Abstract
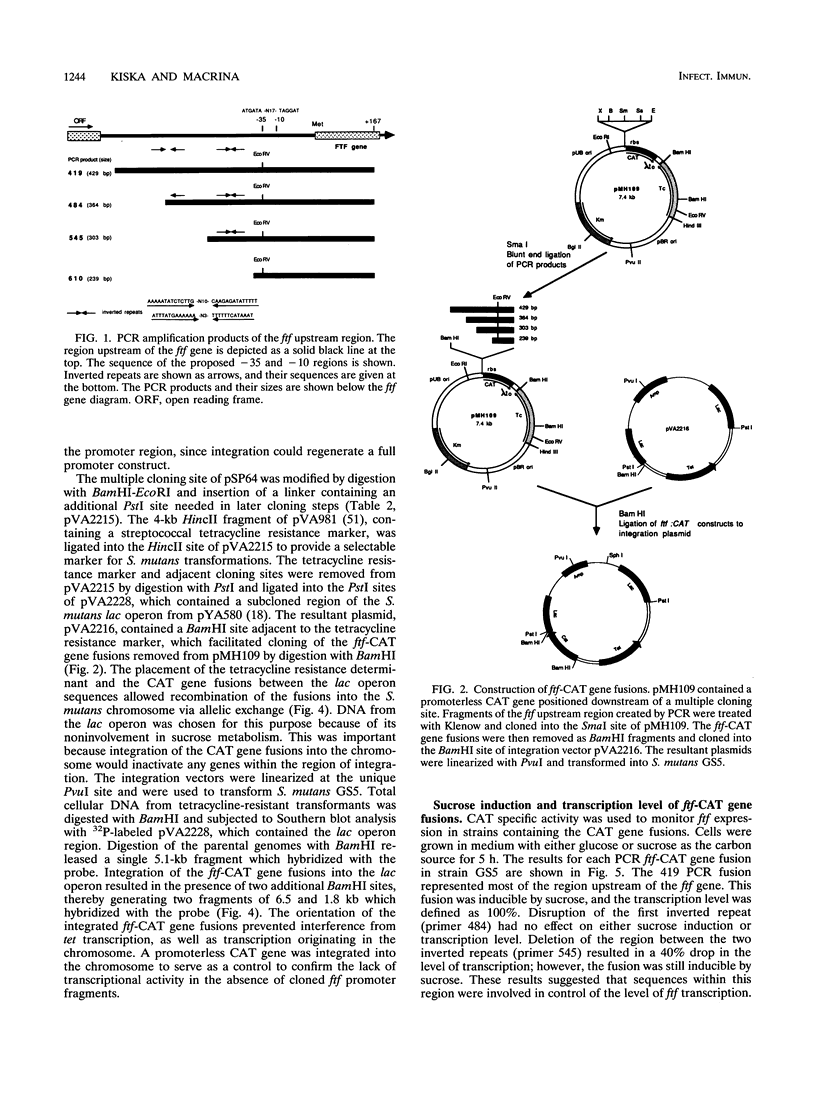
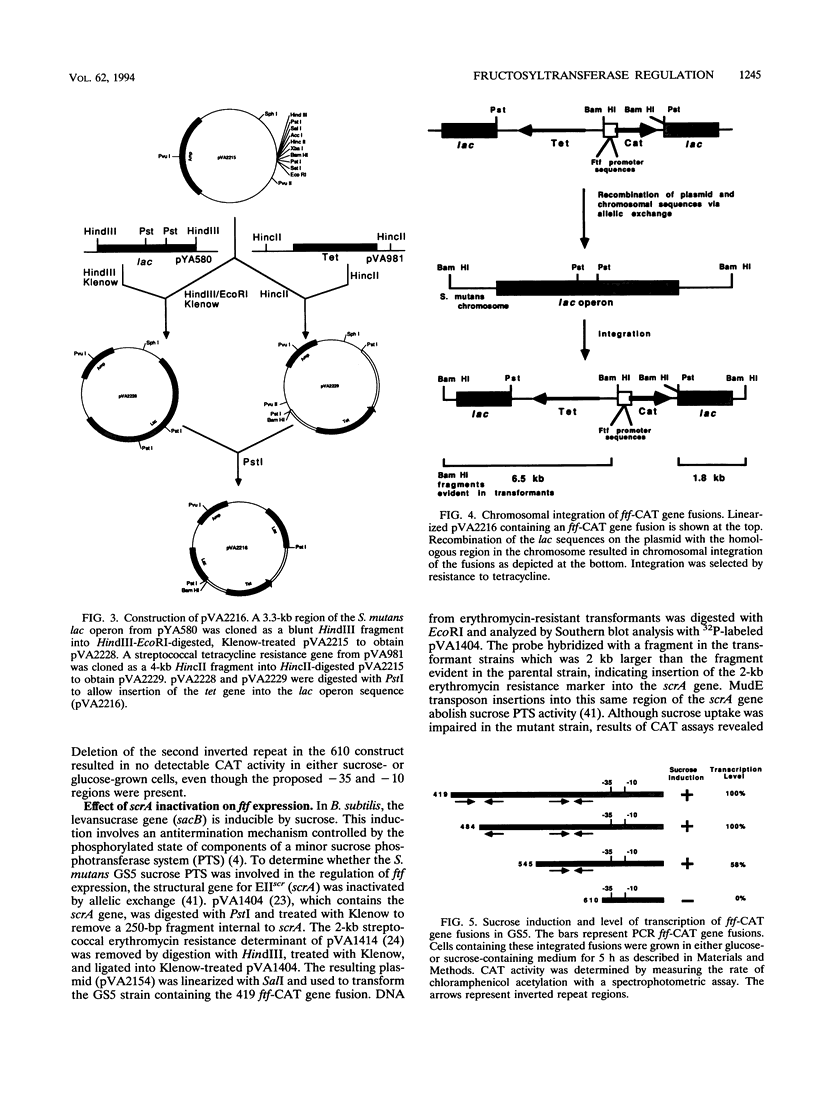
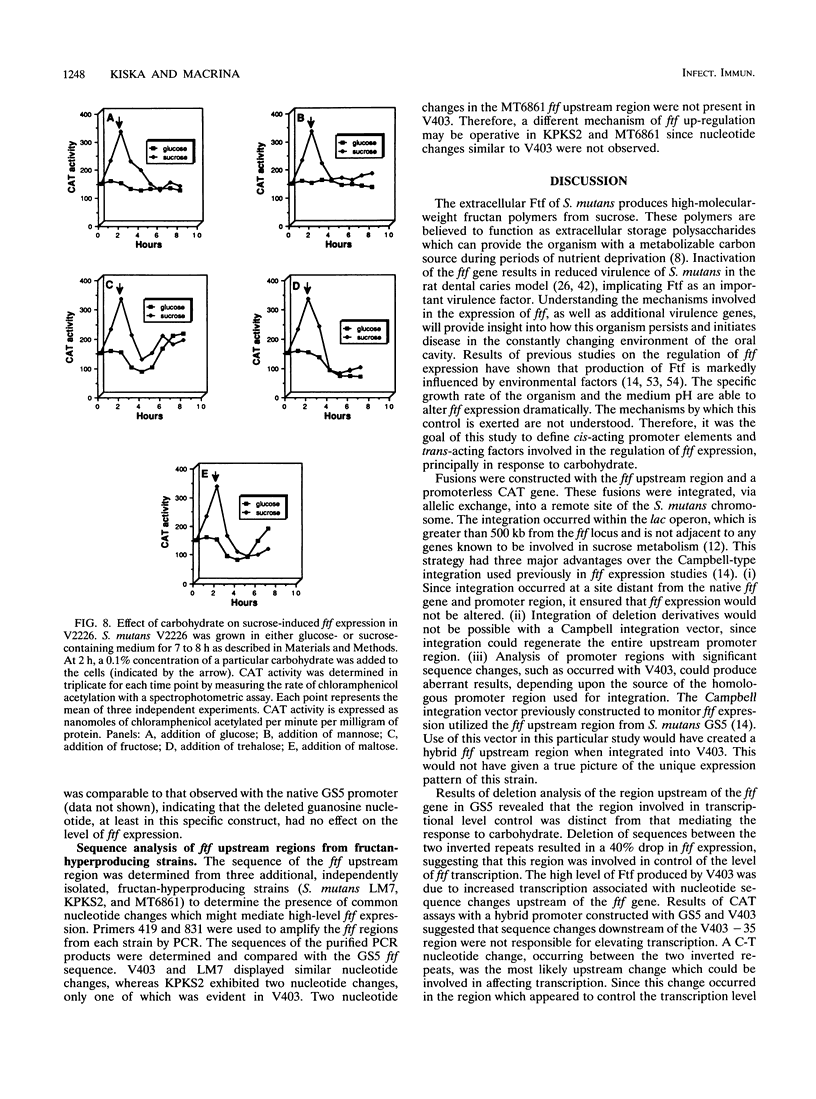
Streptococcus mutans possesses several extracellular sucrose-metabolizing enzymes which have been implicated as important virulence factors in dental caries. This study was initiated to investigate the genetic regulation of one of these enzymes, the extracellular fructosyltransferase (Ftf). Fusions were constructed with the region upstream of the S. mutans GS5 Ftf gene (ftf) and a promoterless chloramphenicol acetyltransferase (CAT) gene. The fusions were integrated at a remote site in the chromosome, and transcriptional activity in response to the addition of various carbohydrates to the growth medium was measured. A significant increase in CAT activity was observed when glucose-grown cells were shifted to sucrose-containing medium. Sucrose-induced expression was repressed immediately upon addition of phosphoenolpyruvate phosphotransferase system sugars to the growth media. Deletion analysis of the ftf upstream region revealed that an inverted repeat structure was involved in the control of ftf expression in response to carbohydrate. However, the control of the level of ftf transcription appeared to involve a region distinct from that mediating carbohydrate regulation. CAT gene fusions also were constructed with the ftf upstream region from S. mutans V403, a fructan-hyperproducing strain which synthesizes increased levels of Ftf. Sequence analysis of the upstream ftf region in this strain revealed several nucleotide sequence changes which were associated with high-level ftf expression. Comparison of the GS5 and V403 ftf expression patterns suggested the presence of a trans-acting factor(s) involved in modulation of ftf expression in response to carbohydrate. This factor(s) was either absent or altered in V403, resulting in the inability of this organism to respond to the presence of carbohydrate. The sequences of the ftf regions from three additional fructan-hyperproducing strains were determined and compared with that of V403. Only one strain displayed nucleotide changes similar to those of V403. Two additional strains did not have these changes, suggesting that several mechanisms for up-regulation of ftf expression exist.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aoki H., Shiroza T., Hayakawa M., Sato S., Kuramitsu H. K. Cloning of a Streptococcus mutans glucosyltransferase gene coding for insoluble glucan synthesis. Infect Immun. 1986 Sep;53(3):587–594. doi: 10.1128/iai.53.3.587-594.