Abstract
The inability to taste phenylthiocarbamide (PTC; “taste-blindness”) has been associated with a number of medical and neurological illnesses not typically related to taste. We examined PTC sensitivity in 67 schizophrenia patients, 30 healthy controls, and 30 first-degree relatives to determine whether taster status could represent a simple vulnerability marker. A higher prevalence of non-tasters was seen in patients and family members relative to healthy controls. Among patients, non-tasters exhibited increased levels of negative and first-rank symptoms as well as poorer right-nostril odor identification skills relative to PTC tasters. These differences were not explained by age, sex, education, smoking, or intensity differences. Phenotypic variation in PTC sensitivity is thought to be genetic in origin and suggests greater illness risk for those subjects with recessive taster alleles.
Keywords: Schizophrenia, Family, Taste, Phenylthiocarbamide, PTC, Genetics
1. Introduction
Sensitivity to the bitter-tasting compound phenylthiocarbamide (PTC) has long been believed to be a simple Mendelian recessive trait, with tasters defined as having at least one dominant allele, (i.e., TT or Tt) and non-tasters displaying a double recessive genotype (i.e., tt) (Bartoshuk et al. 1994; Blakeslee 1932; Drayna 2005; Fox 1931; Kim et al. 2003; Snyder 1931). The strong genetic basis for sensitivity to PTC has been used as a tool to trace family lineages and population migration patterns (Guo and Reed 2001; Mattes 2004). Previous studies have demonstrated that in the U.S. population, approximately 30% are non-tasters of PTC, whereas 70% are tasters of this substance (Drayna 2005; Guo and Reed 2001). In a landmark study of PTC sensitivity in the Utah Genetic Reference Project families, a single major locus on chromosome 7 was found to be responsible for most of the phenotypic variation that exists in the population (Drayna et al. 2003). In addition, a modest linkage score between PTC taste ability and markers on chromosome 16p has also been reported (Reed 2000).
Recent investigations of PTC tasting have focused on the intracellular activation of various G proteins. In an extensive genetic survey of the chromosome 7 linkage region, Kim and colleagues found that the PTC gene encodes for a specific G protein taste receptor, TAS2R38(7q35-36), which accounts for all of the bimodality in PTC taste perception (Kim et al. 2003). The observed differences in taste perception of PTC are hypothesized to be due to variations in types of G proteins activated by TAS2R38 haplotypes (Bufe et al. 2005).
In addition to taste, G protein-dysregulation has been strongly linked to mental illnesses such as major depressive disorder, manic depression, and schizophrenia (Schreiber and Avissar 2000). Indeed, heterotrimeric G proteins are crucial elements in post-receptor information transduction and have been implicated in the biochemical mechanism of many of the drugs used to treat psychiatric disorders (Schreiber and Avissar 2003). In studies of the role of G proteins in mental illness, the chief focus has been on the role of dopamine (DA) in the cortex. Dopamine receptor subtypes couple to multiple G proteins intracellularly, and it is hypothesized that slight alterations in DA subtypes during critical developmental periods may underlie the imbalance displayed in schizophrenia (Gainetdinov et al. 2004; Sidhu and Niznik 2000). These G protein-coupling imbalances have been mimicked in animal models, producing cognitive deficits that closely resemble many endophenotypes of schizophrenia. For example, transgenic mice expressing constitutively active Gsα-coupled dopamine receptors displayed deficits in pre-pulse inhibition, which modeled deficits seen in patients with schizophrenia (Gould et al. 2004). Additionally, studies of schizophrenia patients prior to drug treatment also showed an increase in Gs when compared to healthy controls as well as increases in overall positive and negative symptom presentation when compared within patient groups (Schreiber and Avissar 2003).
PTC tasting status has been linked to a myriad of medical illnesses ranging from epilepsy to gastrointestinal ulcers, though consistent findings among these disorders have been rare (Guo and Reed 2001; Pal et al. 2004). In contrast, the handful of studies examining PTC perception in schizophrenia have been generally consistent in showing an increase in the prevalence of nontasters in patients (Constantinidis 1958; Freire-Maia et al. 1968; Schlosberg and Baruch 1992). We recently reported an increased prevalence of PTC non-tasters among schizophrenia patients and their non-ill first-degree relatives compared to healthy controls (Moberg et al. 2005). We hypothesized that the increased number of non-tasters in patients and non-ill first-degree family members might reflect abnormalities in the function and/or expression of G protein-signaling that interact with other genetic or environmental factors to produce an increased vulnerability to illness.
