Abstract
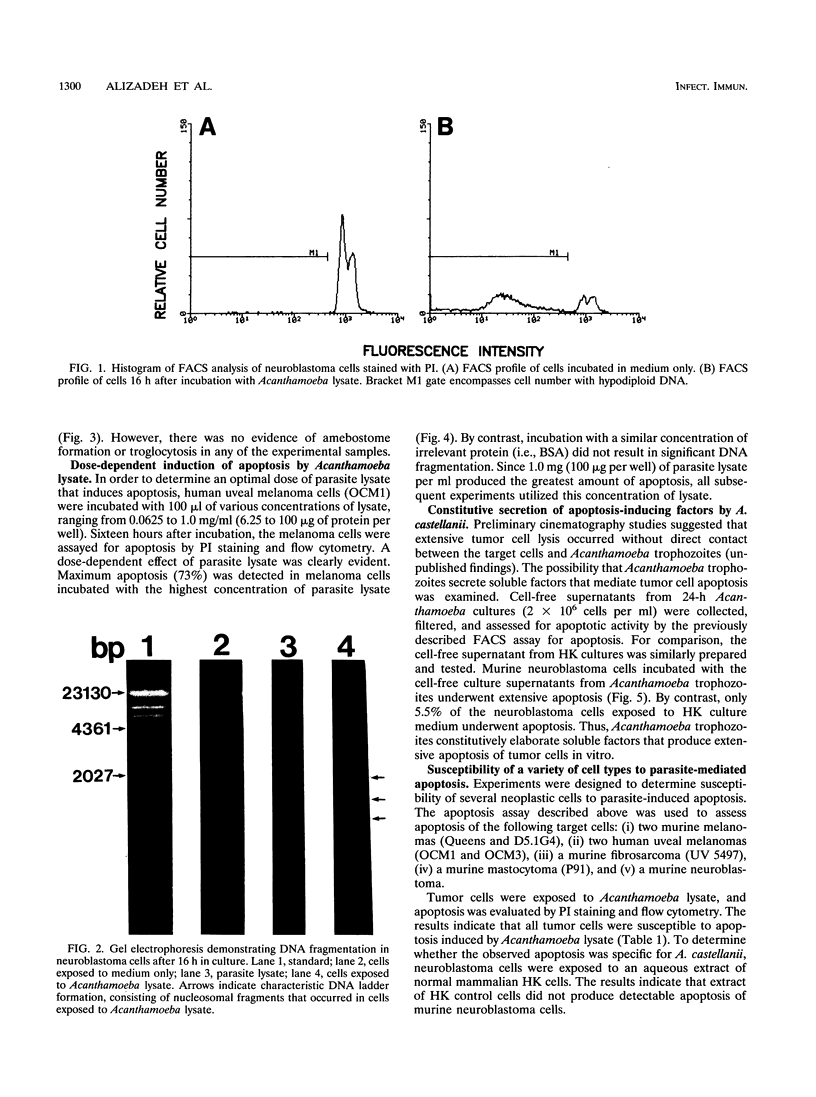
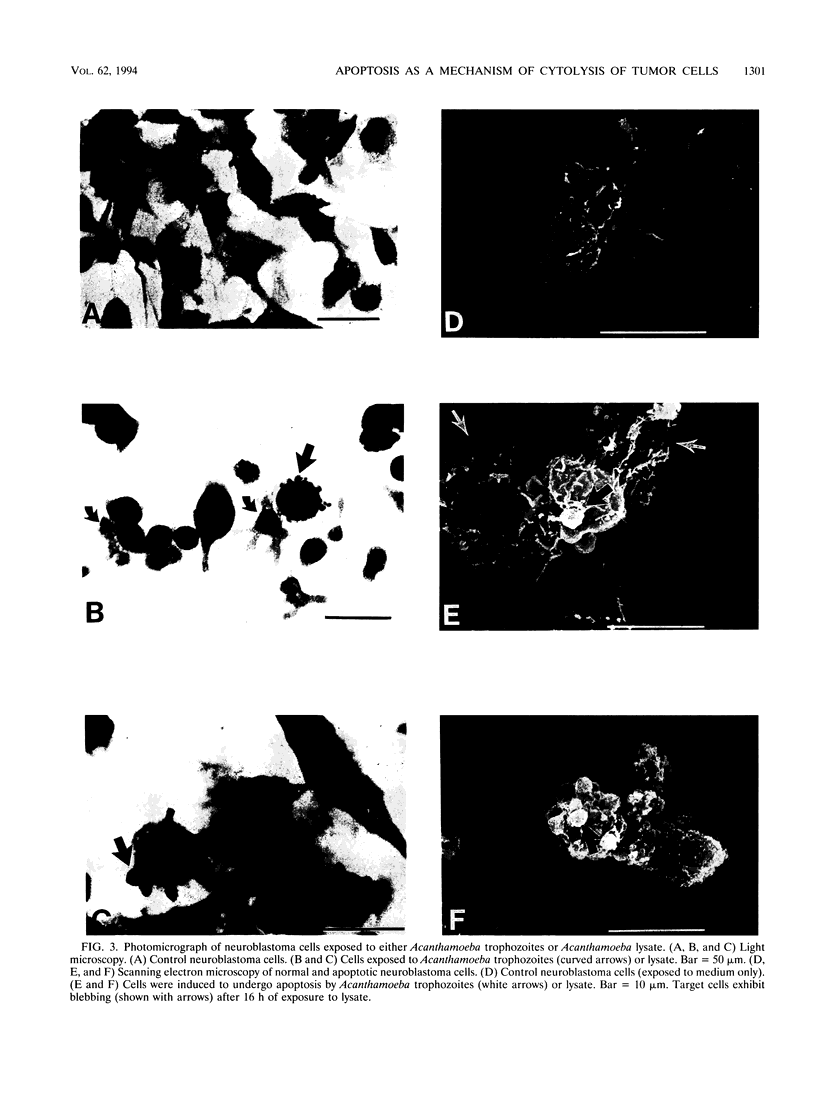
Previous studies have shown that trophozoites of the pathogenic free-living amoeba Acanthamoeba castellanii rapidly lysed a variety of tumor cells in vitro. Tumor cells undergoing parasite-mediated lysis displayed characteristic cell membrane blebbing reminiscent of apoptosis. The present investigation examined the role of apoptosis (programmed cell death) in Acanthamoeba-mediated tumor cell lysis. The results showed that more than 70% of tumor cell DNA was fragmented following exposure to Acanthamoeba cell extracts. By contrast, only 7% of untreated control cells underwent DNA fragmentation. DNA fragmentation increased significantly in a dose-dependent fashion following concentration of the parasite extract. Apoptosis was also confirmed by DNA ladder formation. Characteristic DNA ladders, consisting of multimers of approximately 180 to 200 bp, were produced by tumor cells exposed to Acanthamoeba cell extracts. The morphology of tumor cell lysis was examined by light and scanning electron microscopy. Tumor cells exposed to parasite extract displayed morphological features characteristic of apoptosis including cell shrinkage, cell membrane blebbing, formation of apoptotic bodies, and nuclear condensation. By contrast, similar effects were not found in tumor cells exposed to extract similarly prepared from normal mammalian cells (i.e., human keratocytes). The results suggest that at least one species of pathogenic free-living amoeba is able to lyse tumor cells by a process that culminates in apoptosis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auran J. D., Starr M. B., Jakobiec F. A. Acanthamoeba keratitis. A review of the literature. Cornea. 1987;6(1):2–26. [PubMed] [Google Scholar]
- Berke G. T-cell-mediated cytotoxicity. Curr Opin Immunol. 1991 Jun;3(3):320–325. doi: 10.1016/0952-7915(91)90031-u. [DOI] [PubMed] [Google Scholar]
- Brown T. Observations by immunofluorescence microscopy and electron microscopy on the cytopathogenicity of Naegleria fowleri in mouse embryo-cell cultures. J Med Microbiol. 1979 Aug;12(3):363–371. doi: 10.1099/00222615-12-3-363. [DOI] [PubMed] [Google Scholar]
- Cohen J. J., Duke R. C. Glucocorticoid activation of a calcium-dependent endonuclease in thymocyte nuclei leads to cell death. J Immunol. 1984 Jan;132(1):38–42. [PubMed] [Google Scholar]