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkhed D., Rosell K. G., Granath K. Structure of extracellular water-soluble polysaccharides synthesized from sucrose by oral strains of Streptococcus mutans, Streptococcus salivarius, Streptococcus sanguis and Actinomyces viscosus. Arch Oral Biol. 1979;24(1):53–61. doi: 10.1016/0003-9969(79)90175-4. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crutz A. M., Steinmetz M., Aymerich S., Richter R., Le Coq D. Induction of levansucrase in Bacillus subtilis: an antitermination mechanism negatively controlled by the phosphotransferase system. J Bacteriol. 1990 Feb;172(2):1043–1050. doi: 10.1128/jb.172.2.1043-1050.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dills S. S., Seno S. Regulation of hexitol catabolism in Streptococcus mutans. J Bacteriol. 1983 Feb;153(2):861–866. doi: 10.1128/jb.153.2.861-866.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebisu S., Kato K., Kotani S., Misaki A. Structural differences in fructans elaborated by streptococcus mutans and Strep. salivarius. J Biochem. 1975 Nov;78(5):879–887. doi: 10.1093/oxfordjournals.jbchem.a130993. [DOI] [PubMed] [Google Scholar]
- Fouet A., Sonenshein A. L. A target for carbon source-dependent negative regulation of the citB promoter of Bacillus subtilis. J Bacteriol. 1990 Feb;172(2):835–844. doi: 10.1128/jb.172.2.835-844.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J. Formation and significance of bacterial polysaccharides in caries etiology. Caries Res. 1968;2(2):164–171. doi: 10.1159/000259554. [DOI] [PubMed] [Google Scholar]
- Hamada S., Slade H. D. Biology, immunology, and cariogenicity of Streptococcus mutans. Microbiol Rev. 1980 Jun;44(2):331–384. doi: 10.1128/mr.44.2.331-384.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanada N., Kuramitsu H. K. Isolation and characterization of the Streptococcus mutans gtfC gene, coding for synthesis of both soluble and insoluble glucans. Infect Immun. 1988 Aug;56(8):1999–2005. doi: 10.1128/iai.56.8.1999-2005.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanada N., Kuramitsu H. K. Isolation and characterization of the Streptococcus mutans gtfD gene, coding for primer-dependent soluble glucan synthesis. Infect Immun. 1989 Jul;57(7):2079–2085. doi: 10.1128/iai.57.7.2079-2085.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hantman M. J., Sun S., Piggot P. J., Daneo-Moore L. Chromosome organization of Streptococcus mutans GS-5. J Gen Microbiol. 1993 Jan;139(1):67–77. doi: 10.1099/00221287-139-1-67. [DOI] [PubMed] [Google Scholar]
