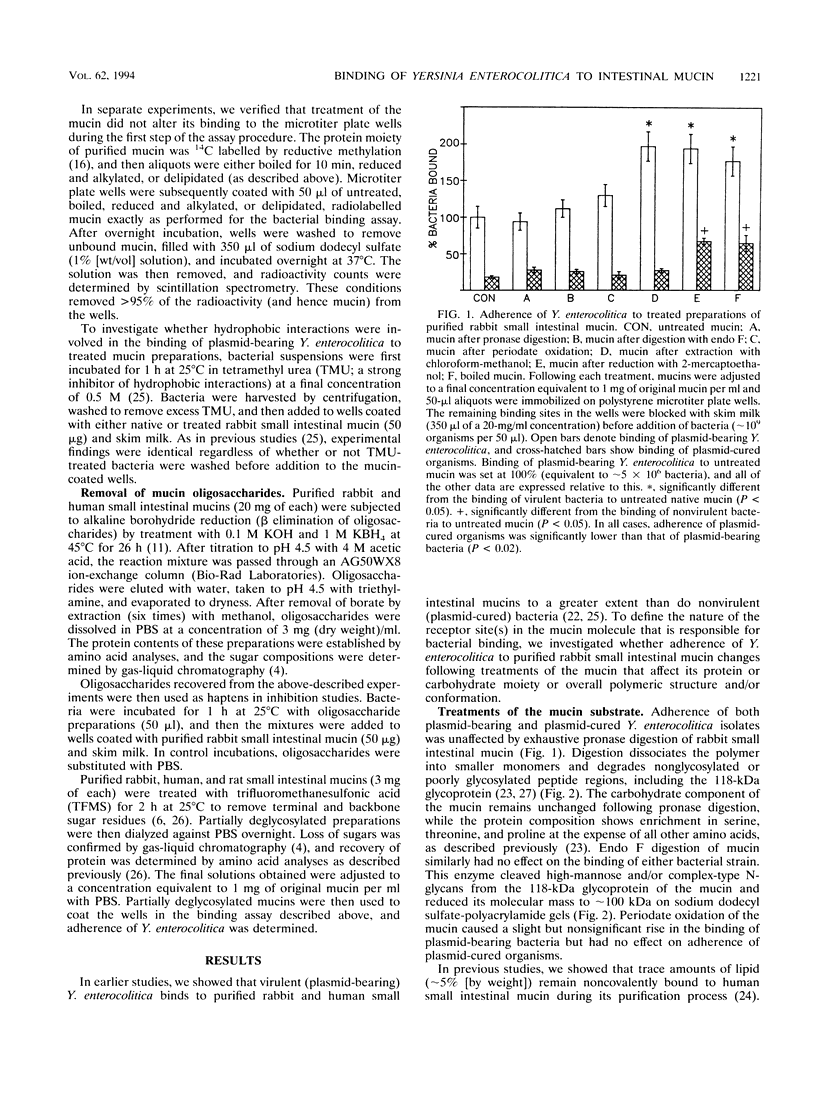
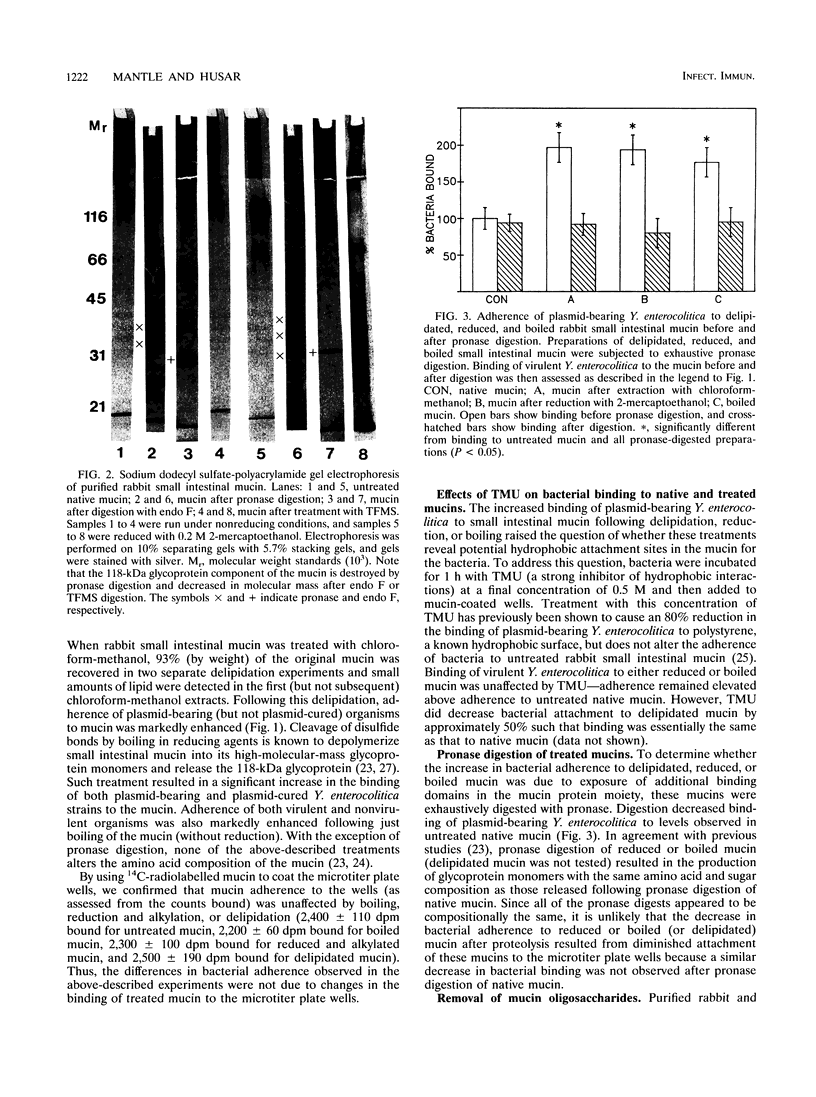
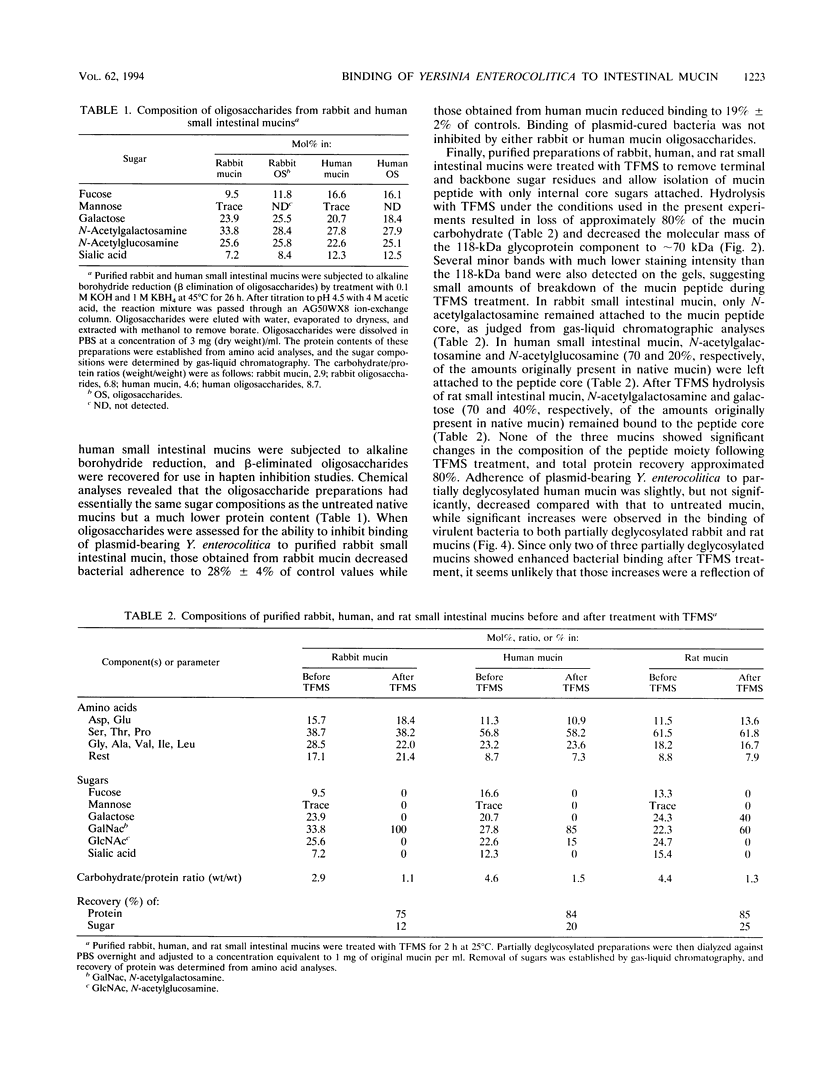
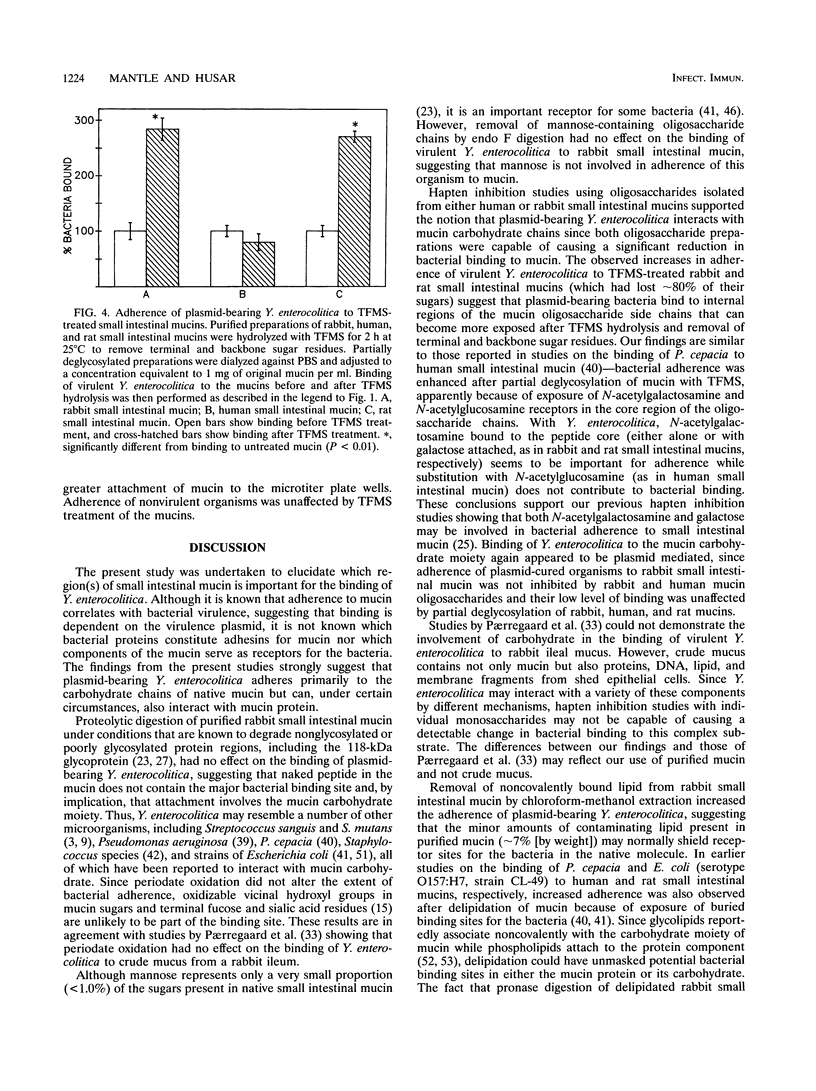
Plasmid-bearing (but not plasmid-cured) Yersinia enterocolitica is known to bind to purified small intestinal mucins from rabbits and humans. This study examined which region(s) of the mucin molecule is important for bacterial adherence. Pronase digestion of mucin and removal of nonglycosylated or poorly glycosylated peptide regions had no effect on bacterial binding, suggesting that plasmid-bearing Y. enterocolitica interacts with mucin carbohydrate. Periodate oxidation also did not alter bacterial adherence, indicating that vicinal hydroxyl groups in the mucin sugars are not important for binding. Boiling of mucin, depolymerization by reduction of disulfide bonds, or removal of noncovalently associated lipid actually enhanced bacterial adherence, suggesting that plasmid-bearing Y. enterocolitica can interact with additional domains in the mucin molecule revealed by these treatments. These domains were destroyed by pronase digestion. In delipidated mucin (but not in reduced or boiled mucin), binding to these domains appeared to be hydrophobic since it could be prevented by treatment of bacteria with tetramethyl urea. Oligosaccharides obtained from both human and rabbit small intestinal mucins were capable of inhibiting attachment of plasmid-bearing (but not plasmid-cured) Y. enterocolitica to mucin. After removal of terminal and backbone sugar residues by treatment of mucin with trifluoromethanesulfonic acid, binding of plasmid-bearing bacteria increased significantly when N-acetylgalactosamine, either alone or with galactose attached, was revealed, indicating that core regions of the sugar side chains are involved in bacterial binding. Adherence of plasmid-cured organisms was unaffected by trifluoromethanesulfonic acid treatment of mucin. We concluded that virulent Y. enterocolitica interacts with the carbohydrate moiety of native small intestinal mucin through a plasmid-mediated process. When mucin becomes denatured, binding of the organism can increase through hydrophobic and nonhydrophobic interactions with (most likely) the mucin protein.