Based on these prior findings, we sought to replicate the finding of an increased prevalence of non-tasters in patients and family members in a larger independent sample, and to examine any possible clinical and symptom correlates of taster status in patients with schizophrenia. In addition, because of the propinquity of smell and taste functions as well as similarities in the underlying neuroanatomy, it was hypothesized that that the well-documented deficits in olfactory function seen in schizophrenia might cluster together with PTC taster status.
2. Method
2.1 Participants
Sixty-seven patients with DSM-IV (1994) criteria for schizophrenia (49 male, 18 female), 30 healthy controls (18 male, 12 female), and 30 healthy first-degree relatives of patients (15 male, 15 female), were recruited from the Schizophrenia Research Center (SRC) at the University of Pennsylvania Medical Center, Philadelphia, PA. The non-ill family members consisted of 8 parents, 19 siblings, and 3 children of probands. The groups did not differ in age (F[2,124]=1.3, p=0.26) or in sex distribution (χ2=5.2, df=2, p=0.074). They did, however, differ with regard to ethnic background (χ2=15.2, df=4, p=0.004), largely driven by a greater proportion of African-American subjects in the patient group. As expected, patients and family members had lower educational attainment than controls (F[2,119]=12.3, p<0.001). Smoking was evaluated by computing a pack-years score, based upon the number of cigarettes smoked per day and the number of years a particular individual had been a smoker. Since pack-years of smoking were not normally distributed, the nonparametric Kruskal-Wallis test was used to compare smoking history among the three groups. A significant difference in smoking exposure was noted (χ2 =2.83, df=2, p<0.001), with both patients and family members smoking more than healthy controls. Demographic and clinical information for the study sample is presented in Table 1.
Table 1.
Sample characteristics for healthy comparison (HC), schizophrenia (SZ), and firstdegree relative (RL) groups. Mean (±standard deviation).
| HC (N=30) | SZ (N=67) | RL (N=30) | |
|---|---|---|---|
| Variable | |||
| Age (years) | 39.3 (15.7) | 37.0 (11.1) | 42.2 (20.1) |
| Sex (male/female) | 18/12 | 49/18 | 15/15 |
| Race (Caucasian/African-American/Other | 17/10/3 | 19/47/1 | 12/18/0* |
| Smoking (pack-years) | 1.8 (5.9) | 14.1 (22.0) | 7.8 (11.5)** |
| Education (years) | 14.6 (2.5) | 12.2 (2.0) | 12.3 (2.4)** |
| Duration of Illness (years) | 13.9 (9.4) | ||
| Age of Onset of Illness (years) | 22.2 (7.5) | ||
| Deficit/Non-Deficit | 8/36 | ||
| BPRS (total) | 31.0 (8.7) | ||
| SANS (total) | 32.4 (17.9) | ||
| SAPS (total) | 22.8 (20.6) |
Note. Duration of illness = difference in years between date of diagnosis and psychophysical assessment; BPRS = Brief Psychiatric Rating Scale; SANS = Scale for the Assessment of Negative Symptoms; SAPS = Scale for the Assessment of Positive Symptoms.
p=.004
p<.001
All subjects received a psychiatric interview with the Structured Clinical Interview for DSM-IV, Patient Edition (i.e., probands) or Nonpatient Edition (i.e., healthy controls, first-degree family members) (First and al 1996; First et al. 1995) and physical examination, including routine laboratory tests. Patients were rated on the Brief Psychiatric Rating Scale (BPRS) (Overall and Gorham 1980), the Scale for Assessment of Negative Symptoms (SANS) (Andreasen 1984a), and Scale for Assessment of Positive Symptoms (SAPS) (Andreasen 1984b). A factor analytic approach, to derive the fewest separable symptom dimensions from these ratings (Gur et al. 1994), yielded four symptom scales: (1) Negative Symptoms, (2) Disorganization, (3) Schneiderian Delusions and Hallucinations and, (4) Suspicion-Hostility. These dimensions have been shown previously to have adequate reliabilities and are statistically independent of one another (intercorrelations ranging from -0.16 to 0.15, p>0.05). Details of this procedure, item composition of the scales, and their item-scale correlations have been presented elsewhere (Gur et al. 1994). Patients were also subtyped into deficit and nondeficit categories following established criteria (Carpenter et al. 1988). Eight patients were categorized as having the deficit syndrome, and 36 as having the nondeficit syndrome.
All probands met DSM-IV criteria for schizophrenia and were free of any concurrent illness. On the date of testing 36 patients were receiving second-generation antipsychotic medications, 17 were receiving first-generation antipsychotic medications, and 14 patients were on a combination of both types.
