Abstract
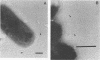
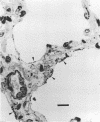
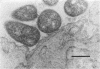
The ability of an Actinobacillus pleuropneumoniae serotype 2 strain to associate in vivo with the epithelium of the porcine respiratory tract was investigated in a sequential study after intranasal inoculation of hysterectomy-derived and colostrum-deprived pigs. At 30 min postinoculation more than 95% of the bacteria present in the lungs were intimately associated with the epithelium of the alveoli or the cilia of the terminal bronchioli, as observed by light and electron microscopy. At 90 and 180 min postinoculation multiple focal early inflammatory lesions in which histologically different, more or less concentric zones could be distinguished were observed. In the center of these pneumonic areas bacteria were associated with infiltrated cells and exudate. In the zone surrounding the center, approximately 95% of the bacteria were lying with their longest side in close apposition to the epithelial cells of alveoli and the cilia of the terminal bronchioli. Bacteria were only sporadically associated with the cilia or the epithelium of the bronchi and trachea. Bacteria were not observed in tonsils or conchae. In view of the findings presented here, we propose the hypothesis that adherence of the A. pleuropneumoniae serotype 2 strain to epithelial cells of the lower respiratory tract constitutes an important initial step in pathogenesis.
Full text
PDF
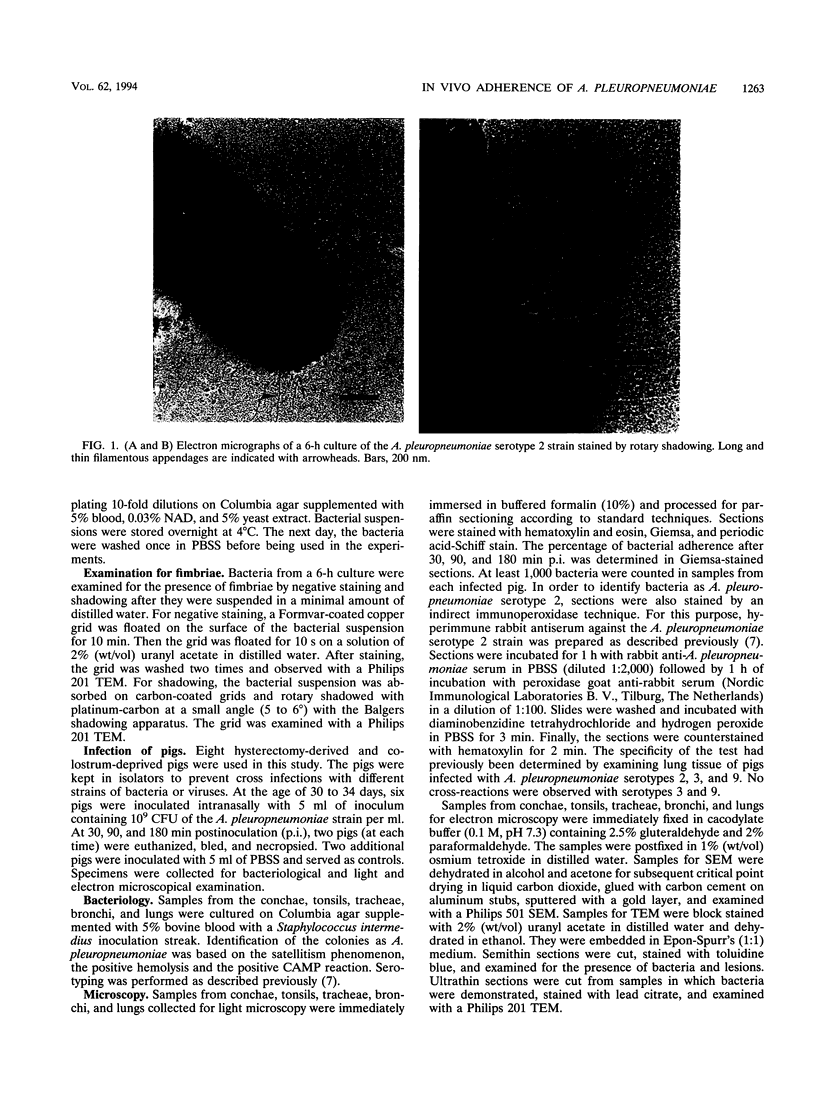
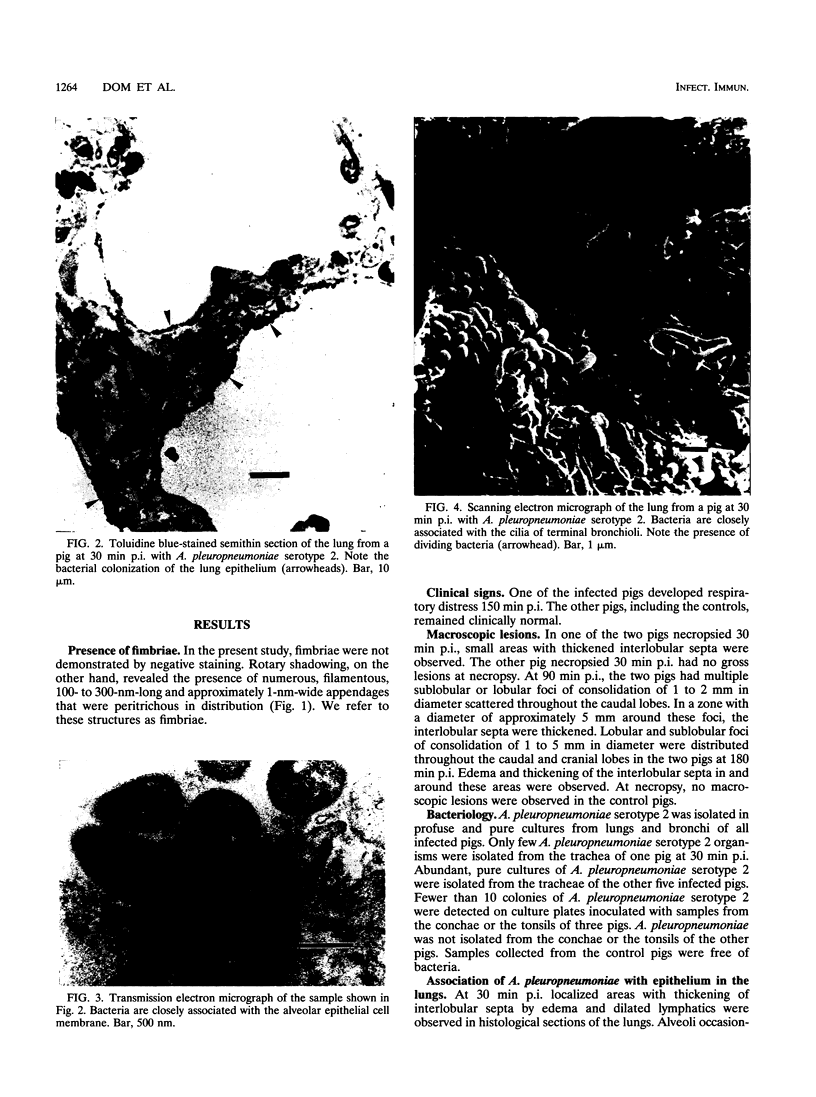
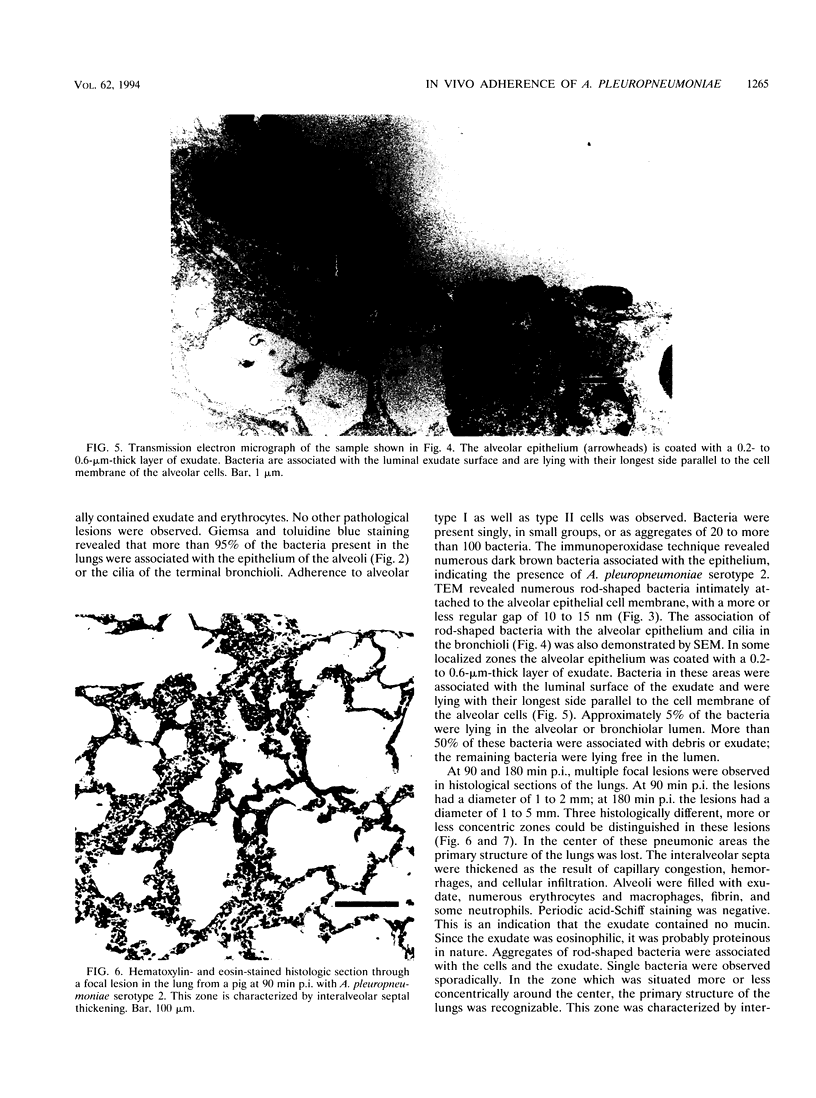