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell A. E., Sellers L. A., Allen A., Cunliffe W. J., Morris E. R., Ross-Murphy S. B. Properties of gastric and duodenal mucus: effect of proteolysis, disulfide reduction, bile, acid, ethanol, and hypertonicity on mucus gel structure. Gastroenterology. 1985 Jan;88(1 Pt 2):269–280. doi: 10.1016/s0016-5085(85)80180-3. [DOI] [PubMed] [Google Scholar]
- Bergey E. J., Levine M. J., Reddy M. S., Bradway S. D., Al-Hashimi I. Use of the photoaffinity cross-linking agent N-hydroxysuccinimidyl-4-azidosalicylic acid to characterize salivary-glycoprotein-bacterial interactions. Biochem J. 1986 Feb 15;234(1):43–48. doi: 10.1042/bj2340043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplin M. F. A rapid and sensitive method for the analysis of carbohydrate components in glycoproteins using gas-liquid chromatography. Anal Biochem. 1982 Jul 1;123(2):336–341. doi: 10.1016/0003-2697(82)90455-9. [DOI] [PubMed] [Google Scholar]
- Cover T. L., Aber R. C. Yersinia enterocolitica. N Engl J Med. 1989 Jul 6;321(1):16–24. doi: 10.1056/NEJM198907063210104. [DOI] [PubMed] [Google Scholar]
- Edge A. S., Faltynek C. R., Hof L., Reichert L. E., Jr, Weber P. Deglycosylation of glycoproteins by trifluoromethanesulfonic acid. Anal Biochem. 1981 Nov 15;118(1):131–137. doi: 10.1016/0003-2697(81)90168-8. [DOI] [PubMed] [Google Scholar]
- Fahim R. E., Forstner G. G., Forstner J. F. Heterogeneity of rat goblet-cell mucin before and after reduction. Biochem J. 1983 Jan 1;209(1):117–124. doi: 10.1042/bj2090117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemski P., Lazere J. R., Casey T. Plasmid associated with pathogenicity and calcium dependency of Yersinia enterocolitica. Infect Immun. 1980 Feb;27(2):682–685. doi: 10.1128/iai.27.2.682-685.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Qureshi J. V. Selective binding of blood group-reactive salivary mucins by Streptococcus mutans and other oral organisms. Infect Immun. 1978 Dec;22(3):665–671. doi: 10.1128/iai.22.3.665-671.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwozdzinski K., Slomiany A., Nishikawa H., Okazaki K., Slomiany B. L. Gastric mucin hydrophobicity: effects of associated and covalently bound lipids, proteolysis, and reduction. Biochem Int. 1988 Nov;17(5):907–917. [PubMed] [Google Scholar]