Family members were assessed for Axis II psychopathology with the Structured Clinical Interview for Personality Disorders (First et al. 1997). In order to be included as a non-ill first-degree family member, the individual had to meet specific criteria 1) be the father, mother, offspring, or full sibling of an individual affected with a sole diagnosis of schizophrenia; 2) if a sibling, the individual must have shared the same mother and father as the individual affected with schizophrenia; 3) be currently and historically free of any Axis I or II psychiatric disorder; 4) meet all other inclusion criteria for participation in olfactory assessment. In addition to the SCID, healthy comparison subjects also received a structured interview for the assessment of Axis II psychopathology (SCID-II)(First et al. 1997). Based on these assessments, healthy comparison subjects were free of any Axis I or Axis II disorder and were negative for any family history of psychiatric illness.
Exclusion criteria for all subjects included: history of neurological disorder, including head trauma with loss of consciousness, history of substance abuse or dependence (as assessed by history, record review and urine toxicology), any medical condition that might alter cerebral function, a recent respiratory infection or any other condition that could affect olfactory or gustatory performance (e.g., common cold, allergies, significant septal deviation). All study procedures were approved by the University of Pennsylvania Institutional Review Board (IRB) and complied with the Declaration of Helsinki’s ethical standards in the treatment of human research participants. Written informed consent was obtained after the procedures had been fully explained.
2.2 Materials
2.21 Phenylthiocarbamide (PTC) Perception Testing
Subjects were administered a 3.80cm × 1.43cm strip of filter paper impregnated with 0.007 mg of PTC (Carolina Biological Supply Company, Burlington, N.C.). Each subject was instructed to moisten their tongue with saliva and to place the filter paper in the middle of their tongue. After approximately 5-8 seconds, they were instructed to expectorate the PTC paper and asked if they tasted anything. After recording their response, each subject was then asked to rate the intensity of their perception of the strip on a 100-mm visual analog line, ranging from 0 mm (no taste) to 100 mm (extremely strong taste). This method and psychophysical approach for testing PTC perception is consistent with other studies in the literature (Driscol et al. 2006; Enoch et al. 2001; Joiner and Perez 2004; Whittemore 1990). Any subject who reported an inability to taste the filter paper and reported an intensity of less than 30mm was classified as a non-taster. The 30mm value denoted the break-point in the bimodal distribution of intensity ratings between tasters and non-tasters.
2.22 Psychophysical Olfactory Assessment
Both odor identification and detection threshold sensitivity tasks were administered unilaterally (each nostril separately), with the contralateral naris occluded using a piece of Durapore™ tape (3M Corporation, Minneapolis, MN) fitted tightly over the edges of the nostril. This procedure effectively isolated the nostril being examined and prevented retronasal airflow (Bromley and Doty 1995).
Odor Identification Testing
Odor identification performance was assessed using the University of Pennsylvania Smell Identification Test (UPSIT) (Doty et al. 1989; Doty et al. 1984). The UPSIT is a standardized, four-alternative, forced-choice test of olfactory identification comprised of four booklets containing ten odorants apiece, one odorant per page. The stimuli are embedded in “scratch and sniff” microcapsules fixed and positioned on strips at the bottom of each page. A multiple-choice question with four response alternatives for each item is located above each odorant strip. The specific stimuli, basis for their selection, and the reliability and sensitivity of this test have been described in detail elsewhere (Doty et al. 1989; Doty et al. 1984). The UPSIT was administered individually by a trained technician, who released the microencapsulated stimuli, placed them under each participant’s nostril, and recorded the answer following the participant’s response. Two booklets of 20 items were presented to the right nostril and the remaining two booklets were presented to the left nostril; the order of booklet and nostril presentation was systematically counterbalanced to avoid position effects.
Odor Detection Threshold Sensitivity Testing
All subjects received a single staircase, forced-choice odor detection threshold test to estimate basal detection sensitivity to phenyl ethyl alcohol (PEA: Gold Label Grade; Aldrich Chemical Co., Milwaukee, WI), a compound with low trigeminal stimulation properties (Doty et al. 1978; Doty et al. 1984) in each nostril. In this procedure, the staircase began at the -6.00 log concentration step of a half-log step (vol/vol) dilution series extending from the weakest -10.00 log concentration to the strongest -2.00 log concentration. Initially, it was moved upward in full-log steps until correct detection occurred on five sets of consecutive trials at a given concentration level. If during this initial phase, an incorrect response was given on any trial, the staircase was moved upward a full-log step. Once the criterion of five consecutive correct responses was made, the staircase was reversed and subsequently moved up or down in half-log increments, depending upon the subject’s performance on two pairs of trials (i.e., each pair consisting of a choice between diluent and odorant) at each concentration step. The geometric mean of the last four staircase reversal points of a total of seven served as the estimate of threshold sensitivity (Doty et al. 1986).