- Honda O., Kato C., Kuramitsu H. K. Nucleotide sequence of the Streptococcus mutans gtfD gene encoding the glucosyltransferase-S enzyme. J Gen Microbiol. 1990 Oct;136(10):2099–2105. doi: 10.1099/00221287-136-10-2099. [DOI] [PubMed] [Google Scholar]
- Hudson M. C., Curtiss R., 3rd Regulation of expression of Streptococcus mutans genes important to virulence. Infect Immun. 1990 Feb;58(2):464–470. doi: 10.1128/iai.58.2.464-470.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson M. C., Stewart G. C. Differential utilization of Staphylococcus aureus promoter sequences by Escherichia coli and Bacillus subtilis. Gene. 1986;48(1):93–100. doi: 10.1016/0378-1119(86)90355-0. [DOI] [PubMed] [Google Scholar]
- Jacobson G. R., Lodge J., Poy F. Carbohydrate uptake in the oral pathogen Streptococcus mutans: mechanisms and regulation by protein phosphorylation. Biochimie. 1989 Sep-Oct;71(9-10):997–1004. doi: 10.1016/0300-9084(89)90103-x. [DOI] [PubMed] [Google Scholar]
- Jagusztyn-Krynicka E. K., Hansen J. B., Crow V. L., Thomas T. D., Honeyman A. L., Curtiss R., 3rd Streptococcus mutans serotype c tagatose 6-phosphate pathway gene cluster. J Bacteriol. 1992 Oct;174(19):6152–6158. doi: 10.1128/jb.174.19.6152-6158.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Liberman E. S., Bleiweis A. S. Role of the phosphoenolpyruvate-dependent glucose phosphotransferase system of Streptococcus mutans GS5 in the regulation of lactose uptake. Infect Immun. 1984 Feb;43(2):536–542. doi: 10.1128/iai.43.2.536-542.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindler L. E., Macrina F. L. Characterization of genetic transformation in Streptococcus mutans by using a novel high-efficiency plasmid marker rescue system. J Bacteriol. 1986 May;166(2):658–665. doi: 10.1128/jb.166.2.658-665.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loesche W. J. Role of Streptococcus mutans in human dental decay. Microbiol Rev. 1986 Dec;50(4):353–380. doi: 10.1128/mr.50.4.353-380.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macrina F. L., Jones K. R., Alpert C. A., Chassy B. M., Michalek S. M. Repeated DNA sequence involved in mutations affecting transport of sucrose into Streptococcus mutans V403 via the phosphoenolpyruvate phosphotransferase system. Infect Immun. 1991 Apr;59(4):1535–1543. doi: 10.1128/iai.59.4.1535-1543.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macrina F. L., Jones K. R., Welch R. A. Transformation of Streptococcus sanguis with monomeric pVA736 plasmid deoxyribonucleic acid. J Bacteriol. 1981 May;146(2):826–830. doi: 10.1128/jb.146.2.826-830.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macrina F. L., Tobian J. A., Jones K. R., Evans R. P., Clewell D. B. A cloning vector able to replicate in Escherichia coli and Streptococcus sanguis. Gene. 1982 Oct;19(3):345–353. doi: 10.1016/0378-1119(82)90025-7. [DOI] [PubMed] [Google Scholar]