- Hansson G. C., Bouhours J. F., Karlsson H., Carlstedt I. Analysis of sialic acid-containing mucin oligosaccharides from porcine small intestine by high-temperature gas chromatography-mass spectrometry of their dimethylamides. Carbohydr Res. 1991 Dec 16;221:179–189. doi: 10.1016/0008-6215(91)80055-r. [DOI] [PubMed] [Google Scholar]
- Isberg R. R., Swain A., Falkow S. Analysis of expression and thermoregulation of the Yersinia pseudotuberculosis inv gene with hybrid proteins. Infect Immun. 1988 Aug;56(8):2133–2138. doi: 10.1128/iai.56.8.2133-2138.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isberg R. R., Voorhis D. L., Falkow S. Identification of invasin: a protein that allows enteric bacteria to penetrate cultured mammalian cells. Cell. 1987 Aug 28;50(5):769–778. doi: 10.1016/0092-8674(87)90335-7. [DOI] [PubMed] [Google Scholar]
- Jabbal I., Forstner G., Forstner J., Kells D. I. Sedimentation velocity studies on microgram quantities of rat intestinal goblet cell mucin. Anal Biochem. 1975 Dec;69(2):558–571. doi: 10.1016/0003-2697(75)90161-x. [DOI] [PubMed] [Google Scholar]
- Jentoft N., Dearborn D. G. Labeling of proteins by reductive methylation using sodium cyanoborohydride. J Biol Chem. 1979 Jun 10;254(11):4359–4365. [PubMed] [Google Scholar]
- Kapperud G., Namork E., Skurnik M., Nesbakken T. Plasmid-mediated surface fibrillae of Yersinia pseudotuberculosis and Yersinia enterocolitica: relationship to the outer membrane protein YOP1 and possible importance for pathogenesis. Infect Immun. 1987 Sep;55(9):2247–2254. doi: 10.1128/iai.55.9.2247-2254.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachica R. V., Zink D. L. Plasmid-associated cell surface charge and hydrophobicity of Yersinia enterocolitica. Infect Immun. 1984 May;44(2):540–543. doi: 10.1128/iai.44.2.540-543.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lian C. J., Hwang W. S., Kelly J. K., Pai C. H. Invasiveness of Yersinia enterocolitica lacking the virulence plasmid: an in-vivo study. J Med Microbiol. 1987 Nov;24(3):219–226. doi: 10.1099/00222615-24-3-219. [DOI] [PubMed] [Google Scholar]