3. Results
In congruence with our previous findings, significant differences in the distribution of tasters and non-tasters among the three groups was observed (χ2=10.9, df=2, p=0.004), with both patients (χ2=9.2, df=1, p=0.0023) and family members (χ2=8.2, df=1, p=0.003) showing an increased prevalence of non-tasters to PTC relative to controls (Figure 1). Notably, first-degree family members and probands did not differ significantly from each other (χ2=0.09, df=1, p=0.76). Among tasters, there was no difference in intensity ratings between controls and patients (F[1,93]=2.8, p=0.09) or family members (F[1,93]=1.3, p=0.25). No effects of sex or diagnosis-by-sex interaction were observed (all ps>0.20). Linear regression analyses with age, sex, education, and smoking history as predictors did not alter the observed differences in taster status or PTC intensity ratings.
Figure 1.
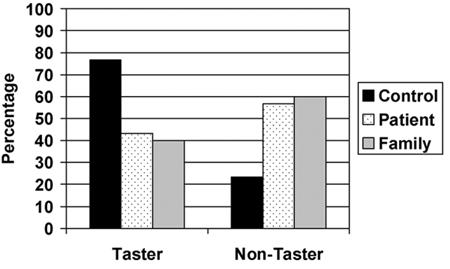
Distribution of taster and non-taster status across patients, controls and first-degree family members.
In order to probe the clinical significance of these genetically-mediated differences in taster status within the proband group, we contrasted tasters and non-tasters on symptom composites derived from the SANS, SAPS, and BPRS. General linear analysis revealed a significant taster status by symptom composite interaction (F[3,117]=3.62, p=0.015). Post-hoc contrasts revealed that non-tasters showed higher negative symptom ratings than tasters (F[1,39]=6.4, p=0.016), as well as higher levels of Schneiderian or first-rank symptoms (F[1,39]=4.7, p=0.036) (Figure 2).
Figure 2.
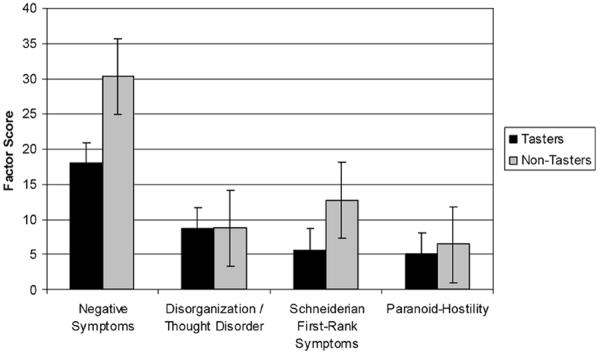
Comparisons of tasters and nontasters of PTC on symptom factor scores in patients with schizophrenia.
In patients, PTC tasters and non-tasters did not differ with regard to age of onset (F[1,52]=0.03, p=0.86), duration of illness (F[1,52]=0.27, p=0.60), illness severity (F[1,41]=0.33, p=0.57), number of hospitalizations (F[1,45]=0.26, p=0.61), or deficit vs. non-deficit classification (χ2=2.02, df=1, p=0.15).
Given the tight anatomical and functional interrelationships between taste and smell, we compared tasters and non-tasters in the patient group on psychophysical measures of odor identification and detection threshold sensitivity. In patients, a significant taster status by nostril interaction on the UPSIT was observed (F[1,57]=4.2, p=0.045), with non-tasters showing poorer right nostril odor identification performance relative to tasters. In contrast, no significant differences in PEA detection thresholds were observed between tasters and non-tasters in the patient group (all p’s >0.18). No differences between PTC tasters and non-tasters on the UPSIT and PEA were seen in either non-ill family members or healthy controls.