- Munro C., Michalek S. M., Macrina F. L. Cariogenicity of Streptococcus mutans V403 glucosyltransferase and fructosyltransferase mutants constructed by allelic exchange. Infect Immun. 1991 Jul;59(7):2316–2323. doi: 10.1128/iai.59.7.2316-2323.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oskouian B., Stewart G. C. Cloning and characterization of the repressor gene of the Staphylococcus aureus lactose operon. J Bacteriol. 1987 Dec;169(12):5459–5465. doi: 10.1128/jb.169.12.5459-5465.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oskouian B., Stewart G. C. Repression and catabolite repression of the lactose operon of Staphylococcus aureus. J Bacteriol. 1990 Jul;172(7):3804–3812. doi: 10.1128/jb.172.7.3804-3812.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postma P. W., Lengeler J. W. Phosphoenolpyruvate:carbohydrate phosphotransferase system of bacteria. Microbiol Rev. 1985 Sep;49(3):232–269. doi: 10.1128/mr.49.3.232-269.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poy F., Jacobson G. R. Evidence that a low-affinity sucrose phosphotransferase activity in Streptococcus mutans GS-5 is a high-affinity trehalose uptake system. Infect Immun. 1990 May;58(5):1479–1480. doi: 10.1128/iai.58.5.1479-1480.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pucci M. J., Jones K. R., Kuramitsu H. K., Macrina F. L. Molecular cloning and characterization of the glucosyltransferase C gene (gtfC) from Streptococcus mutans LM7. Infect Immun. 1987 Sep;55(9):2176–2182. doi: 10.1128/iai.55.9.2176-2182.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reizer J., Peterkofsky A., Romano A. H. Evidence for the presence of heat-stable protein (HPr) and ATP-dependent HPr kinase in heterofermentative lactobacilli lacking phosphoenolpyruvate:glycose phosphotransferase activity. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2041–2045. doi: 10.1073/pnas.85.7.2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reizer J., Romano A. H., Deutscher J. The role of phosphorylation of HPr, a phosphocarrier protein of the phosphotransferase system, in the regulation of carbon metabolism in gram-positive bacteria. J Cell Biochem. 1993 Jan;51(1):19–24. doi: 10.1002/jcb.240510105. [DOI] [PubMed] [Google Scholar]