- Gitler C., Calef E., Rosenberg I. Cytopathogenicity of Entamoeba histolytica. Philos Trans R Soc Lond B Biol Sci. 1984 Nov 13;307(1131):73–85. doi: 10.1098/rstb.1984.0110. [DOI] [PubMed] [Google Scholar]
- Green D. R., Cotter T. G. Introduction: apoptosis in the immune system. Semin Immunol. 1992 Dec;4(6):355–362. [PubMed] [Google Scholar]
- He Y. G., Niederkorn J. Y., McCulley J. P., Stewart G. L., Meyer D. R., Silvany R., Dougherty J. In vivo and in vitro collagenolytic activity of Acanthamoeba castellanii. Invest Ophthalmol Vis Sci. 1990 Nov;31(11):2235–2240. [PubMed] [Google Scholar]
- John D. T., Cole T. B., Jr, Marciano-Cabral F. M. Sucker-like structures on the pathogenic amoeba Naegleria fowleri. Appl Environ Microbiol. 1984 Jan;47(1):12–14. doi: 10.1128/aem.47.1.12-14.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John D. T., John R. A. Cytopathogenicity of Naegleria fowleri in mammalian cell cultures. Parasitol Res. 1989;76(1):20–25. doi: 10.1007/BF00931066. [DOI] [PubMed] [Google Scholar]
- Kan-Mitchell J., Mitchell M. S., Rao N., Liggett P. E. Characterization of uveal melanoma cell lines that grow as xenografts in rabbit eyes. Invest Ophthalmol Vis Sci. 1989 May;30(5):829–834. [PubMed] [Google Scholar]
- Knisely T. L., Niederkorn J. Y. Immunologic evaluation of spontaneous regression of an intraocular murine melanoma. Invest Ophthalmol Vis Sci. 1990 Feb;31(2):247–257. [PubMed] [Google Scholar]
- Larkin D. F., Berry M., Easty D. L. In vitro corneal pathogenicity of Acanthamoeba. Eye (Lond) 1991;5(Pt 5):560–568. doi: 10.1038/eye.1991.98. [DOI] [PubMed] [Google Scholar]
- Ma P., Visvesvara G. S., Martinez A. J., Theodore F. H., Daggett P. M., Sawyer T. K. Naegleria and Acanthamoeba infections: review. Rev Infect Dis. 1990 May-Jun;12(3):490–513. doi: 10.1093/clinids/12.3.490. [DOI] [PubMed] [Google Scholar]
- Marciano-Cabral F. M., Fulford D. E. Cytopathology of pathogenic and nonpathogenic Naegleria species for cultured rat neuroblastoma cells. Appl Environ Microbiol. 1986 May;51(5):1133–1137. doi: 10.1128/aem.51.5.1133-1137.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marciano-Cabral F., Zoghby K. L., Bradley S. G. Cytopathic action of Naegleria fowleri amoebae on rat neuroblastoma target cells. J Protozool. 1990 Mar-Apr;37(2):138–144. doi: 10.1111/j.1550-7408.1990.tb05884.x. [DOI] [PubMed] [Google Scholar]