4. Discussion
These data replicate and extend prior findings of an increased prevalence of PTC non-tasters or “taste blindness” in patients with schizophrenia and their non-ill first-degree relatives, and further indicates that the ability to taste PTC may represent an endophenotypic marker of an inherited neuronal abnormality that conveys risk for the development of schizophrenia. Among healthy controls, 77% were classified as tasters of PTC, in contrast to only 43% among patients and 40% among first-degree family members. The prevalence of non-tasters in the patient and family samples is generally consistent with the four other reports of PTC perception where non-taster prevalence ranged from 34% to 57% among patients with schizophrenia (Constantinidis 1958; Freire-Maia et al. 1968; Moberg et al. 2005; Schlosberg and Baruch 1992). This increased prevalence of non-tasters could not be explained by basal differences in intensity perception, age, sex, education, or smoking history. Similarly, a higher prevalence of non-tasters among patients could not be explained by a greater proportion of African-Americans because this ethnic group has typically been shown to exhibit a lower prevalence of non-tasters than other populations (Barnicot 1950; Guo and Reed 2001; Mourant et al. 1976; Yackinous and Guinard 2001).
Analysis of clinical symptom ratings revealed that non-tasting patients exhibited higher levels of both negative and first-rank symptoms relative to patients who were tasters of PTC. These findings differ somewhat from our prior investigation which found that, among probands, non-tasters of PTC had higher ratings on the hallucinations and delusions subscale of the SAPS but not any differences in negative symptomatology as measured by the SANS (Moberg et al. 2005). In the only other study to address clinical symptoms, Schlosberg and Baruch (1992) found that non-paranoid patients were more likely to be non-tasters of PTC (60%) than those with a diagnosis of paranoia (24%). Some of the differences between the current findings and those of prior studies likely are attributable to the fact that a factor analytic approach was used to derive four orthogonal symptom “factors” from the various clinical symptom rating scales as opposed to prior approaches of using raw total scores from the SANS, SAPS, or BPRS. In our factor analytic strategy, each factor contains items from each of the three scales, and as such, would be psychometrically different than the total scores from each of the three rating scales alone. Along this same line, the study by Schlosberg and Baruch (1992) took an entirely different approach, instead clustering patients by clinical subtypes using only DSM-III-R criteria (i.e., paranoid, non-paranoid, etc.). Each of these approaches differs on a number of levels and likely explains the observed heterogeneity of findings. Regardless, the current data suggest that non-tasters show higher levels of productive and nonproductive psychotic symptoms in general, perhaps reflecting a greater genetic basis for these symptoms.
It is notable that previous work investigating deficit and non-deficit patients for peripheral G protein levels found a significant relationship between these assays and degree of negative symptoms in both groups of patients (Monteleone et al. 2002). We speculate that the difference in PTC tasting status reflects underlying G protein-coupled signal transduction abnormalities that have some contribution to the clinical symptoms of the illness. Given some of the stated inconsistencies in the literature thus far, further studies are clearly needed to address the relationships between PTC tasting status, G protein abnormalities, and clinical symptomatology.
In addition to the aforementioned clinical symptoms, probands who were unable to taste PTC were also impaired in right nostril odor identification performance relative to those patients who could taste PTC. A number of studies have recently discovered that odorant transduction combines unique receptive molecules (a novel family of G protein-coupled receptors) with common G protein-mediated transduction cascades (G protein-coupled adenylyl cyclase cascade) to detect odorants (Ronnett and Moon 2002). In light of these mechanisms, the observed link between odor identification abilities and PTC tasting status might be expected. While chromosome 7q has not been identified as a target in schizophrenia research, it is notable that this chromosome also contains odorant receptor-like (OR-like) genes (Buck and Axel 1991; Kim et al. 2003) that may have implications for the significant deficits in olfactory function seen in patients (Moberg et al. 1999). Indeed, a recent study found that tasters of the bitter compound 6-n-propylthiouracil (PROP) had better detection thresholds to the odor diacetyl than nonasters (Yackinous and Guinard 2001). Similarly, Lawless and colleagues reported that suprathreshold intensity ratings to this odorant by healthy controls fell into a bimodal distribution, suggesting a specific anosmia or “smell-blindness” for this compound (Lawless et al. 1994). These data, in concert with the current findings suggest that PTC tasting status may reflect a general sensory ability or “perceptual tuning” not limited solely to gustatory function. Possible relationships between PTC sensitivity and other sensory markers require further investigation.
Preliminary data from our lab on schizophrenia spectrum- and bipolar-disorder patients suggest that these findings may be specific to patients with schizophrenia. A small independent sample of six patients with a DSM-IV diagnosis of schizoaffective disorder-depressed type were all tasters of PTC. When compared to other schizophrenia patients in the current dataset, this difference in taster status was significant (χ2=6.92, df=1, p=0.009). Also, of three bipolar patients assessed in our lab, all three were tasters of PTC. This group also displayed a trend towards significance when compared to our schizophrenia cohort (χ2=3.63, df=1, p=0.06). While these data on schizophrenia spectrum- and bipolar-disorder are quite limited, it does suggest that an increased prevalence of PTC non-tasters may be particular to schizophrenia. Further investigation into these hypotheses using larger samples is warranted.