- Reizer J., Sutrina S. L., Saier M. H., Stewart G. C., Peterkofsky A., Reddy P. Mechanistic and physiological consequences of HPr(ser) phosphorylation on the activities of the phosphoenolpyruvate:sugar phosphotransferase system in gram-positive bacteria: studies with site-specific mutants of HPr. EMBO J. 1989 Jul;8(7):2111–2120. doi: 10.1002/j.1460-2075.1989.tb03620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reizer J., Sutrina S. L., Wu L. F., Deutscher J., Reddy P., Saier M. H., Jr Functional interactions between proteins of the phosphoenolpyruvate:sugar phosphotransferase systems of Bacillus subtilis and Escherichia coli. J Biol Chem. 1992 May 5;267(13):9158–9169. [PubMed] [Google Scholar]
- Rosell K. G., Birkhed D. An inulin-like fructan produced by Streptococcus mutans, strain JC2. Acta Chem Scand B. 1974;28(5):589–589. doi: 10.3891/acta.chem.scand.28b-0589. [DOI] [PubMed] [Google Scholar]
- Roseman S., Meadow N. D. Signal transduction by the bacterial phosphotransferase system. Diauxie and the crr gene (J. Monod revisited). J Biol Chem. 1990 Feb 25;265(6):2993–2996. [PubMed] [Google Scholar]
- Rosey E. L., Stewart G. C. Nucleotide and deduced amino acid sequences of the lacR, lacABCD, and lacFE genes encoding the repressor, tagatose 6-phosphate gene cluster, and sugar-specific phosphotransferase system components of the lactose operon of Streptococcus mutans. J Bacteriol. 1992 Oct;174(19):6159–6170. doi: 10.1128/jb.174.19.6159-6170.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y., Poy F., Jacobson G. R., Kuramitsu H. K. Characterization and sequence analysis of the scrA gene encoding enzyme IIScr of the Streptococcus mutans phosphoenolpyruvate-dependent sucrose phosphotransferase system. J Bacteriol. 1989 Jan;171(1):263–271. doi: 10.1128/jb.171.1.263-271.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder V. A., Michalek S. M., Macrina F. L. Biochemical characterization and evaluation of virulence of a fructosyltransferase-deficient mutant of Streptococcus mutans V403. Infect Immun. 1989 Nov;57(11):3560–3569. doi: 10.1128/iai.57.11.3560-3569.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw W. V. Chloramphenicol acetyltransferase from chloramphenicol-resistant bacteria. Methods Enzymol. 1975;43:737–755. doi: 10.1016/0076-6879(75)43141-x. [DOI] [PubMed] [Google Scholar]
- Shimamura A., Tsuboi K., Nagase T., Ito M., Tsumori H., Mukasa H. Structural determination of D-fructans from Streptococcus mutans, serotype b, c, e, and f strains, by 13C-n.m.r. spectroscopy. Carbohydr Res. 1987 Jul 15;165(1):150–154. doi: 10.1016/0008-6215(87)80091-5. [DOI] [PubMed] [Google Scholar]