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bertram T. A. Quantitative morphology of peracute pulmonary lesions in swine induced by Haemophilus pleuropneumoniae. Vet Pathol. 1985 Nov;22(6):598–609. doi: 10.1177/030098588502200615. [DOI] [PubMed] [Google Scholar]
- Bélanger M., Dubreuil D., Harel J., Girard C., Jacques M. Role of lipopolysaccharides in adherence of Actinobacillus pleuropneumoniae to porcine tracheal rings. Infect Immun. 1990 Nov;58(11):3523–3530. doi: 10.1128/iai.58.11.3523-3530.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dom P., Haesebrouck F. Comparative virulence of NAD-dependent and NAD-independent Actinobacillus pleuropneumoniae strains. Zentralbl Veterinarmed B. 1992 Jun;39(4):303–306. doi: 10.1111/j.1439-0450.1992.tb01173.x. [DOI] [PubMed] [Google Scholar]
- Dom P., Haesebrouck F., De Baetselier P. Stimulation and suppression of the oxygenation activity of porcine pulmonary alveolar macrophages by Actinobacillus pleuropneumoniae and its metabolites. Am J Vet Res. 1992 Jul;53(7):1113–1118. [PubMed] [Google Scholar]
- Dom P., Haesebrouck F., Kamp E. M., Smits M. A. Influence of Actinobacillus pleuropneumoniae serotype 2 and its cytolysins on porcine neutrophil chemiluminescence. Infect Immun. 1992 Oct;60(10):4328–4334. doi: 10.1128/iai.60.10.4328-4334.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dom P., Hommez J., Castryck F., Devriese L. A., Haesebrouck F. Serotyping and quantitative determination of in vitro antibiotic susceptibility of Actinobacillus pleuropneumoniae strains isolated in Belgium (July 1991-August 1992). Vet Q. 1994 Mar;16(1):10–13. doi: 10.1080/01652176.1994.9694407. [DOI] [PubMed] [Google Scholar]