- Lian C. J., Hwang W. S., Pai C. H. Plasmid-mediated resistance to phagocytosis in Yersinia enterocolitica. Infect Immun. 1987 May;55(5):1176–1183. doi: 10.1128/iai.55.5.1176-1183.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantle M., Basaraba L., Peacock S. C., Gall D. G. Binding of Yersinia enterocolitica to rabbit intestinal brush border membranes, mucus, and mucin. Infect Immun. 1989 Nov;57(11):3292–3299. doi: 10.1128/iai.57.11.3292-3299.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantle M., Forstner G. G., Forstner J. F. Biochemical characterization of the component parts of intestinal mucin from patients with cystic fibrosis. Biochem J. 1984 Dec 1;224(2):345–354. doi: 10.1042/bj2240345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantle M., Forstner J. F. The effects of delipidation on the major antigenic determinant of purified human intestinal mucin. Biochem Cell Biol. 1986 Mar;64(3):223–228. doi: 10.1139/o86-032. [DOI] [PubMed] [Google Scholar]
- Mantle M., Husar S. D. Adhesion of Yersinia enterocolitica to purified rabbit and human intestinal mucin. Infect Immun. 1993 Jun;61(6):2340–2346. doi: 10.1128/iai.61.6.2340-2346.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantle M., Potier M., Forstner G. G., Forstner J. F. Radiation inactivation of human intestinal mucin: determination of the size of the functional antigenic unit. Biochim Biophys Acta. 1986 Apr 11;881(2):248–257. doi: 10.1016/0304-4165(86)90011-5. [DOI] [PubMed] [Google Scholar]
- Mantle M., Thakore E. Rabbit intestinal and colonic mucins: isolation, partial characterization, and measurement of secretion using an enzyme-linked immunoassay. Biochem Cell Biol. 1988 Oct;66(10):1045–1054. doi: 10.1139/o88-121. [DOI] [PubMed] [Google Scholar]