- Shiroza T., Kuramitsu H. K. Sequence analysis of the Streptococcus mutans fructosyltransferase gene and flanking regions. J Bacteriol. 1988 Feb;170(2):810–816. doi: 10.1128/jb.170.2.810-816.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slee A. M., Tanzer J. M. Effect of growth conditions on sucrose phosphotransferase activity of Streptococcus mutans. Infect Immun. 1980 Mar;27(3):922–927. doi: 10.1128/iai.27.3.922-927.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. E., Summers M. D. The bidirectional transfer of DNA and RNA to nitrocellulose or diazobenzyloxymethyl-paper. Anal Biochem. 1980 Nov 15;109(1):123–129. doi: 10.1016/0003-2697(80)90019-6. [DOI] [PubMed] [Google Scholar]
- Stewart G. C. Catabolite repression in the gram-positive bacteria: generation of negative regulators of transcription. J Cell Biochem. 1993 Jan;51(1):25–28. doi: 10.1002/jcb.240510106. [DOI] [PubMed] [Google Scholar]
- Sutrina S. L., Reddy P., Saier M. H., Jr, Reizer J. The glucose permease of Bacillus subtilis is a single polypeptide chain that functions to energize the sucrose permease. J Biol Chem. 1990 Oct 25;265(30):18581–18589. [PubMed] [Google Scholar]
- Terleckyj B., Willett N. P., Shockman G. D. Growth of several cariogenic strains of oral streptococci in a chemically defined medium. Infect Immun. 1975 Apr;11(4):649–655. doi: 10.1128/iai.11.4.649-655.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobian J. A., Cline M. L., Macrina F. L. Characterization and expression of a cloned tetracycline resistance determinant from the chromosome of Streptococcus mutans. J Bacteriol. 1984 Nov;160(2):556–563. doi: 10.1128/jb.160.2.556-563.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda S., Shiroza T., Kuramitsu H. K. Sequence analysis of the gtfC gene from Streptococcus mutans GS-5. Gene. 1988 Sep 15;69(1):101–109. doi: 10.1016/0378-1119(88)90382-4. [DOI] [PubMed] [Google Scholar]
- Wenham D. G., Hennessey T. D., Cole J. A. Regulation of glucosyl- and fructosyltransferase synthesis by continuous cultures of Streptococcus mutans. J Gen Microbiol. 1979 Sep;114(1):117–124. doi: 10.1099/00221287-114-1-117. [DOI] [PubMed] [Google Scholar]
- Wexler D. L., Hudson M. C., Burne R. A. Streptococcus mutans fructosyltransferase (ftf) and glucosyltransferase (gtfBC) operon fusion strains in continuous culture. Infect Immun. 1993 Apr;61(4):1259–1267. doi: 10.1128/iai.61.4.1259-1267.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]