Although confirming genotyping data for PTC and assays of G protein are lacking in the current study, the use of psychophysical PTC sensitivity measures as an index of disease risk in schizophrenia holds promise. The ease of administration and short testing time (approximately 5 minutes) makes this method ideal for screening large groups of subjects for prodromal or genetic studies. The finding of similar impairment in first-degree relatives suggests that people with at least one dominant allele may be at a lower risk than those with two recessive alleles. As the genes for this very simple perceptual task are known, the examination of patients unable to taste PTC may prove fruitful in narrowing the search of candidate genes for schizophrenia.
Acknowledgements
This research was funded in part by National Institutes of Health Grants MH-63381 to Dr. Moberg, MH-59852 to Dr. Turetsky, MH-67091 to Dr. Kanes, MH-42191 to Dr. Gur, and an Independent Investigator Award from the National Alliance for Research on Schizophrenia and Depression to Dr. Moberg. The authors thank the Hofmann Trust for their support of this research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- APA . Diagnostic and Statistical Manual of Mental Disorders. Fourth Edition American Psychiatric Association; Washington, D.C.: 1994. [Google Scholar]
- Andreasen NC. Scale for the Assessment of Negative Symptoms (SANS) University of Iowa; Iowa City, IA: 1984a. [Google Scholar]
- Andreasen NC. The Scale for the Assessment of Positive Symptoms (SAPS) The University of Iowa; Iowa City, IA: 1984b. [Google Scholar]
- Barnicot NA. Taste deficiency for phenylthiourea in African Negroes and Chinese. Annals of Eugenics. 1950;15:248–254. doi: 10.1111/j.1469-1809.1949.tb02440.x. [DOI] [PubMed] [Google Scholar]
- Bartoshuk LM, Duffy VB, Miller IJ. PTC/PROP tasting: anatomy, psychophysics, and sex effects. Physiology & Behavior. 1994;56:1165–1171. doi: 10.1016/0031-9384(94)90361-1. [DOI] [PubMed] [Google Scholar]
- Blakeslee AF. Genetics of sensory thresholds: taste for phenyl-thio-carbamide. Proceedings of the National Academy of Sciences USA. 1932;18:120–130. doi: 10.1073/pnas.18.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromley SM, Doty RL. Odor recognition memory is better under bilateral than unilateral test conditions. Cortex. 1995;31(1):25–40. doi: 10.1016/s0010-9452(13)80103-7. [DOI] [PubMed] [Google Scholar]
- Buck L, Axel R. A novel multigene family may encode odorant receptors: a molecular basis for odor recognition. Cell. 1991;65:175–187. doi: 10.1016/0092-8674(91)90418-x. [DOI] [PubMed] [Google Scholar]
- Bufe B, Breslin PAS, Kuhn C, Reed DR, Tharp CD, Slack JP, Kim U-K, Drayna D, Meyerhof W. The molecular basis of individual differences in phenylthiocarbamide and propylthiouracil bitterness perception. Current Biology. 2005;15:322–327. doi: 10.1016/j.cub.2005.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter WT, Heinrichs DW, Wagman AM. Deficit and nondeficit forms of schizophrenia: the concept. American Journal of Psychiatry. 1988;145(5):578–583. doi: 10.1176/ajp.145.5.578. [DOI] [PubMed] [Google Scholar]
- Constantinidis J. Les marqueurs de chromosomes chez les schizophrènes et la recherche du linkage entre ces caractères et la schizophrenie par la méthode de Penrose. Journal de Genetique Humaine. 1958;7:189–242. [Google Scholar]
- Doty RL, Brugger WE, Jurs PC, Orndorff MA, Synder PJ, Lowry D. Intranasal trigeminal stimulation from odorous volatiles: psychometric responses from anosmic and normal humans. Physiology & Behavior. 1978;20(2):175–185. doi: 10.1016/0031-9384(78)90070-7. [DOI] [PubMed] [Google Scholar]
- Doty RL, Frye RE, Agrawal U. Internal consistency reliability of the fractionated and whole University of Pennsylvania Smell Identification Test. Perception and Psychophysics. 1989;45(5):381–384. doi: 10.3758/bf03210709. [DOI] [PubMed] [Google Scholar]