- Martinez R. J. Plasmid-mediated and temperature-regulated surface properties of Yersinia enterocolitica. Infect Immun. 1983 Sep;41(3):921–930. doi: 10.1128/iai.41.3.921-930.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez R. J. Thermoregulation-dependent expression of Yersinia enterocolitica protein 1 imparts serum resistance to Escherichia coli K-12. J Bacteriol. 1989 Jul;171(7):3732–3739. doi: 10.1128/jb.171.7.3732-3739.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michiels T., Wattiau P., Brasseur R., Ruysschaert J. M., Cornelis G. Secretion of Yop proteins by Yersiniae. Infect Immun. 1990 Sep;58(9):2840–2849. doi: 10.1128/iai.58.9.2840-2849.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller V. L., Farmer J. J., 3rd, Hill W. E., Falkow S. The ail locus is found uniquely in Yersinia enterocolitica serotypes commonly associated with disease. Infect Immun. 1989 Jan;57(1):121–131. doi: 10.1128/iai.57.1.121-131.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paerregaard A., Espersen F., Baker N. The influence of properties encoded by the Yersinia virulence plasmid on adhesion of Yersinia enterocolitica to ileal brush border membrane vesicles. APMIS. 1990 Oct;98(10):927–932. [PubMed] [Google Scholar]
- Paerregaard A., Espersen F., Jensen O. M., Skurnik M. Interactions between Yersinia enterocolitica and rabbit ileal mucus: growth, adhesion, penetration, and subsequent changes in surface hydrophobicity and ability to adhere to ileal brush border membrane vesicles. Infect Immun. 1991 Jan;59(1):253–260. doi: 10.1128/iai.59.1.253-260.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pai C. H., DeStephano L. Serum resistance associated with virulence in Yersinia enterocolitica. Infect Immun. 1982 Feb;35(2):605–611. doi: 10.1128/iai.35.2.605-611.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pai C. H., Mors V., Seemayer T. A. Experimental Yersinia enterocolitica enteritis in rabbits. Infect Immun. 1980 Apr;28(1):238–244. doi: 10.1128/iai.28.1.238-244.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierson D. E., Falkow S. Nonpathogenic isolates of Yersinia enterocolitica do not contain functional inv-homologous sequences. Infect Immun. 1990 Apr;58(4):1059–1064. doi: 10.1128/iai.58.4.1059-1064.