- Frey J., Bosse J. T., Chang Y. F., Cullen J. M., Fenwick B., Gerlach G. F., Gygi D., Haesebrouck F., Inzana T. J., Jansen R. Actinobacillus pleuropneumoniae RTX-toxins: uniform designation of haemolysins, cytolysins, pleurotoxin and their genes. J Gen Microbiol. 1993 Aug;139(8):1723–1728. doi: 10.1099/00221287-139-8-1723. [DOI] [PubMed] [Google Scholar]
- Hoepelman A. I., Tuomanen E. I. Consequences of microbial attachment: directing host cell functions with adhesins. Infect Immun. 1992 May;60(5):1729–1733. doi: 10.1128/iai.60.5.1729-1733.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultgren S. J., Abraham S., Caparon M., Falk P., St Geme J. W., 3rd, Normark S. Pilus and nonpilus bacterial adhesins: assembly and function in cell recognition. Cell. 1993 Jun 4;73(5):887–901. doi: 10.1016/0092-8674(93)90269-v. [DOI] [PubMed] [Google Scholar]
- Jacques M., Bélanger M., Roy G., Foiry B. Adherence of Actinobacillus pleuropneumoniae to porcine tracheal epithelial cells and frozen lung sections. Vet Microbiol. 1991 Apr;27(2):133–143. doi: 10.1016/0378-1135(91)90004-y. [DOI] [PubMed] [Google Scholar]
- Jacques M., Roy G., Mittal K. R. Hemagglutinating properties of Actinobacillus pleuropneumoniae. Can J Microbiol. 1988 Sep;34(9):1046–1049. doi: 10.1139/m88-184. [DOI] [PubMed] [Google Scholar]
- Jann K., Hoschützky H. Nature and organization of adhesins. Curr Top Microbiol Immunol. 1990;151:55–70. doi: 10.1007/978-3-642-74703-8_3. [DOI] [PubMed] [Google Scholar]
- Jansen R., Briaire J., Kamp E. M., Smits M. A. Comparison of the cytolysin II genetic determinants of Actinobacillus pleuropneumoniae serotypes. Infect Immun. 1992 Feb;60(2):630–636. doi: 10.1128/iai.60.2.630-636.