- Martz E., Heagy W., Gromkowski S. H. The mechanism of CTL-mediated killing: monoclonal antibody analysis of the roles of killer and target-cell membrane proteins. Immunol Rev. 1983;72:73–96. doi: 10.1111/j.1600-065x.1983.tb01073.x. [DOI] [PubMed] [Google Scholar]
- Nicoletti I., Migliorati G., Pagliacci M. C., Grignani F., Riccardi C. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J Immunol Methods. 1991 Jun 3;139(2):271–279. doi: 10.1016/0022-1759(91)90198-o. [DOI] [PubMed] [Google Scholar]
- Niederkorn J. Y., Ubelaker J. E., McCulley J. P., Stewart G. L., Meyer D. R., Mellon J. A., Silvany R. E., He Y. G., Pidherney M., Martin J. H. Susceptibility of corneas from various animal species to in vitro binding and invasion by Acanthamoeba castellanii [corrected]. Invest Ophthalmol Vis Sci. 1992 Jan;33(1):104–112. [PubMed] [Google Scholar]
- Pidherney M. S., Alizadeh H., Stewart G. L., McCulley J. P., Niederkorn J. Y. In vitro and in vivo tumoricidal properties of a pathogenic/free-living amoeba. Cancer Lett. 1993 Aug 16;72(1-2):91–98. doi: 10.1016/0304-3835(93)90016-3. [DOI] [PubMed] [Google Scholar]
- Russell J. H., Dobos C. B. Mechanisms of immune lysis. II. CTL-induced nuclear disintegration of the target begins within minutes of cell contact. J Immunol. 1980 Sep;125(3):1256–1261. [PubMed] [Google Scholar]
- Sedmak J. J., Grossberg S. E. A rapid, sensitive, and versatile assay for protein using Coomassie brilliant blue G250. Anal Biochem. 1977 May 1;79(1-2):544–552. doi: 10.1016/0003-2697(77)90428-6. [DOI] [PubMed] [Google Scholar]
- Stopak S. S., Roat M. I., Nauheim R. C., Turgeon P. W., Sossi G., Kowalski R. P., Thoft R. A. Growth of acanthamoeba on human corneal epithelial cells and keratocytes in vitro. Invest Ophthalmol Vis Sci. 1991 Feb;32(2):354–359. [PubMed] [Google Scholar]
- Young J. D., Young T. M., Lu L. P., Unkeless J. C., Cohn Z. A. Characterization of a membrane pore-forming protein from Entamoeba histolytica. J Exp Med. 1982 Dec 1;156(6):1677–1690. doi: 10.1084/jem.156.6.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Klink F., Alizadeh H., Stewart G. L., Pidherney M. S., Silvany R. E., He Y., McCulley J. P., Niederkorn J. Y. Characterization and pathogenic potential of a soil isolate and an ocular isolate of Acanthamoeba castellanii in relation to Acanthamoeba keratitis. Curr Eye Res. 1992 Dec;11(12):1207–1220. doi: 10.3109/02713689208999546. [DOI] [PubMed] [Google Scholar]