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portnoy D. A., Moseley S. L., Falkow S. Characterization of plasmids and plasmid-associated determinants of Yersinia enterocolitica pathogenesis. Infect Immun. 1981 Feb;31(2):775–782. doi: 10.1128/iai.31.2.775-782.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portnoy D. A., Wolf-Watz H., Bolin I., Beeder A. B., Falkow S. Characterization of common virulence plasmids in Yersinia species and their role in the expression of outer membrane proteins. Infect Immun. 1984 Jan;43(1):108–114. doi: 10.1128/iai.43.1.108-114.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramphal R., Carnoy C., Fievre S., Michalski J. C., Houdret N., Lamblin G., Strecker G., Roussel P. Pseudomonas aeruginosa recognizes carbohydrate chains containing type 1 (Gal beta 1-3GlcNAc) or type 2 (Gal beta 1-4GlcNAc) disaccharide units. Infect Immun. 1991 Feb;59(2):700–704. doi: 10.1128/iai.59.2.700-704.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajjan S. U., Forstner J. F. Role of the putative "link" glycopeptide of intestinal mucin in binding of piliated Escherichia coli serotype O157:H7 strain CL-49. Infect Immun. 1990 Apr;58(4):868–873. doi: 10.1128/iai.58.4.868-873.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajjan U. S., Corey M., Karmali M. A., Forstner J. F. Binding of Pseudomonas cepacia to normal human intestinal mucin and respiratory mucin from patients with cystic fibrosis. J Clin Invest. 1992 Feb;89(2):648–656. doi: 10.1172/JCI115631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanford B. A., Thomas V. L., Ramsay M. A. Binding of staphylococci to mucus in vivo and in vitro. Infect Immun. 1989 Dec;57(12):3735–3742. doi: 10.1128/iai.57.12.3735-3742.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze-Koops H., Burkhardt H., Heesemann J., von der Mark K., Emmrich F. Plasmid-encoded outer membrane protein YadA mediates specific binding of enteropathogenic yersiniae to various types of collagen. Infect Immun. 1992 Jun;60(6):2153–2159. doi: 10.1128/iai.60.6.2153-2159.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellers L. A., Allen A., Morris E. R., Ross-Murphy S. B. Mechanical characterization and properties of gastrointestinal mucus gel. Biorheology. 1987;24(6):615–623. doi: 10.3233/bir-1987-24614. [DOI] [PubMed] [Google Scholar]