- Doty RL, Gregor T, Monroe C. Quantitative assessment of olfactory function in an industrial setting. Journal of Occupational Medicine. 1986;28(6):457–460. doi: 10.1097/00043764-198606000-00015. [DOI] [PubMed] [Google Scholar]
- Doty RL, Shaman P, Dann M. Development of the University of Pennsylvania Smell Identification Test: a standardized microencapsulated test of olfactory function. Physiology & Behavior. 1984;32(3):489–502. doi: 10.1016/0031-9384(84)90269-5. [DOI] [PubMed] [Google Scholar]
- Drayna D. Human taste genetics. Annual Review of Genomics and Human Genetics. 2005;6:217–235. doi: 10.1146/annurev.genom.6.080604.162340. [DOI] [PubMed] [Google Scholar]
- Drayna D, Coon H, Kim U-K, Elsner T, Cromer K, Otterud B, Baird L, Peiffer AP, Lepport M. Genetic analysis of a complex trait in the Utah Gentic Reference Project: a major locus for PTC taste ability on chromosome 7q and secondary locus on chromosome 16p. Human Genetics. 2003;112:567–572. doi: 10.1007/s00439-003-0911-y. [DOI] [PubMed] [Google Scholar]
- Driscol KA, Perez M, Cukrowicz KC, Butler M, Joiner TE. Associations of phenylthiocarbamide tasting to alcohol problems and family history of alcoholism differ by gender. Psychiatry Research. 2006;143:21–27. doi: 10.1016/j.psychres.2005.07.029. [DOI] [PubMed] [Google Scholar]
- Enoch MA, Harris CR, Goldman D. Does a reduced sensitivity to bitter taste increase the risk of becoming nicotine addicted. Addictive Behaviors. 2001;26:399–404. doi: 10.1016/s0306-4603(00)00117-9. [DOI] [PubMed] [Google Scholar]
- First MB. Structured Clinical Interview for DSM-IV - Patient Edition. New York State Psychiatric Institute; New York: 1996. (SCID-P, Version 2.0) [Google Scholar]
- First MB, Spitzer RL, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders, Non-Patient Edition. State Psychiatric Institute/Biometrics Research Department; New York: 1995. (SCID-NP) [Google Scholar]
- First MB, Spitzer RL, Williams JBW, Benjamin LS. Structured Clinical Interview for DSM-IV Axis II Personality Disorders. American Psychiatric Press; Washington, DC.: 1997. (SCID-II) [Google Scholar]
- Fox AL. Taste blindness. Science. 1931;73:14. [Google Scholar]
- Freire-Maia N, Karam EJ, Mehl H. PTC sensitivity among psychiatric patients. Acta Genetica et Statistica Medica. 1968;18:31–37. doi: 10.1159/000152117. [DOI] [PubMed] [Google Scholar]
- Gainetdinov RR, Premont RT, Bohn LM, Lefkowitz RJ, Caron MG. Desensitization of G protein-coupled receptors and neuronal functions. Annual Review of Neuroscience. 2004;27:107–144. doi: 10.1146/annurev.neuro.27.070203.144206. [DOI] [PubMed] [Google Scholar]
- Gould TJ, Bizily SP, Tokarczyk J, Kelly MP, Siegel SJ, Kanes SJ, Abel T. Sensorimotor gating deficits in transgenic mice expressing a constitutively active form of Gsα. Neuropsychopharmacology. 2004;29:494–501. doi: 10.1038/sj.npp.1300309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo S-W, Reed DR. The genetics of phenylthiocarbamide perception. Annals of Human Biology. 2001;28(2):111–142. doi: 10.1080/03014460151056310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur RE, Mozley PD, Shtasel DL, Cannon TD, Gallacher F, Turetsky B, Grossman R, Gur RC. Clinical subtypes of schizophrenia: differences in brain and CSF volume. American Journal of Psychiatry. 1994;151(3):343–350. doi: 10.1176/ajp.151.3.343. [DOI] [PubMed] [Google Scholar]
- Joiner T, Perez M. Phenylthiocarbamide tasting and family history of depression, revisited: low rates of depression in families of supertasters. Psychiatry Research. 2004;126:83–87. doi: 10.1016/j.psychres.2003.12.020. [DOI] [PubMed] [Google Scholar]