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamp E. M., Popma J. K., Anakotta J., Smits M. A. Identification of hemolytic and cytotoxic proteins of Actinobacillus pleuropneumoniae by use of monoclonal antibodies. Infect Immun. 1991 Sep;59(9):3079–3085. doi: 10.1128/iai.59.9.3079-3085.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kume K., Nakai T., Sawata A. Isolation of Haemophilus pleuropneumoniae from the nasal cavities of healthy pigs. Nihon Juigaku Zasshi. 1984 Oct;46(5):641–647. doi: 10.1292/jvms1939.46.641. [DOI] [PubMed] [Google Scholar]
- Lagergård T., Purvén M., Frisk A. Evidence of Haemophilus ducreyi adherence to and cytotoxin destruction of human epithelial cells. Microb Pathog. 1993 Jun;14(6):417–431. doi: 10.1006/mpat.1993.1041. [DOI] [PubMed] [Google Scholar]
- Liggett A. D., Harrison L. R., Farrell R. L. Sequential study of lesion development in experimental haemophilus pleuropneumonia. Res Vet Sci. 1987 Mar;42(2):204–212. [PubMed] [Google Scholar]
- Serebrin S., Rosendal S., Valdivieso-Garcia A., Little P. B. Endothelial cytotoxicity of Actinobacillus pleuropneumoniae. Res Vet Sci. 1991 Jan;50(1):18–22. doi: 10.1016/0034-5288(91)90047-r. [DOI] [PubMed] [Google Scholar]
- Utrera V., Pijoan C. Fimbriae in A pleuropneumoniae strains isolated from pig respiratory tracts. Vet Rec. 1991 Apr 13;128(15):357–358. doi: 10.1136/vr.128.15.357. [DOI] [PubMed] [Google Scholar]
- Van Leengoed L. A., Kamp E. M., Pol J. M. Toxicity of Haemophilus pleuropneumoniae to porcine lung macrophages. Vet Microbiol. 1989 Apr;19(4):337–349. doi: 10.1016/0378-1135(89)90099-0. [DOI] [PubMed] [Google Scholar]
- Willson P. J., Falk G., Klashinsky S. Detection of Actinobacillus pleuropneumoniae Infection in Pigs. Can Vet J. 1987 Mar;28(3):111–116. [PMC free article] [PubMed] [Google Scholar]