- Skurnik M. Expression of antigens encoded by the virulence plasmid of Yersinia enterocolitica under different growth conditions. Infect Immun. 1985 Jan;47(1):183–190. doi: 10.1128/iai.47.1.183-190.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slomiany B. L., Murty V. L., Sarosiek J., Piotrowski J., Slomiany A. Role of associated and covalently bound lipids in salivary mucin hydrophobicity: effect of proteolysis and disulfide bridge reduction. Biochem Biophys Res Commun. 1988 Mar 30;151(3):1046–1053. doi: 10.1016/s0006-291x(88)80471-6. [DOI] [PubMed] [Google Scholar]
- Smith B. F., LaMont J. T. Hydrophobic binding properties of bovine gallbladder mucin. J Biol Chem. 1984 Oct 10;259(19):12170–12177. [PubMed] [Google Scholar]
- Tertti R., Skurnik M., Vartio T., Kuusela P. Adhesion protein YadA of Yersinia species mediates binding of bacteria to fibronectin. Infect Immun. 1992 Jul;60(7):3021–3024. doi: 10.1128/iai.60.7.3021-3024.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanke C. A., Cronan S., Goss C., Chadee K., Guerrant R. L. Characterization of binding of Escherichia coli strains which are enteropathogens to small-bowel mucin. Infect Immun. 1990 Mar;58(3):794–800. doi: 10.1128/iai.58.3.794-800.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witas H., Sarosiek J., Aono M., Murty V. L., Slomiany A., Slomiany B. L. Lipids associated with rat small-intestinal mucus glycoprotein. Carbohydr Res. 1983 Aug 16;120:67–76. doi: 10.1016/0008-6215(83)88007-0. [DOI] [PubMed] [Google Scholar]
- Witas H., Slomiany B. L., Zdebska E., Kojima K., Liau Y. H., Slomiany A. Lipids associated with dog gastric mucus glycoprotein. J Appl Biochem. 1983 Feb-Apr;5(1-2):16–24. [PubMed] [Google Scholar]
- Xu G., Huan L., Khatri I., Sajjan U. S., McCool D., Wang D., Jones C., Forstner G., Forstner J. Human intestinal mucin-like protein (MLP) is homologous with rat MLP in the C-terminal region, and is encoded by a gene on chromosome 11 p 15.5. Biochem Biophys Res Commun. 1992 Mar 16;183(2):821–828. doi: 10.1016/0006-291x(92)90557-2. [DOI] [PubMed] [Google Scholar]