- Kim U-K, Jorgenson E, Coon H, Leppert M, Risch N, Drayna D. Positional cloning of the human quantitative trait locus underlying taste sensitivity to phenylthiocarbamide. Science. 2003;299:1221–1225. doi: 10.1126/science.1080190. [DOI] [PubMed] [Google Scholar]
- Lawless HT, Antinone MJ, Ledford RA, Johnston M. Olfactory responsiveness to diacetyl. Journal of Sensory Studies. 1994;9:47–56. [Google Scholar]
- Mattes RD. 6-n-propylthiouracil taster status: dietary modifier, marker, or misleader? In: Prescott J, Tepper BJ, editors. Genetic variation in taste sensitivity. Marcel Dekker, Inc.; New York: 2004. pp. 229–250. [Google Scholar]
- Moberg PJ, Agrin R, Gur RE, Gur RC, Turetsky BI, Doty RL. Olfactory dysfunction in schizophrenia: A qualitative and quantitative review. Neuropsychopharmacology. 1999;21:325–340. doi: 10.1016/S0893-133X(99)00019-6. [DOI] [PubMed] [Google Scholar]
- Moberg PJ, Roalf DR, Balderston CC, Kanes SJ, Gur RE, Turetsky BI. Phenylthiocarbamide (PTC) perception in patients with schizophrenia and first-degree relatives. American Journal of Psychiatry. 2005;162:788–790. doi: 10.1176/appi.ajp.162.4.788. [DOI] [PubMed] [Google Scholar]
- Monteleone P, Di Lieto A, Martiadis V, Bartoli L, Maj M. Correlations between negative symptoms and peripheral G protein levels in mononuclear leukocytes of deficit and nondeficit schizophrenics. European Archives of Psychiatry and Clinical Neuroscience. 2002;252(5):214–218. doi: 10.1007/s00406-002-0383-4. [DOI] [PubMed] [Google Scholar]
- Mourant A, Kopec A, Domaniewski-Sobezak K. The distribution of the human blood groups and other polymorphisms. Oxford University Press; Oxford, UK: 1976. [Google Scholar]
- Overall M, Gorham DR. The Brief Psychiatric Rating Scale. Journal of Operational Psychiatry. 1980;11:48–64. [Google Scholar]
- Pal SK, Sharma K, Pathak A, Sawhney IM, Prabhakar S. Possible relationship between phenylthiocarbamide taste sensitivity and epilepsy. Neurology India. 2004;52:206–209. [PubMed] [Google Scholar]
- Reed DR. Gene mapping for taste related phenotypes in humans and mice. Appetite. 2000;35:189–190. doi: 10.1006/appe.2000.0347. [DOI] [PubMed] [Google Scholar]
- Ronnett GV, Moon C. G proteins and olfactory signal transduction. Annual Review of Physiology. 2002;64:189–222. doi: 10.1146/annurev.physiol.64.082701.102219. [DOI] [PubMed] [Google Scholar]
- Schlosberg A, Baruch I. Phenylthiocarbamide (PTC) tasting in paranoid and nonparanoid schizophrenic patients. Perceptual and Motor Skills. 1992;74:383–386. doi: 10.2466/pms.1992.74.2.383. [DOI] [PubMed] [Google Scholar]
- Schreiber G, Avissar S. G proteins as a biochemical tool for diagnosing and monitoring treatments of mental disorders. The Israel Medical Association Journal. 2000;2(8691) [PubMed] [Google Scholar]
- Schreiber G, Avissar S. Application of G-proteins in the molecular diagnosis of psychiatric disorders. Expert Review of Molecular Diagnostics. 2003;3(1):69–80. doi: 10.1586/14737159.3.1.69. [DOI] [PubMed] [Google Scholar]
- Sidhu A, Niznik HB. Coupling of dopamine receptor subtypes to multiple and diverse G proteins. International Journal of Developmental Neuroscience. 2000;18:669–677. doi: 10.1016/s0736-5748(00)00033-2. [DOI] [PubMed] [Google Scholar]
- Snyder LH. Inherited taste deficiency. Science. 1931;74:151–152. doi: 10.1126/science.74.1910.151. [DOI] [PubMed] [Google Scholar]
- Whittemore P. Phenylthiocarbamide (PTC) tasting, genetics, and depression. Journal of Clinical Psychology. 1990;46:262–272. doi: 10.1002/1097-4679(199005)46:3<262::aid-jclp2270460303>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Yackinous C, Guinard JX. Relation between PROP taster status and fat perception, touch, and olfaction. Physiology & Behavior. 2001;72:427–437. doi: 10.1016/s0031-9384(00)00430-3. [DOI] [PubMed] [Google Scholar]


